Mitochondrial Respiration of Platelets: Comparison of Isolation Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Reagents
2.3. Blood Sampling
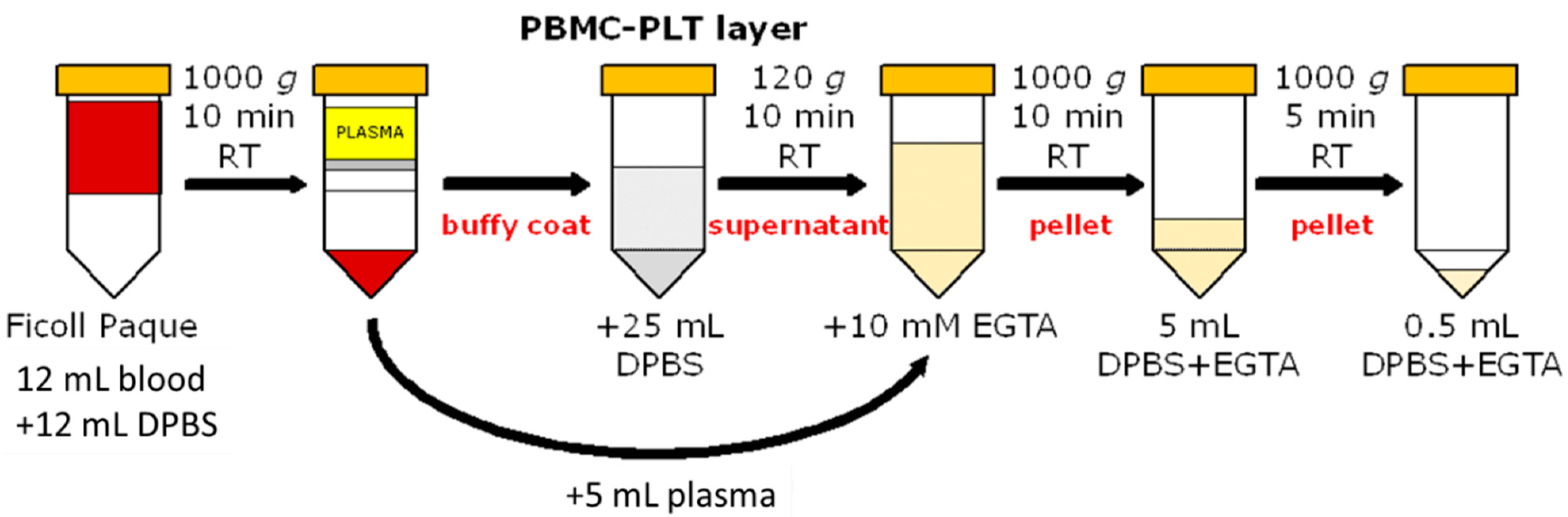
2.4. Platelet Preparation
2.4.1. Density Gradient Centrifugation
2.4.2. Platelets Apheresis from a Single Donor
2.4.3. Apheresis Sample Washing
2.5. Cell Counting
2.6. Flow Cytometry
2.7. Mitochondrial Respiration
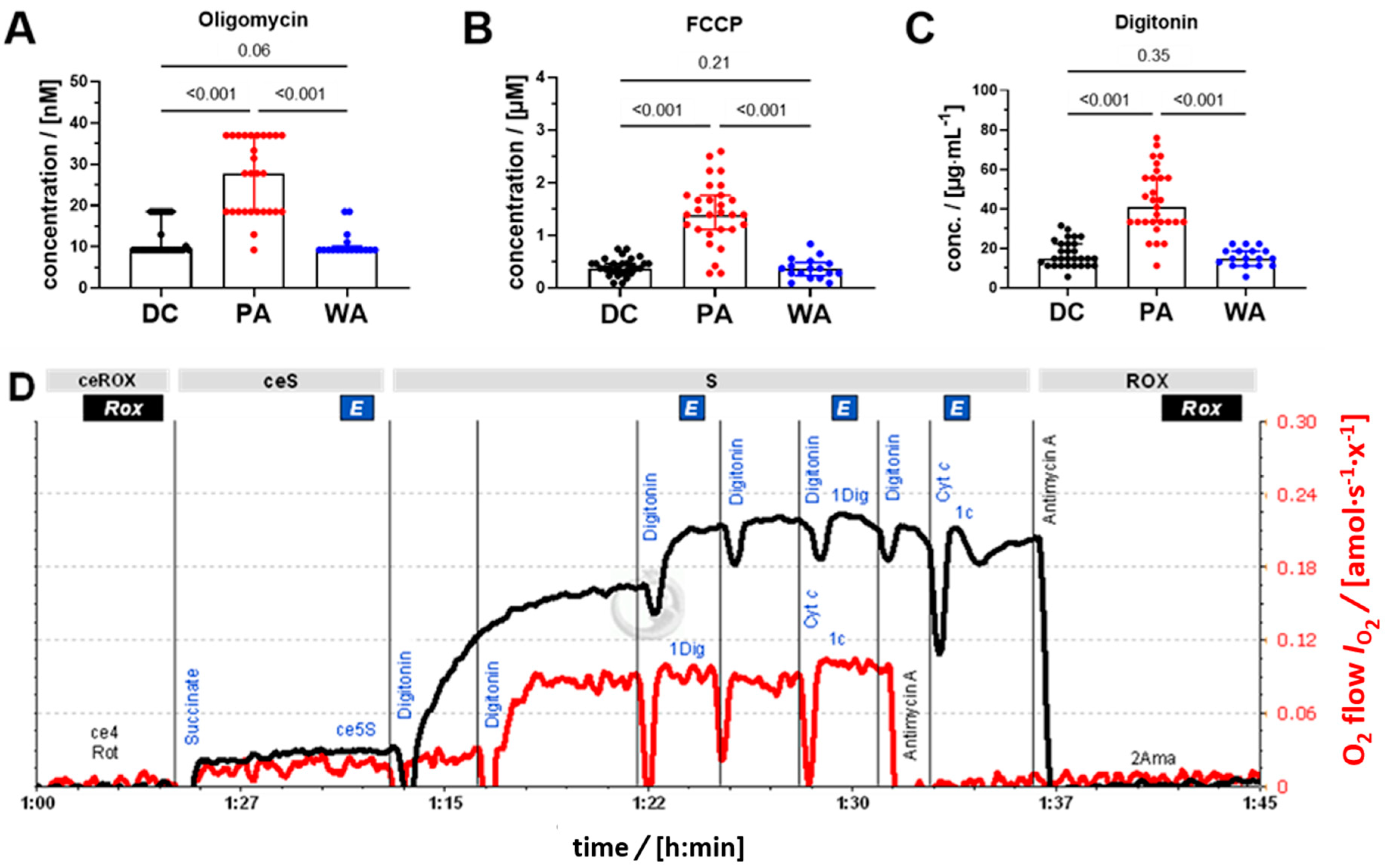
2.8. SUIT Protocol for HRR
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the PLT Donors
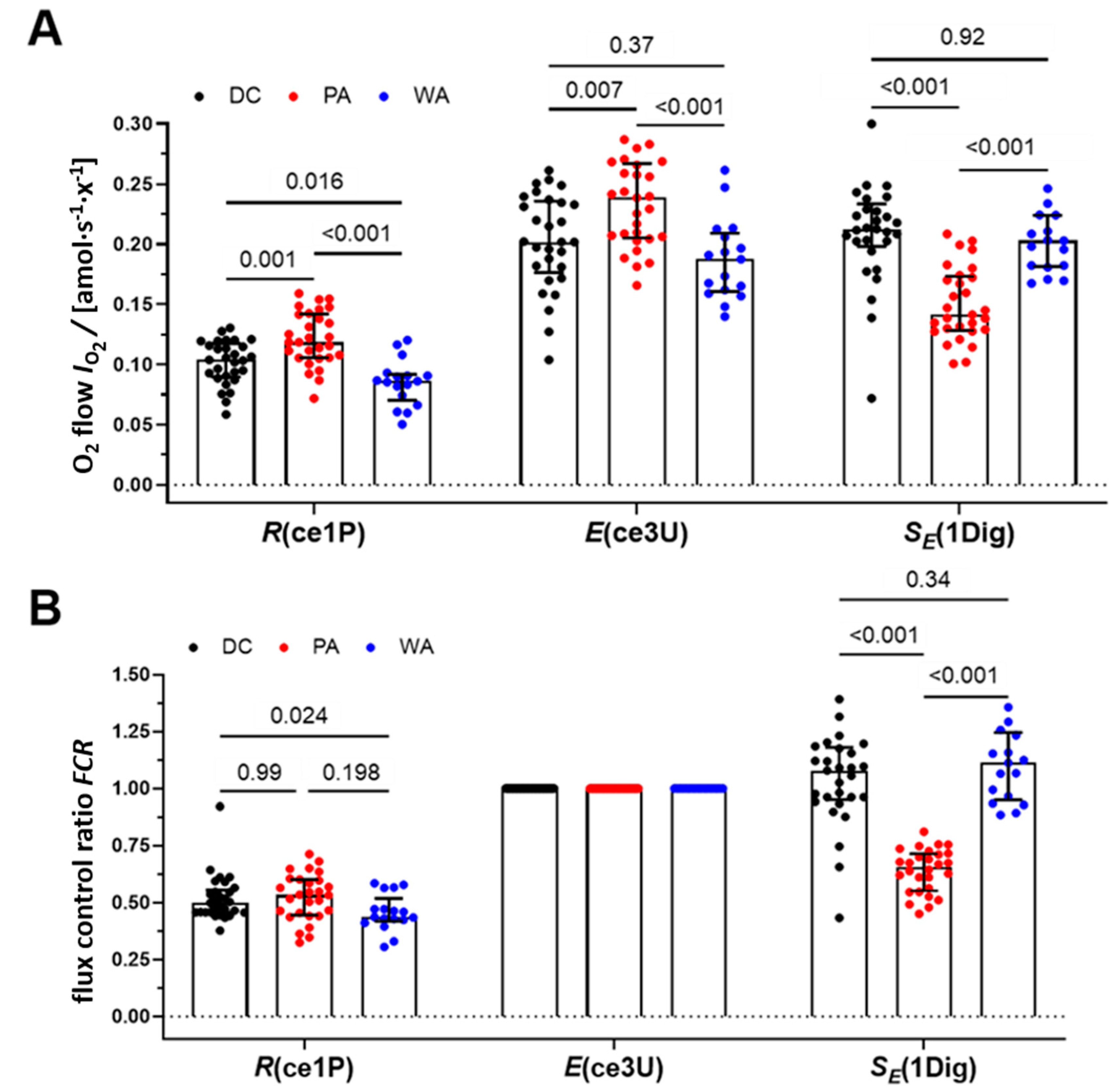
3.2. Mitochondrial Respiration of PLT
3.3. Yield and Sample Quality: Cytochrome C Test and Viability
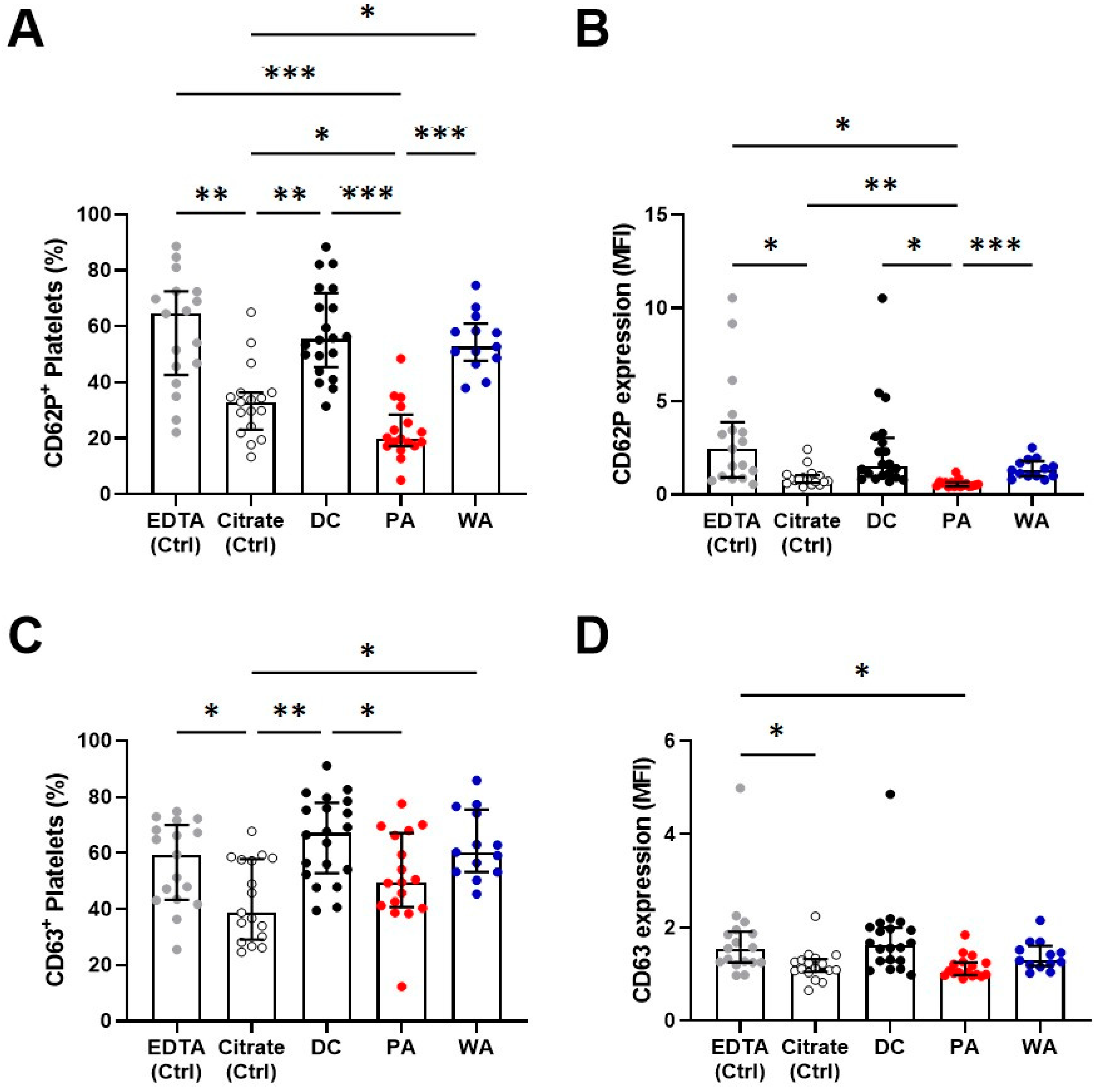
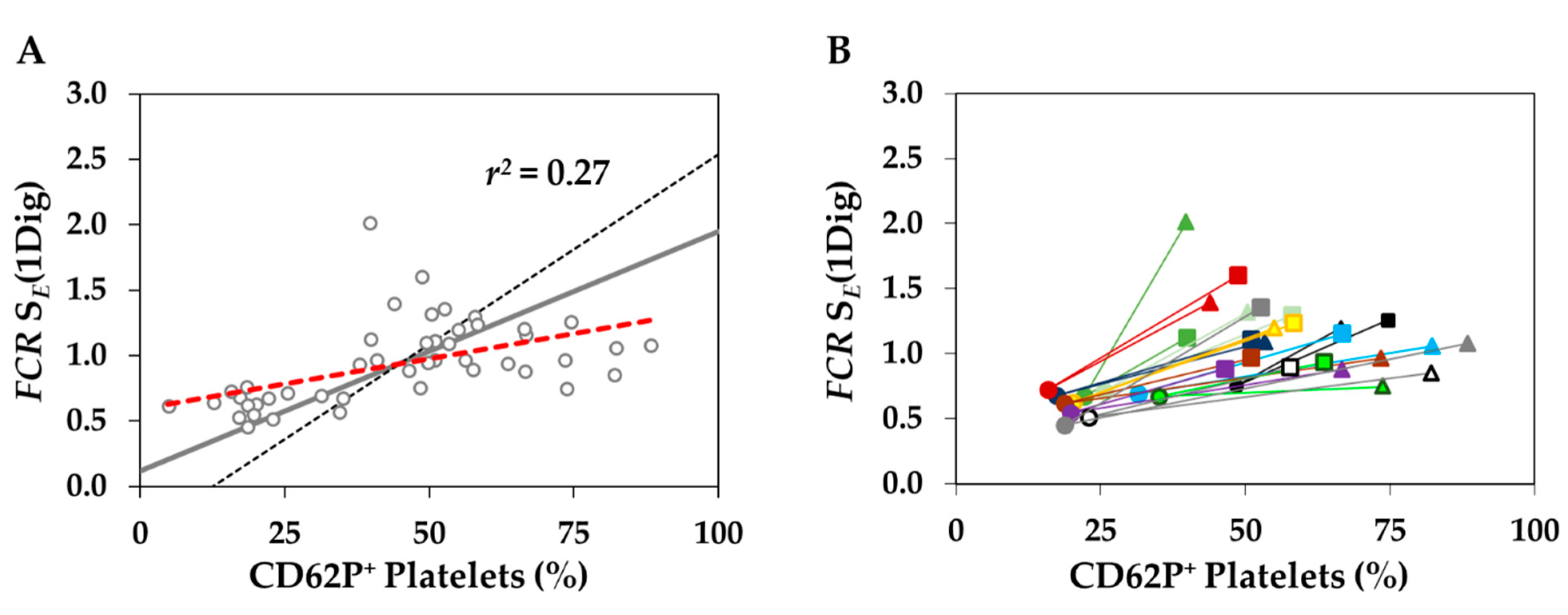
3.4. Platelet Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laki, K. Our Ancient Heritage in Blood Clotting and Some of Its Consequences. Ann. N. Y. Acad. Sci. 1972, 202, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [Green Version]
- Gnaiger, E. Mitochondrial Pathways and Respiratory Control: An Introduction to OXPHOS Analysis. Bioenerg. Commun. 2020, 2020, 112. [Google Scholar] [CrossRef]
- Gnaiger, E.; Steinlechner-Maran, R.; Méndez, G.; Eberl, T.; Margreiter, R. Control of Mitochondrial and Cellular Respiration by Oxygen. J. Bioenerg. Biomembr. 1995, 27, 583–596. [Google Scholar] [CrossRef]
- Sumbalova, Z.; Garcia-Souza, L.F.; Calabria, E.; Volani, C.; Gnaiger, E. O2k-Protocols: Isolation of Peripheral Blood Mononuclear Cells and Platelets from Human Blood for HRFR. Mitochondr. Physiol. Netw. 2020, 21, 1–15. Available online: https://wiki.oroboros.at/index.php/MiPNet21.17_BloodCellsIsolation (accessed on 14 October 2021).
- Villar-Vesga, J.; Grajales, C.; Burbano, C.; Vanegas–García, A.; Muñoz–Vahos, C.H.; Vásquez, G.; Rojas, M.; Castaño, D. Platelet-Derived Microparticles Generated in Vitro Resemble Circulating Vesicles of Patients with Rheumatoid Arthritis and Activate Monocytes. Cell. Immunol. 2019, 336, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Sutton, R.M.; Karlsson, M.; Sjövall, F.; Becker, L.B.; Berg, R.A.; Margulies, S.S.; Kilbaugh, T.J. Increased Platelet Mitochondrial Respiration after Cardiac Arrest and Resuscitation as a Potential Peripheral Biosignature of Cerebral Bioenergetic Dysfunction. J. Bioenerg. Biomembr. 2016, 48, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Puskarich, M.A.; Kline, J.A.; Watts, J.A.; Shirey, K.; Hosler, J.; Jones, A.E. Early Alterations in Platelet Mitochondrial Function Are Associated with Survival and Organ Failure in Patients with Septic Shock. J. Crit. Care 2016, 31, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppel, F.; Calabria, E.; Pesta, D.H.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Effects of Ultramarathon Running on Mitochondrial Function of Platelets and Oxidative Stress Parameters: A Pilot Study. Front. Physiol. 2021, 12, 632664. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Sumbalova, Z.; Kucharska, J.; Komlosi, M.; Rausova, Z.; Vancova, O.; Szamosova, M.; Mojto, V. Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease. Diagnostics 2020, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Gvozdjakova, A.; Sumbalova, Z.; Kucharska, J.; Chladekova, A.; Rausova, Z.; Vancova, O.; Komlosi, M.; Ulicna, O.; Mojto, V. Platelet Mitochondrial Bioenergetic Analysis in Patients with Nephropathies and Non-Communicable Diseases: A New Method. Bratisl. Med. J. 2019, 120, 630–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, C.; Huang, R.; Stevens, M.; Aponte, A.; Tripodi, D.; Kim, K.; Sack, M. Platelet Mitochondrial Dysfunction Is Evident in Type 2 Diabetes in Association with Modifications of Mitochondrial Anti-Oxidant Stress Proteins. Exp. Clin. Endocrinol. Diabetes 2012, 120, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Zharikov, S.; Shiva, S. Platelet Mitochondrial Function: From Regulation of Thrombosis to Biomarker of Disease. Biochem. Soc. Trans. 2013, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Merlo Pich, M.; Bovina, C.; Formiggini, G.; Cometti, G.G.; Ghelli, A.; Parenti Castelli, G.; Genova, M.L.; Marchetti, M.; Semeraro, S.; Lenaz, G. Inhibitor Sensitivity of Respiratory Complex I in Human Platelets: A Possible Biomarker of Ageing. FEBS Lett. 1996, 380, 176–178. [Google Scholar] [CrossRef] [Green Version]
- Braganza, A.; Corey, C.G.; Santanasto, A.J.; Distefano, G.; Coen, P.M.; Glynn, N.W.; Nouraie, S.-M.; Goodpaster, B.H.; Newman, A.B.; Shiva, S. Platelet Bioenergetics Correlate with Muscle Energetics and Are Altered in Older Adults. JCI Insight 2019, 4, e128248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Cardenes, N.; Corey, C.; Erzurum, S.C.; Shiva, S. Platelets from Asthmatic Individuals Show Less Reliance on Glycolysis. PLoS ONE 2015, 10, e0132007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjövall, F.; Morota, S.; Åsander Frostner, E.; Hansson, M.J.; Elmér, E. Cytokine and Nitric Oxide Levels in Patients with Sepsis–Temporal Evolvement and Relation to Platelet Mitochondrial Respiratory Function. PLoS ONE 2014, 9, e97673. [Google Scholar] [CrossRef]
- Protti, A.; Fortunato, F.; Artoni, A.; Lecchi, A.; Motta, G.; Mistraletti, G.; Novembrino, C.; Comi, G.; Gattinoni, L. Platelet Mitochondrial Dysfunction in Critically Ill Patients: Comparison between Sepsis and Cardiogenic Shock. Crit. Care 2015, 19, 39. [Google Scholar] [CrossRef] [Green Version]
- Sjövall, F.; Morota, S.; Hansson, M.J.; Friberg, H.; Gnaiger, E.; Elmér, E. Temporal Increase of Platelet Mitochondrial Respiration Is Negatively Associated with Clinical Outcome in Patients with Sepsis. Crit. Care 2010, 14, R214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente, L.; Martín, M.M.; López-Gallardo, E.; Blanquer, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; Jiménez, A.; Montoya, J.; Ruiz-Pesini, E. Decrease of Oxidative Phosphorylation System Function in Severe Septic Patients. J. Crit. Care 2015, 30, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Fišar, Z.; Jirák, R.; Zvěřová, M.; Setnička, V.; Habartová, L.; Hroudová, J.; Vaníčková, Z.; Raboch, J. Plasma Amyloid Beta Levels and Platelet Mitochondrial Respiration in Patients with Alzheimer’s Disease. Clin. Biochem. 2019, 72, 71–80. [Google Scholar] [CrossRef]
- Magron, A.; Laugier, J.; Provost, P.; Boilard, E. Pathogen Reduction Technologies: The Pros and Cons for Platelet Transfusion. Platelets 2018, 29, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Matsuyama, N.; Kuroishi, A.; Tani, Y.; Furuta, R.A.; Hirayama, F. Mitochondrial Damage-Associated Molecular Patterns as Potential Proinflammatory Mediators in Post-Platelet Transfusion Adverse Effects: Innate Immune Cell Activation by Mitochondrial DAMPs. Transfusion (Paris) 2016, 56, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Perales Villarroel, J.P.; Figueredo, R.; Guan, Y.; Tomaiuolo, M.; Karamercan, M.A.; Welsh, J.; Selak, M.A.; Becker, L.B.; Sims, C. Increased Platelet Storage Time Is Associated with Mitochondrial Dysfunction and Impaired Platelet Function. J. Surg. Res. 2013, 184, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bynum, J.A.; Adam Meledeo, M.; Getz, T.M.; Rodriguez, A.C.; Aden, J.K.; Cap, A.P.; Pidcoke, H.F. Bioenergetic Profiling of Platelet Mitochondria during Storage: 4 °C Storage Extends Platelet Mitochondrial Function and Viability. Transfusion (Paris) 2016, 56, S76–S84. [Google Scholar] [CrossRef]
- Ekaney, M.L.; Grable, M.A.; Powers, W.F.; McKillop, I.H.; Evans, S.L. Cytochrome c and Resveratrol Preserve Platelet Function during Cold Storage. J. Trauma Acute Care Surg. 2017, 83, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Kramer, P.A.; Ravi, S.; Johnson, M.S.; Hardy, R.W.; Ballinger, S.W.; Darley-Usmar, V.M. Methods for Defining Distinct Bioenergetic Profiles in Platelets, Lymphocytes, Monocytes, and Neutrophils, and the Oxidative Burst from Human Blood. Lab. Investig. 2013, 93, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Renner, K.; Geiselhöringer, A.-L.; Fante, M.; Bruss, C.; Färber, S.; Schönhammer, G.; Peter, K.; Singer, K.; Andreesen, R.; Hoffmann, P.; et al. Metabolic Plasticity of Human T Cells: Preserved Cytokine Production under Glucose Deprivation or Mitochondrial Restriction, but 2-Deoxy-Glucose Affects Effector Functions: Cellular Immune Response. Eur. J. Immunol. 2015, 45, 2504–2516. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Blood Transfusion (Council of Europe, Strasbourg, France). Good Practise Guidelines for Blood Establishment Required to Comply with Directive 2005/62/EC. Available online: https://www.edqm.eu/sites/default/files/medias/fichiers/Blood/good_practices_guidelines_for_blood_establisments-blood_guide_20th_may_2020.pdf (accessed on 12 October 2021).
- Gnaiger, E.; Kuznetsov, A.V.; Schneeberger, S.; Seiler, R.; Brandacher, G.; Steurer, W.; Margreiter, R. Mitochondria in the Cold. In Life in the Cold; Heldmaier, G., Klingenspor, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 431–432. [Google Scholar] [CrossRef]
- Sjövall, F.; Ehinger, J.K.H.; Marelsson, S.E.; Morota, S.; Åsander Frostner, E.; Uchino, H.; Lundgren, J.; Arnbjörnsson, E.; Hansson, M.J.; Fellman, V.; et al. Mitochondrial Respiration in Human Viable Platelets—Methodology and Influence of Gender, Age and Storage. Mitochondrion 2013, 13, 7–14. [Google Scholar] [CrossRef]
- SUIT-003 O2 ce-pce D020. Available online: https://wiki.oroboros.at/index.php/SUIT-003_O2_ce-pce_D020 (accessed on 12 October 2021).
- Stadlmann, S.; Renner, K.; Pollheimer, J.; Moser, P.L.; Zeimet, A.G.; Offner, F.A.; Gnaiger, E. Preserved Coupling of Oxidative Phosphorylation but Decreased Mitochondrial Respiratory Capacity in IL-1β-Treated Human Peritoneal Mesothelial Cells. Cell Biochem. Biophys. 2006, 44, 179–186. [Google Scholar] [CrossRef]
- Doerrier, C.; Garcia-Souza, L.F.; Krumschnabel, G.; Wohlfarter, Y.; Mészáros, A.T.; Gnaiger, E. High-Resolution Fluorespirometry and OXPHOS Protocols for Human Cells, Permeabilized Fibers from Small Biopsies of Muscle, and Isolated Mitochondria. In Mitochondrial Bioenergetics; Palmeira, C.M., Moreno, A.J., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1782, pp. 31–70. ISBN 9781493978304. [Google Scholar]
- Gnaiger, E.; Aasander Frostner, E.; Abdul Karim, N.; Abdel-Rahman, E.A.; Abumrad, N.A.; Acuna-Castroviejo, D.; Adiele, R.C.; Ahn, B.; Alencar, M.; Ali, S.S.; et al. MitoEAGLE Task Group. Mitochondrial Physiology. Bioenerg. Commun. 2020, 2020, 1–44. [Google Scholar] [CrossRef]
- Hvas, A.-M.; Favaloro, E.J. Platelet Function Analyzed by Light Transmission Aggregometry. In Hemostasis and Thrombosis; Favaloro, E.J., Lippi, G., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1646, pp. 321–331. ISBN 9781493971947. [Google Scholar]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 Guidelines for Immune Thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wu, Q.; Fan, Z.; Xie, R.; Wang, Z.; Lu, Y. Platelet Mitochondrial Dysfunction and the Correlation with Human Diseases. Biochem. Soc. Trans. 2017, 45, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; McHugh, C.E.; Kirby, M.; Jennaro, T.S.; Jones, A.E.; Stringer, K.A.; Puskarich, M.A. A Multivariate Metabolomics Method for Estimating Platelet Mitochondrial Oxygen Consumption Rates in Patients with Sepsis. Metabolites 2020, 10, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, J.A.; Puskarich, M.A.; Pike, J.W.; Zagorski, J.; Alves, N.J. Inhaled Nitric Oxide to Control Platelet Hyper-Reactivity in Patients with Acute Submassive Pulmonary Embolism. Nitric Oxide 2020, 96, 20–28. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tsai, H.-H.; Fu, T.-C.; Wang, J.-S. Exercise Training Enhances Platelet Mitochondrial Bioenergetics in Stroke Patients: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braganza, A.; Annarapu, G.K.; Shiva, S. Blood-Based Bioenergetics: An Emerging Translational and Clinical Tool. Mol. Asp. Med. 2020, 71, 100835. [Google Scholar] [CrossRef] [PubMed]
- Siewiera, K.; Kassassir, H.; Talar, M.; Wieteska, L.; Watala, C. Higher Mitochondrial Potential and Elevated Mitochondrial Respiration Are Associated with Excessive Activation of Blood Platelets in Diabetic Rats. Life Sci. 2016, 148, 293–304. [Google Scholar] [CrossRef]
- Stadlmann, S.; Rieger, G.; Amberger, A.; Kuznetsov, A.V.; Margreiter, R.; Gnaiger, E. H2O2-Mediated Oxidative Stress versus Cold Ischemia-Reperfusion: Mitochondrial Respiratory Defects in Cultured Human Endothelial Cells: Transplantation. Transplantation 2002, 74, 1800–1803. [Google Scholar] [CrossRef]
- Gnaiger, E.; Kuznetsov, A.V.; Rieger, G.; Amberger, A.; Fuchs, A.; Stadlmann, S.; Eberl, T.; Margreiter, R. Mitochondrial Defects by Intracellular Calcium Overload versus Endothelial Cold Ischemia/Reperfusion Injury. Transpl. Int. 2000, 13, S555–S557. [Google Scholar] [CrossRef] [PubMed]
- Siewiera, K.; Labieniec-Watala, M.; Wolska, N.; Kassassir, H.; Watala, C. Sample Preparation as a Critical Aspect of Blood Platelet Mitochondrial Respiration Measurements—The Impact of Platelet Activation on Mitochondrial Respiration. Int. J. Mol. Sci. 2021, 22, 9332. [Google Scholar] [CrossRef] [PubMed]
- Holme, S.; Sweeney, J.; Sawyer, S.; Elfath, M. The expression of p-selectin during collection, processing, and storage of platelet concentrates: Relationship to loss of in vivo viability. Transfusion 1997, 37, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Watala, C.; Golański, J.; Boncler, M.A.; Pietrucha, T.; Gwoździński, K. Membrane lipid fluidity of blood platelets: A common denominator that underlies the opposing actions of various agents that affect platelet activation in whole blood. Platelets 1998, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Gnaiger, E. Bioenergetic cluster analysis—Mitochondrial respiratory control in human fibroblasts. MitoFit Prepr. 2021, 2021, 1–32. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernerova, A.; Garcia-Souza, L.F.; Soucek, O.; Kostal, M.; Rehacek, V.; Kujovska Krcmova, L.; Gnaiger, E.; Sobotka, O. Mitochondrial Respiration of Platelets: Comparison of Isolation Methods. Biomedicines 2021, 9, 1859. https://doi.org/10.3390/biomedicines9121859
Vernerova A, Garcia-Souza LF, Soucek O, Kostal M, Rehacek V, Kujovska Krcmova L, Gnaiger E, Sobotka O. Mitochondrial Respiration of Platelets: Comparison of Isolation Methods. Biomedicines. 2021; 9(12):1859. https://doi.org/10.3390/biomedicines9121859
Chicago/Turabian StyleVernerova, Andrea, Luiz Felipe Garcia-Souza, Ondrej Soucek, Milan Kostal, Vit Rehacek, Lenka Kujovska Krcmova, Erich Gnaiger, and Ondrej Sobotka. 2021. "Mitochondrial Respiration of Platelets: Comparison of Isolation Methods" Biomedicines 9, no. 12: 1859. https://doi.org/10.3390/biomedicines9121859
APA StyleVernerova, A., Garcia-Souza, L. F., Soucek, O., Kostal, M., Rehacek, V., Kujovska Krcmova, L., Gnaiger, E., & Sobotka, O. (2021). Mitochondrial Respiration of Platelets: Comparison of Isolation Methods. Biomedicines, 9(12), 1859. https://doi.org/10.3390/biomedicines9121859





