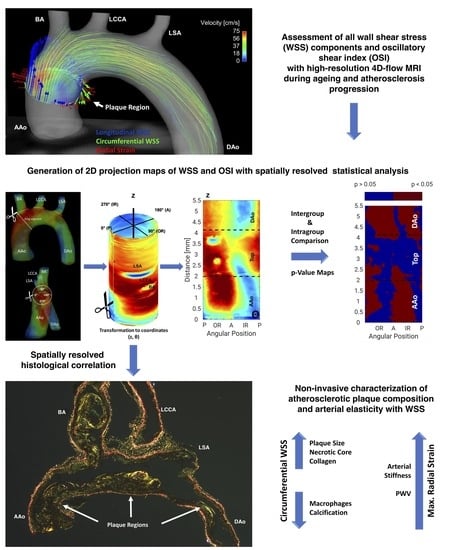
2D Projection Maps of WSS and OSI Reveal Distinct Spatiotemporal Changes in Hemodynamics in the Murine Aorta during Ageing and Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling and Experimental Design
2.2. MRI Measurements
2.3. WSS Calculation
Calculation of 2D Projection Maps
2.4. Calculation of the Oscillatory Shear Index (OSI)
2.5. PWV Analysis
2.6. Aortic Volume Quantification
2.7. Atherosclerotic Lesion Quantification
2.8. Statistical Analysis
3. Results
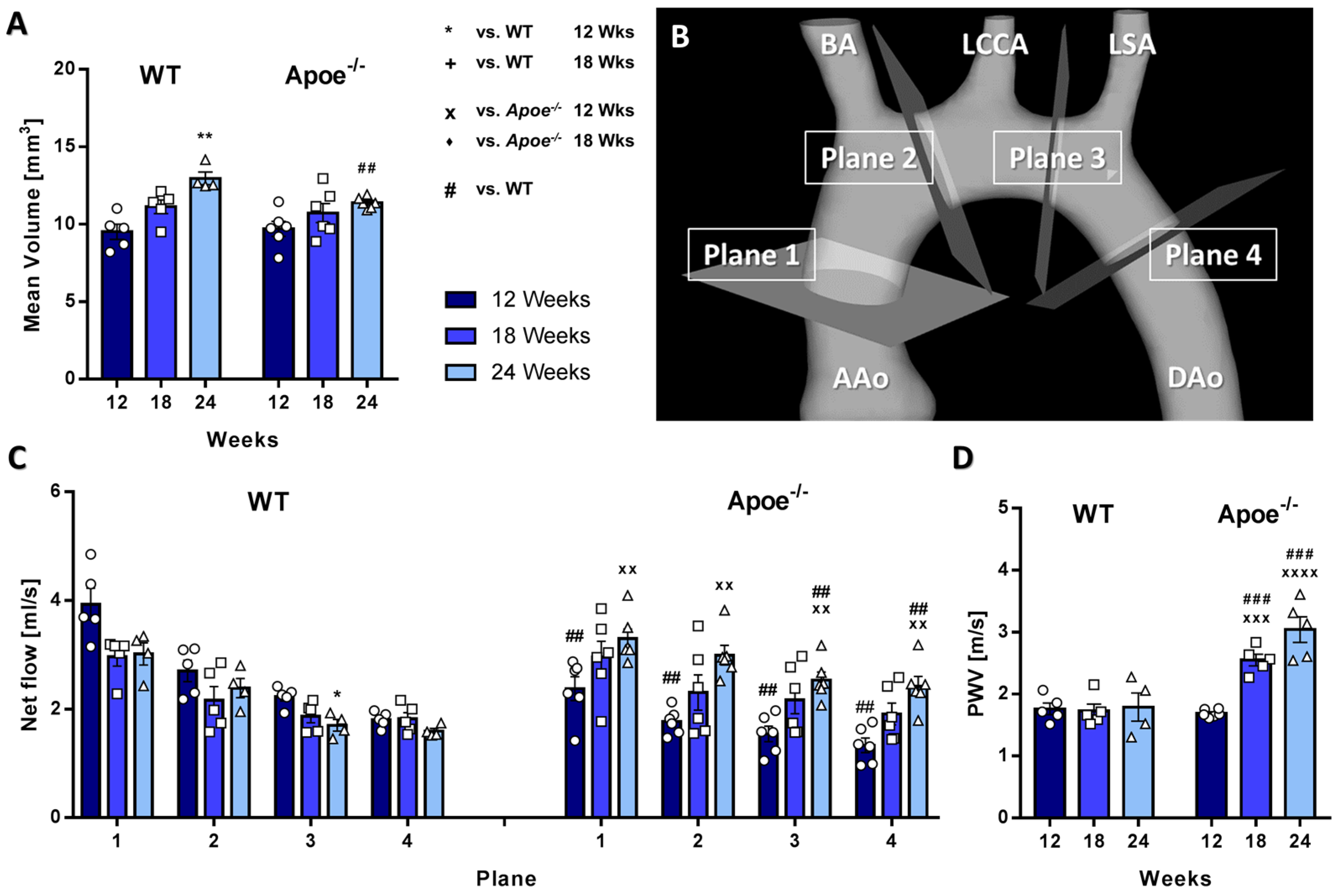
3.1. Aortic Volume Decreases and Aortic Flow and Pulse-Wave Velocity Increases in Atherosclerotic Apoe−/− Mice
3.2. Longitudinal WSS Increases in Apoe−/− mice during Atherosclerosis Progression but Decreases in WT Mice over Time
3.3. Circumferential WSS Shows Inverse Changes in Apoe−/− and WT Mice, Whereas Radial Strain Only Shows Local Changes
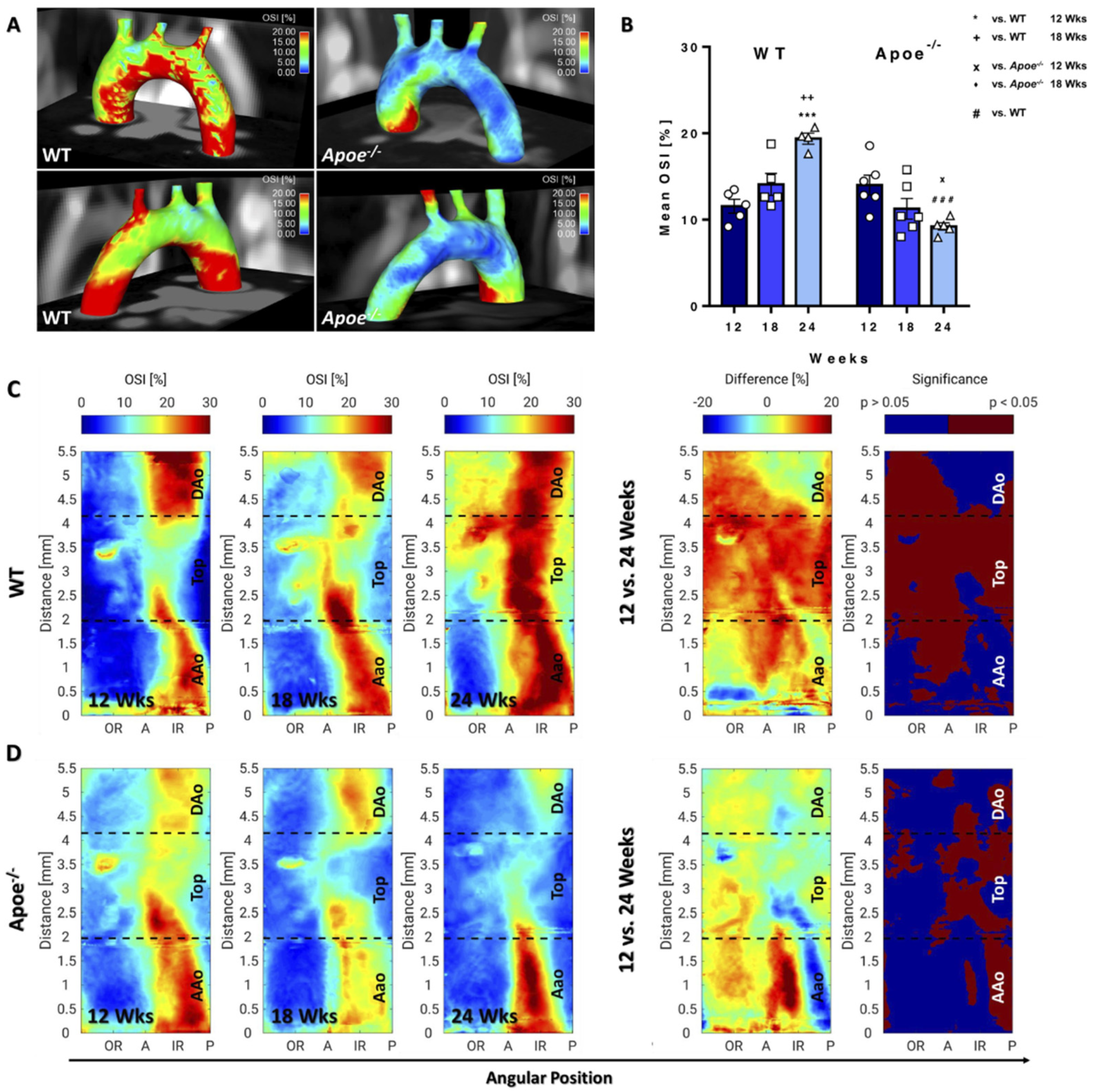
3.4. OSI Decreases in Apoe−/− Mice during Atherosclerosis Progression but Increases in WT Mice during Ageing
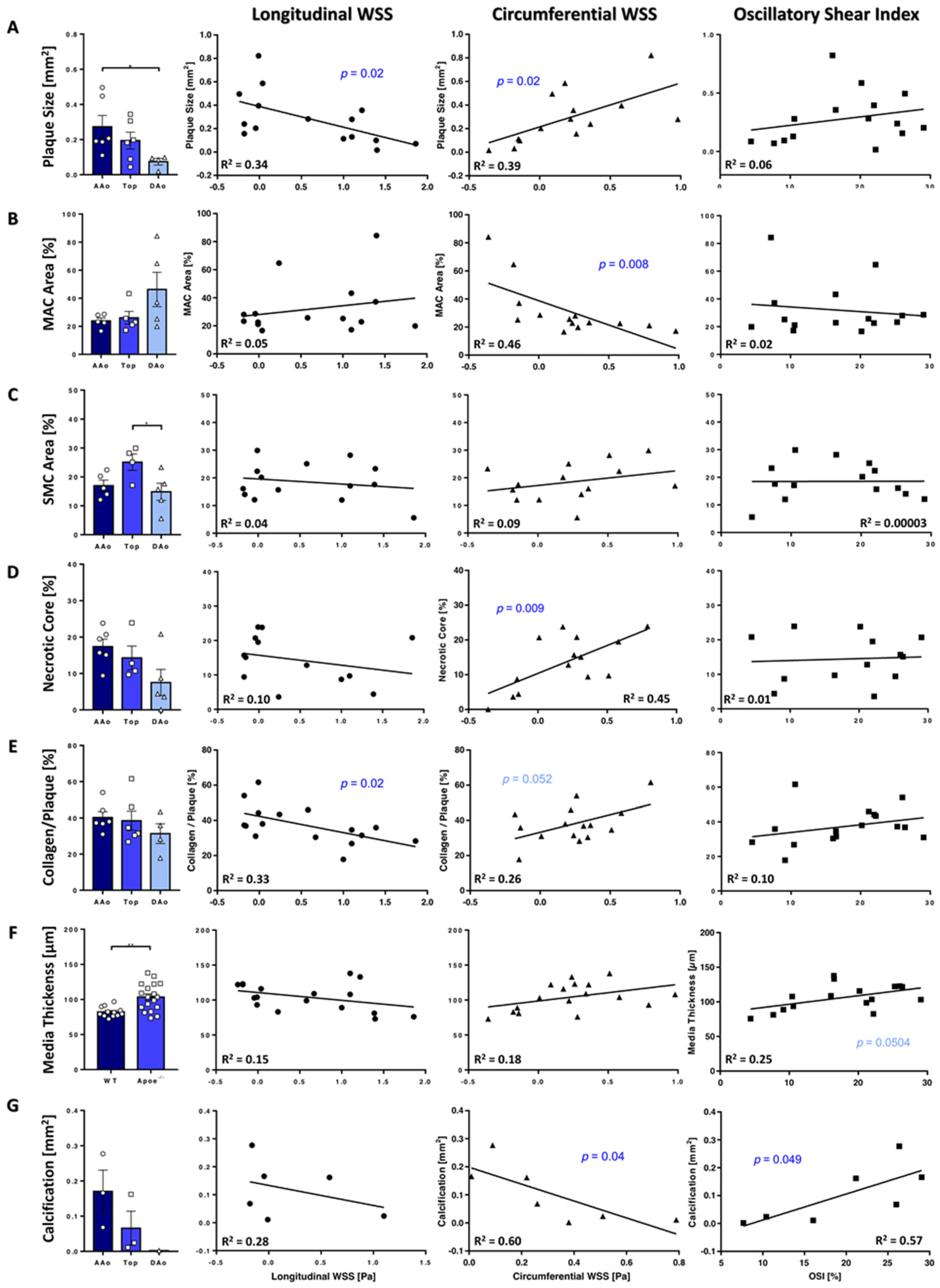
3.5. Plaque Characteristics Correlate with WSS
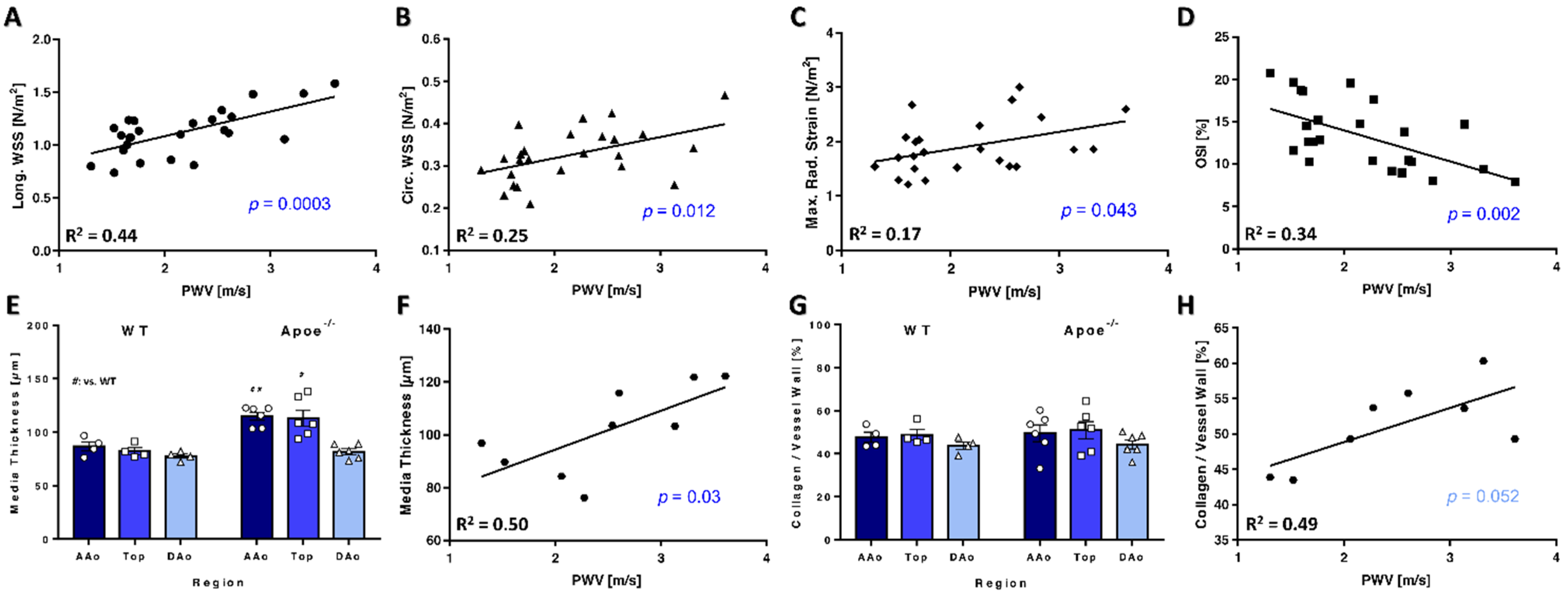
3.6. PWV Correlates with WSS and Vessel Wall Characteristics
4. Discussion
4.1. Accuracy
4.2. Limitations
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 4D | Four-dimensional |
| A | Anterior side |
| AAo | Ascending Aorta |
| Apoe−/− | Apolipoprotein E-deficient |
| BCA | Brachiocephalic artery |
| CircWSS | Circumferential WSS |
| DAo | Descending Aorta |
| DAPI | 4‘,6-Diamidin-2-phenylindol |
| EtOH | Ethanol |
| IR | Inner radius |
| LCCA | Left common carotid artery |
| LongWSS | Longitudinal WSS |
| LSA | Left subclavian artery |
| MAC | Macrophages |
| MRI | Magnetic resonance imaging |
| OR | Outer radius |
| OSI | Oscillatory shear index |
| P | Posterior |
| PBS | Phosphate buffered saline |
| PC | Phase contrast |
| PFA | Paraformaldehyde |
| PWV | Pulse wave velocity |
| RadStrain | Radial strain |
| SD | Standard deviation |
| SEM | Standard error of mean |
| SMCs | Smooth muscle cells |
| WSS | Wall shear stress |
| WT | Wildtype |
References
- Cochain, C.; Zernecke, A. Macrophages and immune cells in atherosclerosis: Recent advances and novel concepts. Basic Res. Cardiol. 2015, 110, 34. [Google Scholar] [CrossRef]
- Zarins, C.K.; Giddens, D.P.; Bharadvaj, B.K.; Sottiurai, V.S.; Mabon, R.F.; Glagov, S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 1983, 53, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Gnasso, A.; Irace, C.; Carallo, C.; De Franceschi, M.S.; Motti, C.; Mattioli, P.L.; Pujia, A. In vivo association between low wall shear stress and plaque in subjects with asymmetrical carotid atherosclerosis. Stroke 1997, 28, 993–998. [Google Scholar] [CrossRef]
- Hoogendoorn, A.; Kok, A.M.; Hartman, E.M.J.; de Nisco, G.; Casadonte, L.; Chiastra, C.; Coenen, A.; Korteland, S.A.; Van der Heiden, K.; Gijsen, F.J.H.; et al. Multidirectional wall shear stress promotes advanced coronary plaque development: Comparing five shear stress metrics. Cardiovasc. Res. 2020, 116, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Fragiadaki, M.; Morris, P.D.; Serbanovic-Canic, J. Shear stress: The dark energy of atherosclerotic plaques. Cardiovasc. Res. 2021, 117, 1811–1813. [Google Scholar] [CrossRef]
- Stalder, A.F.; Russe, M.F.; Frydrychowicz, A.; Bock, J.; Hennig, J.; Markl, M. Quantitative 2D and 3D Phase Contrast MRI: Optimized Analysis of Blood Flow and Vessel Wall Parameters. Magn. Reson. Med. 2008, 60, 1218–1231. [Google Scholar] [CrossRef]
- Winter, P.; Andelovic, K.; Kampf, T.; Gutjahr, F.T.; Heidenreich, J.; Zernecke, A.; Bauer, W.R.; Jakob, P.M.; Herold, V. Fast self-navigated wall shear stress measurements in the murine aortic arch using radial 4D-phase contrast cardiovascular magnetic resonance at 17.6 T. J. Cardiovasc. Magn. Reson. 2019, 21, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harloff, A.; Nußbaumer, A.; Bauer, S.; Stalder, A.F.; Frydrychowicz, A.; Weiller, C.; Hennig, J.; Markl, M. In vivo assessment of wall shear stress in the atherosclerotic aorta using flow-sensitive 4D MRI. Magn. Reson. Med. 2010, 63, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Cho, K.I.; Kim, J.H.; Kim, S.Y.; Kim, C.S.; You, G.I.; Lee, J.Y.; Choi, S.Y.; Lee, S.W.; Kim, H.S.; et al. Wall shear stress in hypertensive patients is associated with carotid vascular deformation assessed by speckle tracking strain imaging. Clin. Hypertens. 2014, 20, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez, J. Arterial stiffness and extracellular matrix. Adv. Cardiol. 2007, 44, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Gotschy, A.; Bauer, E.; Schrodt, C.; Lykowsky, G.; Ye, Y.X.; Rommel, E.; Jakob, P.M.; Bauer, W.R.; Herold, V. Local arterial stiffening assessed by MRI precedes atherosclerotic plaque formation. Circ. Cardiovasc. Imaging 2013, 6, 916–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotschy, A.; Bauer, W.R.; Winter, P.; Nordbeck, P.; Rommel, E.; Jakob, P.M.; Herold, V. Local versus global aortic pulse wave velocity in early atherosclerosis: An animal study in ApoE-/--mice using ultrahigh field MRI. PLoS ONE 2017, 12, e0171603. [Google Scholar] [CrossRef]
- Winter, P.; Kampf, T.; Helluy, X.; Gutjahr, F.T.; Meyer, C.B.; Rommel, E.; Bauer, W.R.; Jakob, P.M.; Herold, V. Fast retrospectively triggered local pulse-wave velocity measurements in mice with CMR-microscopy using a radial trajectory. J. Cardiovasc. Magn. Reson. 2013, 15, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herold, V.; Herz, S.; Winter, P.; Gutjahr, F.T.; Andelovic, K.; Bauer, W.R.; Jakob, P.M. Assessment of local pulse wave velocity distribution in mice using k-t BLAST PC-CMR with semi-automatic area segmentation. J. Cardiovasc. Magn. Reson. 2017, 19, 77. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, J.; Urbina, J.; Valverde, I.; Tejos, C.; Irarrazaval, P.; Andia, M.E.; Uribe, S.; Hurtado, D.E. 3D Quantification of Wall Shear Stress and Oscillatory Shear Index Using a Finite-Element Method in 3D CINE PC-MRI Data of the Thoracic Aorta. IEEE Trans. Med. Imaging 2016, 35, 1475–1487. [Google Scholar] [CrossRef]
- Guala, A.; Teixido-Tura, G.; Dux-Santoy, L.; Granato, C.; Ruiz-Munoz, A.; Valente, F.; Galian-Gay, L.; Gutierrez, L.; Gonzalez-Alujas, T.; Johnson, K.M.; et al. Decreased rotational flow and circumferential wall shear stress as early markers of descending aorta dilation in Marfan syndrome: A 4D flow CMR study. J. Cardiovasc. Magn. Reson. 2019, 21, 63. [Google Scholar] [CrossRef]
- Crouch, A.C.; Cao, A.A.; Scheven, U.M.; Greve, J.M. In Vivo MRI Assessment of Blood Flow in Arteries and Veins from Head-to-Toe Across Age and Sex in C57BL/6 Mice. Ann. Biomed. Eng. 2020, 48, 329–341. [Google Scholar] [CrossRef]
- Janiczek, R.L.; Blackman, B.R.; Roy, R.J.; Meyer, C.H.; Acton, S.T.; Epstein, F.H. Three-Dimensional Phase Contrast Angiography of the Mouse Aortic Arch Using Spiral MRI. Magn. Reson. Med. 2011, 66, 1382–1390. [Google Scholar] [CrossRef] [Green Version]
- Braig, M.; Leupold, J.; Menza, M.; Russe, M.; Ko, C.W.; Hennig, J.; Elverfeldt, D. Preclinical 4D-flow magnetic resonance phase contrast imaging of the murine aortic arch. PLoS ONE 2017, 12, e0187596. [Google Scholar] [CrossRef] [Green Version]
- Braig, M.; Menza, M.; Leupold, J.; LeVan, P.; Feng, L.; Ko, C.W.; von Zur Muhlen, C.; Krafft, A.J.; Hennig, J.; von Elverfeldt, D. Analysis of accelerated 4D flow MRI in the murine aorta by radial acquisition and compressed sensing reconstruction. NMR Biomed. 2020, 33, e4394. [Google Scholar] [CrossRef]
- Zhao, X.; Pratt, R.; Wansapura, J. Quantification of aortic compliance in mice using radial phase contrast MRI. J. Magn. Reson. Imaging 2009, 30, 286–291. [Google Scholar] [CrossRef]
- Wentland, A.L.; Wieben, O.; Shanmuganayagam, D.; Krueger, C.G.; Meudt, J.J.; Consigny, D.; Rivera, L.; McBride, P.E.; Reed, J.D.; Grist, T.M. Measurements of wall shear stress and aortic pulse wave velocity in swine with familial hypercholesterolemia. J. Magn. Reson. Imaging 2015, 41, 1475–1485. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.; Andelovic, K.; Kampf, T.; Hansmann, J.; Jakob, P.M.; Bauer, W.R.; Zernecke, A.; Herold, V. Simultaneous measurements of 3D wall shear stress and pulse wave velocity in the murine aortic arch. J. Cardiovasc. Magn. Reson. 2021, 23, 34. [Google Scholar] [CrossRef]
- Andelovic, K.; Winter, P.; Jakob, P.M.; Bauer, W.R.; Herold, V.; Zernecke, A. Evaluation of Plaque Characteristics and Inflammation Using Magnetic Resonance Imaging. Biomedicines 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- van Ooij, P.; Potters, W.V.; Nederveen, A.J.; Allen, B.D.; Collins, J.; Carr, J.; Malaisrie, S.C.; Markl, M.; Barker, A.J. A methodology to detect abnormal relative wall shear stress on the full surface of the thoracic aorta using four-dimensional flow MRI. Magn. Reson. Med. 2015, 73, 1216–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalska, M.; Machtoub, L.; Manthey, H.D.; Bauer, E.; Herold, V.; Krohne, G.; Lykowsky, G.; Hildenbrand, M.; Kampf, T.; Jakob, P.; et al. Visualization of vascular inflammation in the atherosclerotic mouse by ultrasmall superparamagnetic iron oxide vascular cell adhesion molecule-1-specific nanoparticles. Arter. Thromb. Vasc. Biol. 2012, 32, 2350–2357. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, A.C.; Liu, X.; Hadoke, P.W.; Miller, M.R.; Newby, D.E.; Sethi, T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology 2013, 23, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Hartley, C.J.; Reddy, A.K.; Madala, S.; Martin-McNulty, B.; Vergona, R.; Sullivan, M.E.; Halks-Miller, M.; Taffet, G.E.; Michael, L.H.; Entman, M.L.; et al. Hemodynamic changes in apolipoprotein E-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2326–H2334. [Google Scholar] [CrossRef] [Green Version]
- Herold, V.; Parczyk, M.; Morchel, P.; Ziener, C.H.; Klug, G.; Bauer, W.R.; Rommel, E.; Jakob, P.M. In vivo measurement of local aortic pulse-wave velocity in mice with MR microscopy at 17.6 Tesla. Magn. Reson. Med. 2009, 61, 1293–1299. [Google Scholar] [CrossRef]
- Herold, V.; Wellen, J.; Ziener, C.H.; Weber, T.; Hiller, K.H.; Nordbeck, P.; Rommel, E.; Haase, A.; Bauer, W.R.; Jakob, P.M.; et al. In vivo comparison of atherosclerotic plaque progression with vessel wall strain and blood flow velocity in apoE−/− mice with MR microscopy at 17.6 T. Magn. Reson. Mater. Phys. Biol. Med. 2009, 22, 159–166. [Google Scholar] [CrossRef]
- Parczyk, M.; Herold, V.; Klug, G.; Bauer, W.R.; Rommel, E.; Jakob, P.M. Regional in vivo transit time measurements of aortic pulse wave velocity in mice with high-field CMR at 17.6 Tesla. J. Cardiovasc. Magn. Reson. 2010, 12, 72. [Google Scholar] [CrossRef] [Green Version]
- Xing, R.; Moerman, A.M.; Ridwan, Y.; Daemen, M.J.; van der Steen, A.F.W.; Gijsen, F.J.H.; van der Heiden, K. Temporal and spatial changes in wall shear stress during atherosclerotic plaque progression in mice. R. Soc. Open Sci. 2018, 5, 171447. [Google Scholar] [CrossRef] [Green Version]
- Pedrigi, R.M.; Mehta, V.V.; Bovens, S.M.; Mohri, Z.; Poulsen, C.B.; Gsell, W.; Tremoleda, J.L.; Towhidi, L.; de Silva, R.; Petretto, E.; et al. Influence of shear stress magnitude and direction on atherosclerotic plaque composition. R. Soc. Open Sci. 2016, 3, 160588. [Google Scholar] [CrossRef] [Green Version]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Sung, J.Y.; Woo, C.H.; Choi, H.C. Laminar shear stress suppresses vascular smooth muscle cell proliferation through nitric oxide-AMPK pathway. Biochem. Biophys. Res. Commun. 2017, 490, 1369–1374. [Google Scholar] [CrossRef]
- Haga, M.; Yamashita, A.; Paszkowiak, J.; Sumpio, B.E.; Dardik, A. Oscillatory shear stress increases smooth muscle cell proliferation and Akt phosphorylation. J. Vasc. Surg. 2003, 37, 1277–1284. [Google Scholar] [CrossRef] [Green Version]
- Kojima, K.; Hiro, T.; Koyama, Y.; Ohgaku, A.; Fujito, H.; Ebuchi, Y.; Arai, R.; Monden, M.; Migita, S.; Morikawa, T.; et al. High Wall Shear Stress Is Related to Atherosclerotic Plaque Rupture in the Aortic Arch of Patients with Cardiovascular Disease: A Study with Computational Fluid Dynamics Model and Non-Obstructive General Angioscopy. J. Atheroscler. Thromb. 2020, 28, 742–753. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Thondapu, V.; Mamon, C.; Poon, E.K.W.; Kurihara, O.; Kim, H.O.; Russo, M.; Araki, M.; Shinohara, H.; Yamamoto, E.; Dijkstra, J.; et al. High Spatial Endothelial Shear Stress Gradient Independently Predicts Site of Acute Coronary Plaque Rupture and Erosion. Cardiovasc. Res. 2020, 117, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.; Dux-Santoy, L.; Guala, A.; Rodríguez-Palomares, J.; Evangelista, A.; Sing-Long, C.; Urbina, J.; Mura, J.; Hurtado, D.E.; Uribe, S. 3D axial and circumferential wall shear stress from 4D flow MRI data using a finite element method and a laplacian approach. Magn. Reson. Med. 2018, 79, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; et al. Aortic flow patterns and wall shear stress maps by 4D-flow cardiovascular magnetic resonance in the assessment of aortic dilatation in bicuspid aortic valve disease. J. Cardiovasc. Magn. Reson. 2018, 20, 28. [Google Scholar] [CrossRef]
- Truong, M.; Lennartsson, F.; Bibic, A.; Sundius, L.; Persson, A.; Siemund, R.; Zandt, R.I.; Goncalves, I.; Wassélius, J. Classifications of atherosclerotic plaque components with T1 and T2* mapping in 11.7 T MRI. Eur. J. Radiol. Open 2021, 8, 100323. [Google Scholar] [CrossRef]
- Becher, T.; Riascos-Bernal, D.F.; Kramer, D.J.; Almonte, V.M.; Chi, J.; Tong, T.; Oliveira-Paula, G.H.; Koleilat, I.; Chen, W.; Cohen, P.; et al. Three-Dimensional Imaging Provides Detailed Atherosclerotic Plaque Morphology and Reveals Angiogenesis After Carotid Artery Ligation. Circ. Res. 2020, 126, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Flassbeck, S.; Schmelter, S.; Schmeyer, E.; Ladd, M.E.; Schmitter, S. The impact of 4D flow displacement artifacts on wall shear stress estimation. Magn. Reson. Med. 2021, 85, 3154–3168. [Google Scholar] [CrossRef]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Winter, P.; Andelovic, K.; Jakob, P.M.; Bauer, W.R.; Herold, V. Fast self-navigated PC-MRI for human cardiac flow measurements using retrospective radial sampling. In Proceedings of the ISMRM, Honolulu, HI, USA, 22–27 April 2017; Volume 25, p. 3213. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andelovic, K.; Winter, P.; Kampf, T.; Xu, A.; Jakob, P.M.; Herold, V.; Bauer, W.R.; Zernecke, A. 2D Projection Maps of WSS and OSI Reveal Distinct Spatiotemporal Changes in Hemodynamics in the Murine Aorta during Ageing and Atherosclerosis. Biomedicines 2021, 9, 1856. https://doi.org/10.3390/biomedicines9121856
Andelovic K, Winter P, Kampf T, Xu A, Jakob PM, Herold V, Bauer WR, Zernecke A. 2D Projection Maps of WSS and OSI Reveal Distinct Spatiotemporal Changes in Hemodynamics in the Murine Aorta during Ageing and Atherosclerosis. Biomedicines. 2021; 9(12):1856. https://doi.org/10.3390/biomedicines9121856
Chicago/Turabian StyleAndelovic, Kristina, Patrick Winter, Thomas Kampf, Anton Xu, Peter Michael Jakob, Volker Herold, Wolfgang Rudolf Bauer, and Alma Zernecke. 2021. "2D Projection Maps of WSS and OSI Reveal Distinct Spatiotemporal Changes in Hemodynamics in the Murine Aorta during Ageing and Atherosclerosis" Biomedicines 9, no. 12: 1856. https://doi.org/10.3390/biomedicines9121856
APA StyleAndelovic, K., Winter, P., Kampf, T., Xu, A., Jakob, P. M., Herold, V., Bauer, W. R., & Zernecke, A. (2021). 2D Projection Maps of WSS and OSI Reveal Distinct Spatiotemporal Changes in Hemodynamics in the Murine Aorta during Ageing and Atherosclerosis. Biomedicines, 9(12), 1856. https://doi.org/10.3390/biomedicines9121856