Zonal-Layered Chondrocyte Sheets for Repairment of Full-Thickness Articular Cartilage Defect: A Mini-Pig Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Preparation and Chondrocytes Sheets Construction
2.2. Cell Proliferation and Viability
2.3. Gene Expression of Chondrocytes Sheets
2.4. Total Glycosaminoglycan (GAG) Quantification and DNA Content Assays
2.5. Measurement of Humoral Factors
2.6. Immunofluorescence Assay
2.7. In Vivo Implantation of Chondrocyte Sheets
2.8. Macroscopic Evaluation by ICRS Scoring System
2.9. Histology and Immunohistochemical Examination
2.10. Histological Grading Score for the Assessment of Cartilage Repair
2.11. Statistics
3. Results
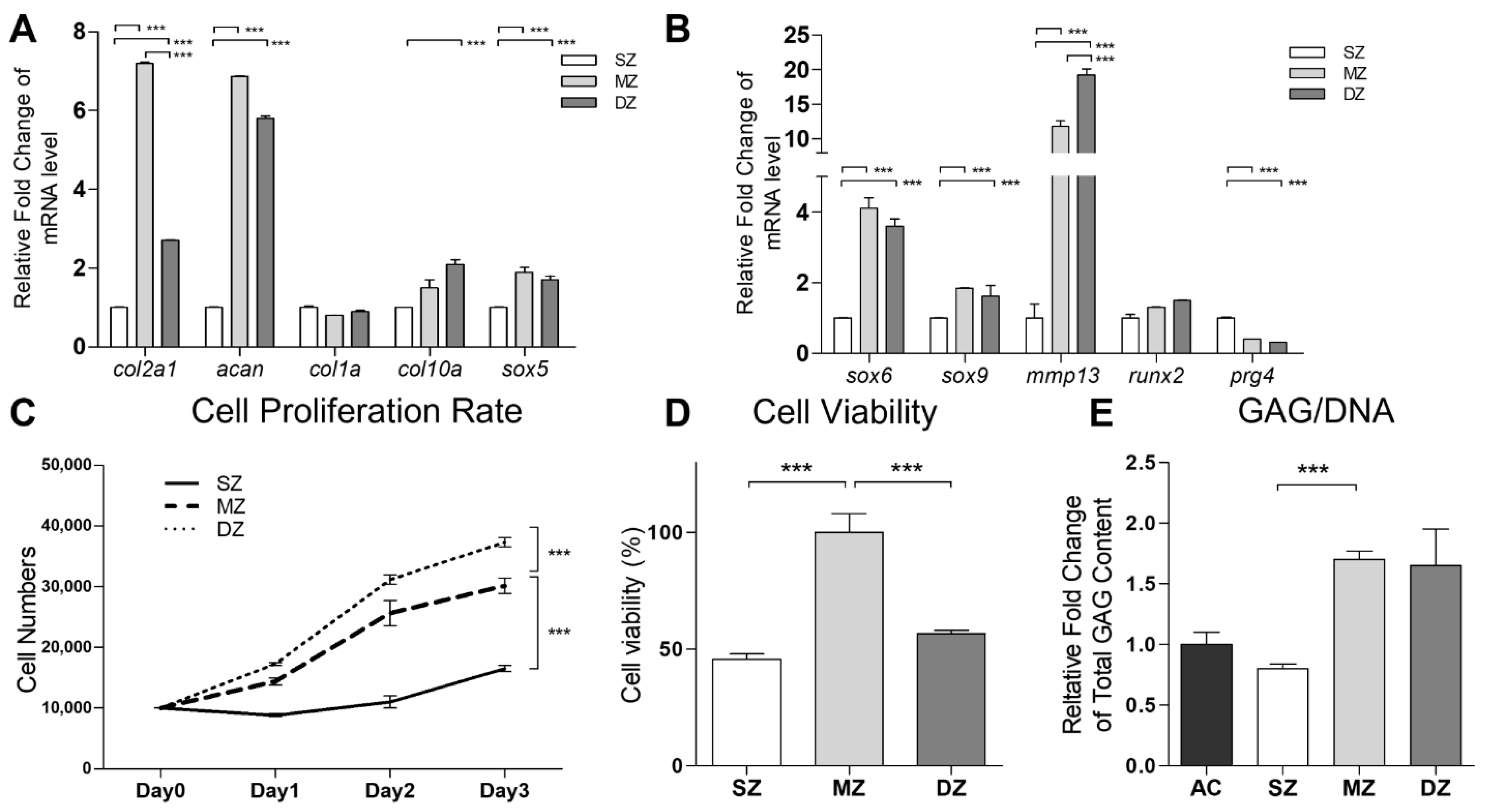
3.1. Functional Properties of SZ, MZ, and DZ Chondrocytes
3.2. Cell Viability, Cell Proliferation, and Expression of Chondrogenic Markers in H-CS and S-CS
3.3. Secretion of ECM Destructive Enzymes in H-CS and S-CS
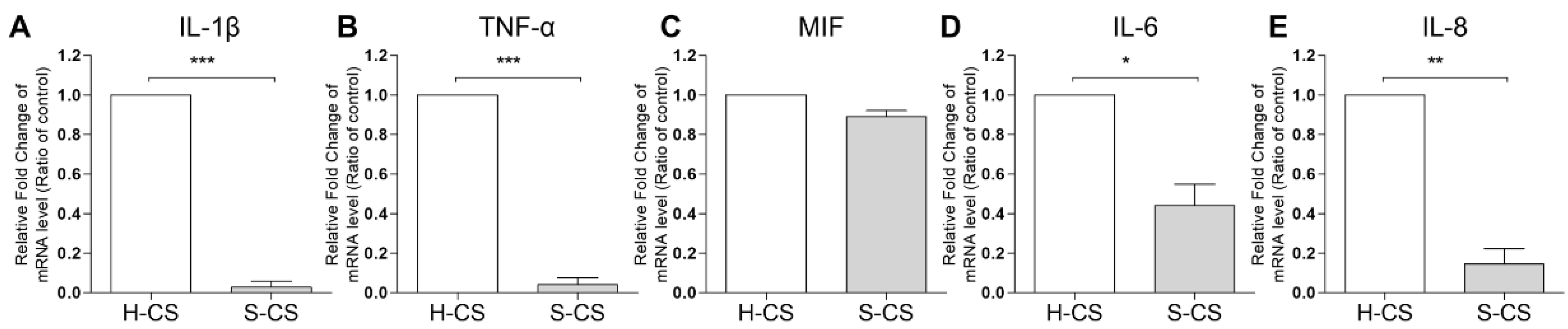
3.4. Pro-Inflammatory Cytokine Gene Expression in H-CS and S-CS
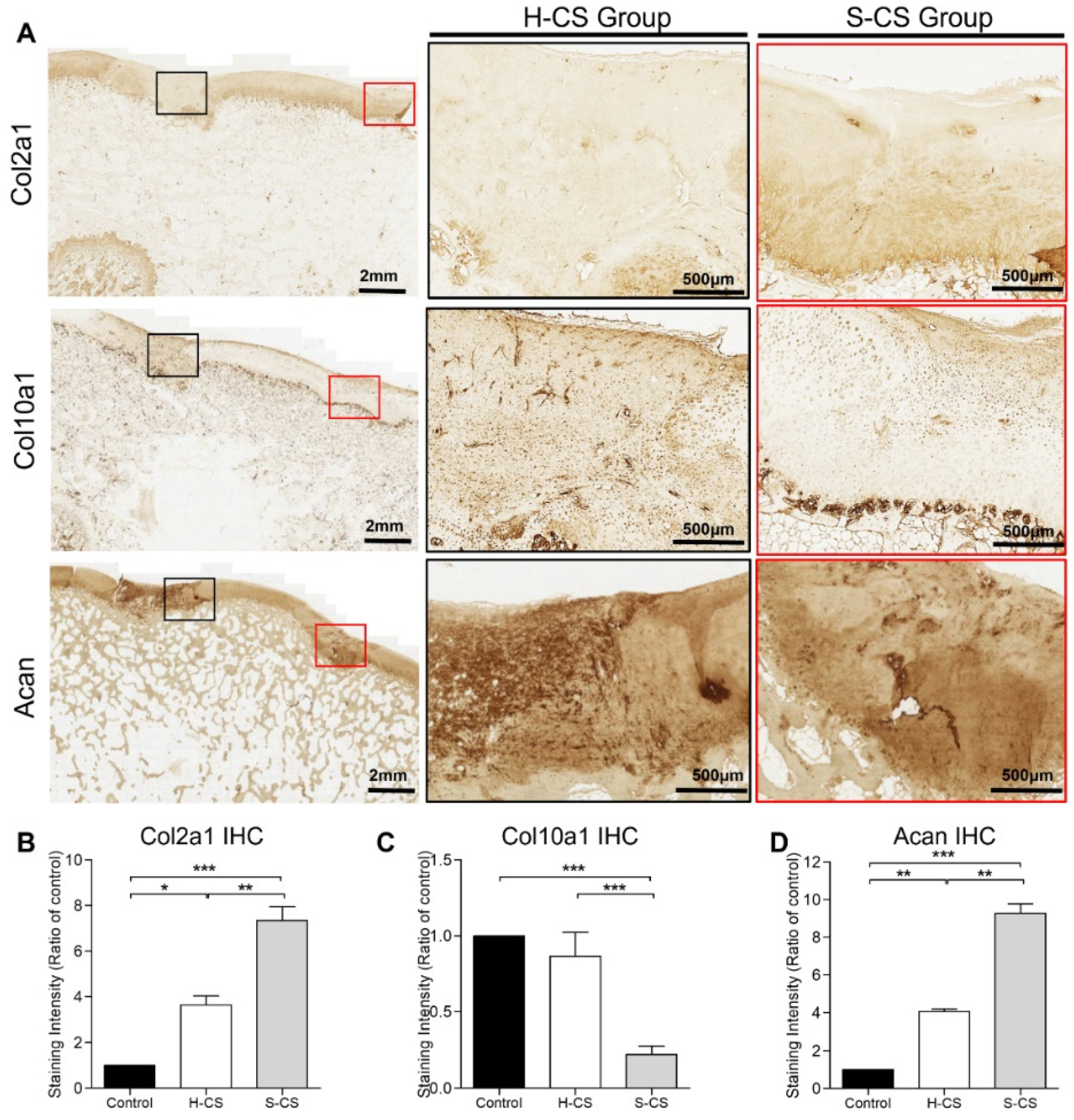
3.5. Comparison of Matrix Production Abilities and Immunohistochemical Analyses of H-CS and S-CS
3.6. In Vivo Repair Evaluation Based on Gross Appearance and Histology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunziker, E. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [Green Version]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Kreuz, P.; Steinwachs, M.; Erggelet, C.; Krause, S.; Konrad, G.; Uhl, M.; Südkamp, N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr. Cartil. 2006, 14, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Marcacci, M.; Filardo, G.; Kon, E. Treatment of cartilage lesions: What works and why? Injury 2013, 44, S11–S15. [Google Scholar] [CrossRef]
- Gillogly, S.D.; Wheeler, K.S. Autologous Chondrocyte Implantation With Collagen Membrane. Sports Med. Arthrosc. Rev. 2015, 23, 118–124. [Google Scholar] [CrossRef]
- Hinckel, B.B.; Gomoll, A.H. Autologous Chondrocytes and Next-Generation Matrix-Based Autologous Chondrocyte Implantation. Clin. Sports Med. 2017, 36, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Basad, E.; Ishaque, B.; Bachmann, G.; Stürz, H.; Steinmeyer, J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: A 2-year randomised study. Knee Surgery, Sports Traumatol. Arthrosc. 2010, 18, 519–527. [Google Scholar] [CrossRef]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical Efficacy of the Microfracture Technique for Articular Cartilage Repair in the Knee: An Evidence-Based Systematic Analysis. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef]
- Riff, A.J.; Huddleston, H.P.; Cole, B.J.; Yanke, A.B. Autologous Chondrocyte Implantation and Osteochondral Allograft Transplantation Render Comparable Outcomes in the Setting of Failed Marrow Stimulation. Am. J. Sports Med. 2020, 48, 861–870. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, T.; Fan, W. Osteochondral autograft transplantation or autologous chondrocyte implantation for large cartilage defects of the knee: A meta-analysis. Cell Tissue Bank. 2015, 17, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Jeuken, R.M.; van Hugten, P.P.; Roth, A.K.; Timur, U.T.; Boymans, T.A.; van Rhijn, L.W.; Bugbee, W.D.; Emans, P.J. A Systematic Review of Focal Cartilage Defect Treatments in Middle-Aged Versus Younger Patients. Orthop. J. Sports Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.L.; Kuiper, N.J. Regenerative Medicine: A Review of the Evolution of Autologous Chondrocyte Implantation (ACI) Therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Heal. A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xia, Y.; Lu, M. Concentration profiles of collagen and proteoglycan in articular cartilage by Fourier transform infrared imaging and principal component regression. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 88, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rucklidge, G.J.; Milne, G.; Robins, S.P. Collagen Type X: A Component of the Surface of Normal Human, Pig, and Rat Articular Cartilage. Biochem. Biophys. Res. Commun. 1996, 224, 297–302. [Google Scholar] [CrossRef]
- Greene, G.W.; Banquy, X.; Lee, D.W.; Lowrey, D.D.; Yu, J.; Israelachvili, J.N. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc. Natl. Acad. Sci. USA 2011, 108, 5255–5259. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.; Wuethrich, P.; Eggli, P.; Hunziker, E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: A new method using confocal microscopic stereology and quantitative autoradiography. J. Orthop. Res. 1996, 14, 424–432. [Google Scholar] [CrossRef]
- Ng, K.W.; Ateshian, G.A.; Hung, C.T. Zonal Chondrocytes Seeded in a Layered Agarose Hydrogel Create Engineered Cartilage with Depth-Dependent Cellular and Mechanical Inhomogeneity. Tissue Eng. Part A 2009, 15, 2315–2324. [Google Scholar] [CrossRef]
- Sharma, B.; Williams, C.G.; Kim, T.K.; Sun, D.; Malik, A.; Khan, M.; Leong, K.; Elisseeff, J.H. Designing Zonal Organization into Tissue-Engineered Cartilage. Tissue Eng. 2007, 13, 405–414. [Google Scholar] [CrossRef]
- Visser, J.; Gawlitta, D.; Benders, K.E.; Toma, S.M.; Pouran, B.; van Weeren, P.R.; Dhert, W.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Tong, X.; Trinh, P.; Yang, F. Mimicking Cartilage Tissue Zonal Organization by Engineering Tissue-Scale Gradient Hydrogels as 3D Cell Niche. Tissue Eng. Part A 2018, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Cheng, A.W.-M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage Tissue Engineering Application of Injectable Gelatin Hydrogel with In Situ Visible-Light-Activated Gelation Capability in Both Air and Aqueous Solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Liu, Y.; Zhang, Z.-Y.; Tao, R.; Liu, Y.; He, A.; Yin, Z.; Li, D.; Zhang, W.; Liu, W.; et al. Long-term functional reconstruction of segmental tracheal defect by pedicled tissue-engineered trachea in rabbits. Biomaterials 2013, 34, 3336–3344. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yin, Z.; Luo, X.; Liu, W.; Zhang, W.; Zhang, Z.-Y.; Cao, Y.; Liu, Y.; Zhou, G. Prolonged in vitro precultivation alleviates post-implantation inflammation and promotes stable subcutaneous cartilage formation in a goat model. Biomed. Mater. 2016, 12, 15006. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamato, M.; Akutsu, T.; Shibata, T.; Isoi, Y.; Kikuchi, A.; Umezu, M.; Okano, T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J. Biomed. Mater. Res. 2002, 60, 110–117. [Google Scholar] [CrossRef]
- Oie, Y.; Komoto, S.; Kawasaki, R. Systematic review of clinical research on regenerative medicine for the cornea. Jpn. J. Ophthalmol. 2021, 65, 169–183. [Google Scholar] [CrossRef]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin-Darby canine kidney (MDCK) cell sheets. J. Biomed. Mater. Res. 2000, 51, 216–223. [Google Scholar] [CrossRef]
- Min, B.-H.; Kim, H.J.; Lim, H.; Park, S.R. CHARACTERIZATION OF SUBPOPULATED ARTICULAR CHONDROCYTES SEPARATED BY PERCOLL DENSITY GRADIENT. Vitr. Cell. Dev. Biol.-Anim. 2002, 38, 35. [Google Scholar] [CrossRef]
- Ralphs, J.R.; Evans, L.; Ali, S.Y. Separation of rabbit epiphyseal chondrocytes in various stages of differentiation. Cell Tissue Res. 1988, 254, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Utsumi, M.; Kushida, A.; Konno, C.; Kikuchi, A.; Okano, T. Thermo-Responsive Culture Dishes Allow the Intact Harvest of Multilayered Keratinocyte Sheets without Dispase by Reducing Temperature. Tissue Eng. 2001, 7, 473–480. [Google Scholar] [CrossRef]
- Shen, P.-C.; Chou, S.-H.; Lu, C.-C.; Huang, H.-T.; Chien, S.-H.; Huang, P.-J.; Liu, Z.-M.; Shih, C.-L.; Su, S.-J.; Chen, L.-M.; et al. Shockwave Treatment Enhanced Extracellular Matrix Production in Articular Chondrocytes Through Activation of the ROS/MAPK/Nrf2 Signaling Pathway. CARTILAGE 2021. [Google Scholar] [CrossRef] [PubMed]
- Mainil-Varlet, P.; Aigner, T.; Brittberg, M.; Bullough, P.; Hollander, A.; Hunziker, E.; Kandel, R.; Nehrer, S.; Pritzker, K.; Roberts, S.; et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J. Bone Jt. Surg. Am. 2003, 85, 45–57. [Google Scholar] [CrossRef]
- Mankin, H.J.; Dorfman, H.; Lippiello, L.; Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Jt. Surgery. Am. 1971, 53, 523–537. [Google Scholar] [CrossRef]
- Albrecht, C.; Tichy, B.; Zak, L.; Aldrian, S.; Nürnberger, S.; Marlovits, S. Influence of Cell Differentiation and IL-1β Expression on Clinical Outcomes After Matrix-Associated Chondrocyte Transplantation. Am. J. Sports Med. 2013, 42, 59–69. [Google Scholar] [CrossRef]
- Archer, C.; McDowell, J.; Bayliss, M.; Stephens, M.; Bentley, G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J. Cell Sci. 1990, 97, 361–371. [Google Scholar] [CrossRef]
- Bayliss, M.T.; Venn, M.; Maroudas, A.; Ali, S.Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem. J. 1983, 209, 387–400. [Google Scholar] [CrossRef]
- Siczkowski, M.; Watt, F. Subpopulations of chondrocytes from different zones of pig articular cartilage. Isolation, growth and proteoglycan synthesis in culture. J. Cell Sci. 1990, 97, 349–360. [Google Scholar] [CrossRef]
- Kim, T.-K.; Sharma, B.; Williams, C.; Ruffner, M.; Malik, A.; McFarland, E.; Elisseeff, J. Experimental Model for Cartilage Tissue Engineering to Regenerate the Zonal Organization of Articular Cartilage. Osteoarthr. Cartil. 2003, 11, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Tee, C.A.; Yang, Z.; Yin, L.; Wu, Y.; Han, J.; Lee, E.H. Improved zonal chondrocyte production protocol integrating size-based inertial spiral microchannel separation and dynamic microcarrier culture for clinical application. Biomaterials 2019, 220, 119409. [Google Scholar] [CrossRef]
- Lui, J.C.; Chau, M.; Chen, W.; Cheung, C.S.F.; Hanson, J.; Rodriguez-Canales, J.; Nilsson, O.; Baron, J. Spatial regulation of gene expression during growth of articular cartilage in juvenile mice. Pediatr. Res. 2014, 77, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Athanasiou, K.A. Chondrocytes from Different Zones Exhibit Characteristic Differences in High Density Culture. Connect. Tissue Res. 2006, 47, 133–140. [Google Scholar] [CrossRef]
- Wang, F.; Hu, Y.; He, D.; Zhou, G.; Ellis, E. Scaffold-free cartilage cell sheet combined with bone-phase BMSCs-scaffold regenerate osteochondral construct in mini-pig model. Am. J. Transl. Res. 2018, 10, 2997–3010. [Google Scholar] [PubMed]
- Angele, P.; Fritz, J.; Albrecht, D.; Koh, J.; Zellner, J. Defect type, localization and marker gene expression determines early adverse events of matrix-associated autologous chondrocyte implantation. Injury 2015, 46, S2–S9. [Google Scholar] [CrossRef]
- Niemeyer, P.; Pestka, J.M.; Salzmann, G.M.; Südkamp, N.P.; Schmal, H. Influence of Cell Quality on Clinical Outcome After Autologous Chondrocyte Implantation. Am. J. Sports Med. 2011, 40, 556–561. [Google Scholar] [CrossRef]
- Blewis, M.E.; Schumacher, B.L.; Klein, T.J.; Schmidt, T.A.; Voegtline, M.S.; Sah, R.L. Microenvironment regulation of PRG4 phenotype of chondrocytes. J. Orthop. Res. 2007, 25, 685–695. [Google Scholar] [CrossRef]
- Ding, J.; Bao, S.; Qian, W.; Zhao, H. Subcutaneous Regeneration of Engineered Cartilage: A Comparison of Cell Sheets and Chondrocyte-Scaffold Constructs in a Porcine Model. Plast. Reconstr. Surg. 2021, 147, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, Y.; Yang, Z.; Denslin, V.; Ren, X.; Tee, C.A.; Lai, Z.; Lim, C.T.; Han, J.; Lee, E.H. Characterization and application of size-sorted zonal chondrocytes for articular cartilage regeneration. Biomaterials 2018, 165, 66–78. [Google Scholar] [CrossRef]
- Chawla, K.; Klein, T.J.; Schumacher, B.L.; Jadin, K.D.; Shah, B.H.; Nakagawa, K.; Wong, V.W.; Chen, A.C.; Masuda, K.; Sah, R.L. Short-Term Retention of Labeled Chondrocyte Subpopulations in Stratified Tissue-Engineered Cartilaginous Constructs Implanted In Vivo in Mini-Pigs. Tissue Eng. 2007, 13, 1525–1537. [Google Scholar] [CrossRef]
- Radin, E.L.; Rose, R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 1986, 5, 34–40. [Google Scholar] [CrossRef]




| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| col2a1 | ACTCCTGGCACGGATGGTC | CTTTCTCACCAACATCGCCC |
| aggrecan | CCCAACCAGCCTGACAACTT | CCTTCTCGTGCCAGATCATCA |
| col10a1 | TGAACTTGGTTCATGGAGTGTTTTA | TGCCTTGGTGTTGGATGGT |
| sox5 | GGCCAAGCAGCAGCAAGAACAG | AGCTGAAGCCTGGAGGAAGGAG |
| sox6 | CAGCCCTGTCAGTCTGCCTAACA | GCATCTTCCGAGCCTCCTGAATAGC |
| sox9 | GGCAATCCCAGGGTCCACCAAC | TGGTCGAACTCGTTGACGTCGAAG |
| mmp13 | ACCCAGGAGCCCTCATGTTTCC | CAGGGTTTCTCCTCGGAGACTG |
| runx2 | CCAGACCAGCAGCACTCCATAC | GGGAACTGCTGTGGCTTCCATC |
| prg4 | CTCCCAAGGAGCAGCTTCTAC | GGTGGTGGGAGCTGGTTCCTTG |
| pcna | GCGCCTGGTCCAGGGC | TCACGCCCATGGCCAAATTGC |
| IL-1 | GTACATGGTTGCTGCCTGAA | CTAGTGTGCCATGGTTTCCA |
| IL-6 | GGCAGAAAACAACCTGAACC | GTGGTGGCTTTGTCTGGATT |
| IL-8 | TAGGACCAGAGCCAGGAAGA | CAGTGGGGTCCACTCTCAAT |
| TNF | ACTGCACTTCGAGGTTATCG | GCTGGTTGTCTTTCAGCTTC |
| MIF | CGTGCGCCCTTTGCAGTCTG | TGGCCGCGTTCATGTCGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.-C.; Lu, C.-C.; Chou, S.-H.; Liu, Z.-M.; Su, S.-J.; Tien, Y.-C. Zonal-Layered Chondrocyte Sheets for Repairment of Full-Thickness Articular Cartilage Defect: A Mini-Pig Model. Biomedicines 2021, 9, 1806. https://doi.org/10.3390/biomedicines9121806
Shen P-C, Lu C-C, Chou S-H, Liu Z-M, Su S-J, Tien Y-C. Zonal-Layered Chondrocyte Sheets for Repairment of Full-Thickness Articular Cartilage Defect: A Mini-Pig Model. Biomedicines. 2021; 9(12):1806. https://doi.org/10.3390/biomedicines9121806
Chicago/Turabian StyleShen, Po-Chih, Cheng-Chang Lu, Shih-Hsiang Chou, Zi-Miao Liu, Shu-Jem Su, and Yin-Chun Tien. 2021. "Zonal-Layered Chondrocyte Sheets for Repairment of Full-Thickness Articular Cartilage Defect: A Mini-Pig Model" Biomedicines 9, no. 12: 1806. https://doi.org/10.3390/biomedicines9121806
APA StyleShen, P.-C., Lu, C.-C., Chou, S.-H., Liu, Z.-M., Su, S.-J., & Tien, Y.-C. (2021). Zonal-Layered Chondrocyte Sheets for Repairment of Full-Thickness Articular Cartilage Defect: A Mini-Pig Model. Biomedicines, 9(12), 1806. https://doi.org/10.3390/biomedicines9121806






