The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Study Group and Design
2.3. Blood Sampling and Cell Isolation
2.4. Flow Cytometry for PBMCs or Peripheral Blood Lymphocytes
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Demographics of the Participants
3.2. Immune Checkpoint Expression in LTBI Status
3.3. Immune Checkpoint Expression after LTBI Treatment
3.4. Predictors for LTBI in Logistic Regression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Rose, D.N. Benefits of screening for latent Mycobacterium tuberculosis infection. Arch. Intern. Med. 2000, 160, 1513–1521. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinsson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.D.; Stein, C.M.; Seshadri, C.; Campo, M.; Alter, G.; Fortune, S.; Schurr, E.; Wallis, R.S.; Churchyard, G.; Mayanja-Kizza, H.; et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 2018, 18, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, G.; Zhong, J.; Zheng, K.; Xiao, H.; Li, C.; Song, X.; Li, Y.; Xu, C.; Wu, H.; et al. Immune Checkpoints in Viral Infections. Viruses 2020, 12, 1051. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mohan, A.; Dey, A.B.; Mitra, D.K. Programmed death-1+ T cells inhibit effector T cells at the pathological site of miliary tuberculosis. Clin. Exp. Immunol. 2017, 187, 269–283. [Google Scholar] [CrossRef]
- Singh, A.; Mohan, A.; Dey, A.B.; Mitra, D.K. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon gamma-producing T cells from apoptosis in patients with pulmonary tuberculosis. J. Infect. Dis. 2013, 208, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2, 16076. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Asai, A.; Yasuoka, H.; Matsui, M.; Tsuchimoto, Y.; Fukunishi, S.; Higuchi, K. Programmed Death 1 Ligand Expression in the Monocytes of Patients with Hepatocellular Carcinoma Depends on Tumor Progression. Cancers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Druszczynska, M.; Wlodarczyk, M.; Janiszewska-Drobinska, B.; Kielnierowski, G.; Zawadzka, J.; Kowalewicz-Kulbat, M.; Fol, M.; Szpakowski, P.; Rudnicka, K.; Chmiela, M.; et al. Monocyte signal transduction receptors in active and latent tuberculosis. Clin. Dev. Immunol. 2013, 2013, 851452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Girndt, M.; Sester, U.; Sester, M.; Kaul, H.; Köhler, H. Impaired cellular immune function in patients with end-stage renal failure. Nephrol. Dial. Transplant. 1999, 14, 2807–2810. [Google Scholar] [CrossRef]
- Lin, H.H.; Ezzati, M.; Chang, H.Y.; Murray, M. Association between tobacco smoking and active tuberculosis in Taiwan: Prospective cohort study. Am. J. Respir. Crit. Care Med. 2009, 180, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Jasmer, R.M.; Snyder, D.C.; Chin, D.P.; Hopewell, P.C.; Cuthbert, S.S.; Antonio Paz, E.; Daley, C.L. Twelve months of isoniazid compared with four months of isoniazid and rifampin for persons with radiographic evidence of previous tuberculosis: An outcome and cost-effectiveness analysis. Am. J. Respir. Crit. Care Med. 2000, 162, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, M.; Patt, C.; Seckler, B.; Adler, J.J. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis. Chest 1998, 113, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.E., III; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kaul, A.; Tsolaki, A.G.; Kishore, U.; Bhakta, S. Mycobacterium tuberculosis: Immune evasion, latency and reactivation. Immunobiology 2012, 217, 363–374. [Google Scholar] [CrossRef]
- Urdahl, K.B.; Shafiani, S.; Ernst, J.D. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011, 4, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Khan, N.; Kaufmann, E. Beyond Killing Mycobacterium tuberculosis: Disease Tolerance. Front. Immunol. 2018, 9, 2976. [Google Scholar] [CrossRef]
- Langan, E.A.; Graetz, V.; Allerheiligen, J.; Zillikens, D.; Rupp, J.; Terheyden, P. Immune checkpoint inhibitors and tuberculosis: An old disease in a new context. Lancet Oncol. 2020, 21, e55–e65. [Google Scholar] [CrossRef]
- Hasibuan, F.M.; Shiratori, B.; Senoputra, M.A.; Chagan-Yasutan, H.; Koesoemadinata, R.C.; Apriani, L.; Takahashi, Y.; Niki, T.; Alisjahbana, B.; Hattori, T. Evaluation of matricellular proteins in systemic and local immune response to Mycobacterium tuberculosis infection. Microbiol. Immunol. 2015, 59, 623–632. [Google Scholar] [CrossRef]
- Lee, M.R.; Chang, L.Y.; Chang, C.H.; Yan, B.S.; Wang, J.Y.; Lin, W.H. Differed IL-1 Beta Response between Active TB and LTBI Cases by Ex Vivo Stimulation of Human Monocyte-Derived Macrophage with TB-Specific Antigen. Dis. Markers 2019, 2019, 7869576. [Google Scholar] [CrossRef]
- Shu, C.C.; Wu, V.C.; Yang, F.J.; Hsu, C.L.; Pan, S.C.; Wang, J.Y.; Wang, J.T.; Yu, C.J.; Lee, L.N. Dynamic changes in positive interferon-gamma release assay in a dialysis population: An observational cohort study. J. Infect. 2013, 67, 529–535. [Google Scholar] [CrossRef]
- Callahan, M.K.; Wolchok, J.D. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 41–53. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Elkington, P.T.; Bateman, A.C.; Thomas, G.J.; Ottensmeier, C.H. Implications of Tuberculosis Reactivation after Immune Checkpoint Inhibition. Am. J. Respir. Crit. Care Med. 2018, 198, 1451–1453. [Google Scholar] [CrossRef]
- Samelson, A.J.; Jensen, M.K.; Soto, R.A.; Cate, J.H.; Marqusee, S. Quantitative determination of ribosome nascent chain stability. Proc. Natl. Acad. Sci. USA 2016, 113, 13402–13407. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sada-Ovalle, I.; Beladi, S.; Anderson, A.C.; Dardalhon, V.; Hotta, C.; Kuchroo, V.K.; Behar, S.M. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010, 207, 2343–2354. [Google Scholar] [CrossRef]
- Lauer, F.T.; Denson, J.L.; Burchiel, S.W. Isolation, Cryopreservation, and Immunophenotyping of Human Peripheral Blood Mononuclear Cells. Curr. Protoc. Toxicol. 2017, 74, 18.20.11–18.20.16. [Google Scholar] [CrossRef]
- Martikainen, M.V.; Roponen, M. Cryopreservation affected the levels of immune responses of PBMCs and antigen-presenting cells. Toxicol. Vitr. 2020, 67, 104918. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Abrahams, D.A.; Bunjun, R.; Stone, L.; de Kock, M.; Walzl, G.; Wilkinson, R.J.; Burgers, W.A.; Hanekom, W.A. PD-1 Expression on Mycobacterium tuberculosis-Specific CD4 T Cells Is Associated With Bacterial Load in Human Tuberculosis. Front. Immunol. 2018, 9, 1995. [Google Scholar] [CrossRef]
- Takeshita, M.; Suzuki, K.; Kondo, Y.; Morita, R.; Okuzono, Y.; Koga, K.; Kassai, Y.; Gamo, K.; Takiguchi, M.; Kurisu, R.; et al. Multi-dimensional analysis identified rheumatoid arthritis-driving pathway in human T cell. Ann. Rheum. Dis. 2019, 78, 1346–1356. [Google Scholar] [CrossRef] [PubMed]


| Healthy Controls (n = 29) | LTBI-ALL (n = 58) | LTBI-ALL | ||
|---|---|---|---|---|
| LTBI-Non-CKD (n = 19) | LTBI-ESRD (n = 39) | |||
| Age, year | 57.7 ± 15.0 * | 57.4 ± 13.3 | 49.1 ± 15.7 ¶ | 61.5 ± 9.8 |
| Male sex | 17 (58.6) * | 37(63.8) | 9 (47.4) ¶ | 28 (71.8) |
| Smoking | 3 (10.3) #§ | 13(22.4) | 1 (5.3) | 12 (30.8) |
| BMI | 23.6 ± 3.8 | 23.7 ± 3.6 | 23.7 ± 4.1 | 23.7 ± 3.4 |
| Underlying disease | ||||
| ESRD | 0 | 39(67.2) | 0 | 39(100) |
| Malignancy | 0 | 1(1.7) | 1 (5.3) | 0 |
| Cirrhosis of liver | 0 | 0 | 0 | 0 |
| Diabetes mellitus | 6 (20.7) | 8(13.8) | 1 (5.3) | 7 (17.9) |
| Radiographic findings | ||||
| No lesion | 26 (89.7) | 55(94.8) | 18 (94.7) | 37 (92.5) |
| Lesion, not related to TB | 3 (10.3) | 3(5.2) | 1 (5.3) | 3 (7.7) |
| Lesion, prior TB | 0 | 0 | 0 | 0 |
| Hemoglobin, g/dL | 13.8 ± 1.5 *#§ | 11.8 ± 1.7 | 12.5 ± 2.0 ¶ | 11.4 ± 1.5 |
| Blood leukocyte count,/μL | 6920 ± 1398 | 7050 ± 2332 | 7560 ± 2607 | 6801 ± 2178 |
| Healthy Controls (n = 29) | LTBI-non-CKD (n = 19) | LTBI-ESRD (n = 39) | |
|---|---|---|---|
| CD4+ cells in lymphocytes | 43.0 ± 12.9 | 41.9 ± 13.7 | 43.0 ± 11.0 |
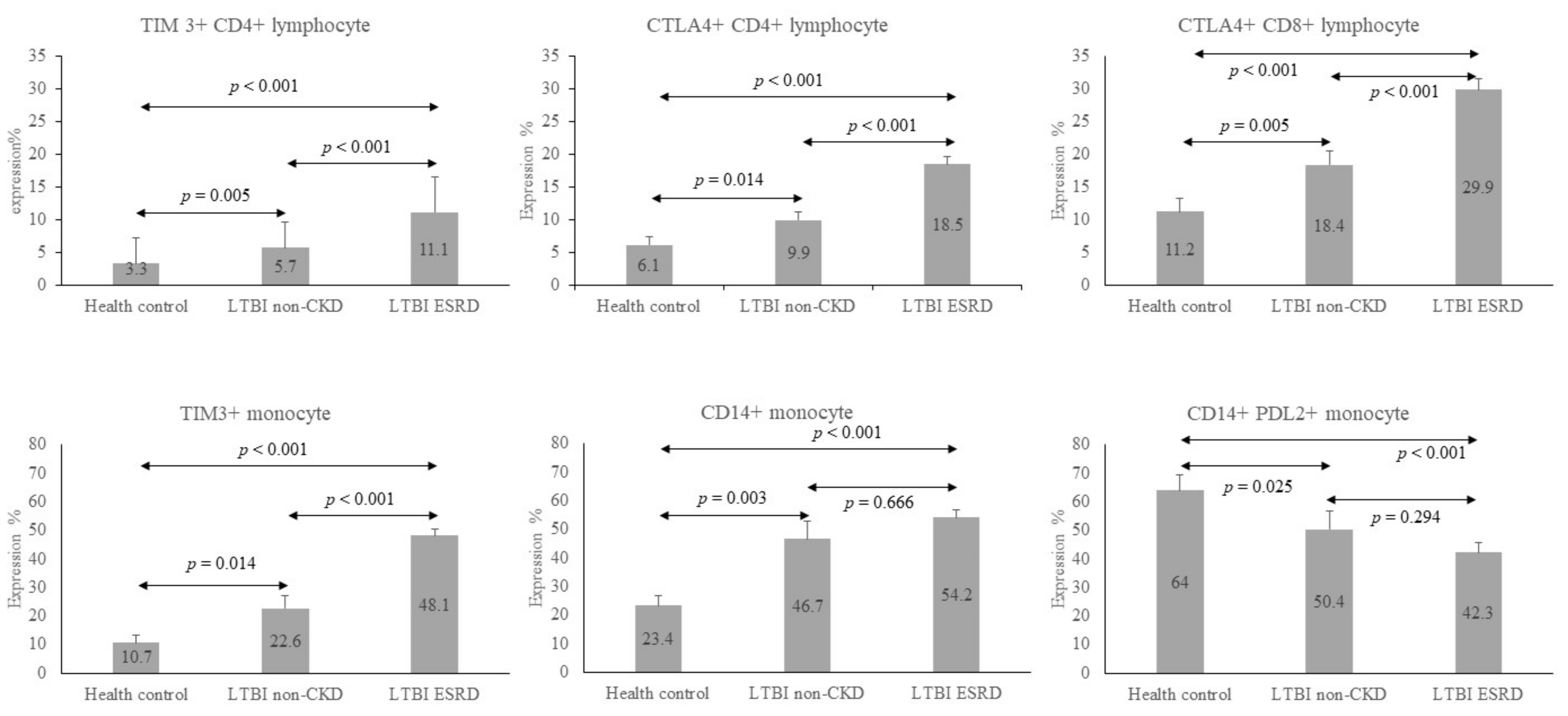
| TIM3 on CD4+ | 3.3 ± 3.9 *# | 5.7 ± 4.0 ¶ | 11.1 ± 5.5 |
| PD-1 on CD4+ | 17.3 ± 9.4 *# | 22.1 ± 11.6 | 25.6 ± 10.1 |
| CTLA-4 on CD4+ | 6.1 ± 6.8 *# | 9.9 ± 5.6 ¶ | 18.5 ± 7.6 |
| CD8+ cells in lymphocytes | 22.1 ± 8.3 | 23.1 ± 11.9 | 22.3 ± 9.1 |
| TIM3 on CD8+ | 8.5 ± 8.6 *# | 12.6 ± 7.0 | 16.0 ± 7.6 |
| PD-1 on CD8+ | 26.8 ± 11.7 * | 33.1 ± 9.0 | 29.9 ± 11.1 |
| CTLA-4 on CD8+ | 11.2 ± 10.7 *# | 18.4 ± 9.4 ¶ | 29.9 ± 10.6 |
| TIM3 in monocytes | 10.7 ± 13.6 *# | 22.6 ± 18.9 ¶ | 48.1 ± 15.0 |
| PD-1 on monocytes | 10.4 ± 10.5 *# | 16.7 ± 10.9 | 18.8 ± 8.8 |
| CLTA-4 on monocytes | 11.8 ± 10.0 # | 6.6 ± 5.3 | 6.6 ± 5.7 |
| CD14+ on monocytes | 23.4 ± 18.4 *# | 46.7 ± 26.3 | 54.2 ± 16.8 |
| PD-L1 on CD14+ cells | 12.7 ± 9.3 # | 12.5 ± 21.6 | 5.4 ± 3.9 |
| PD-L2 on CD14+ cells | 69.0 ± 28.3 *# | 50.4 ± 27.2 | 42.3 ± 20.0 |
| Galectin-9 on CD14+ cells | 16.2 ± 14.8 | 19.0 ± 29.4 | 9.5 ± 7.0 |
| Before LTBI Treatment | After 12 Weeks of LTBI Treatment | p Value | |
|---|---|---|---|
| CD4+ cells in lymphocytes | 42.5 ± 11.2 | 36.6 ± 15.1 | 0.005 |
| TIM3 on CD4+ | 10.7 ± 5.6 | 7.2 ± 5.8 | 0.005 |
| PD-1 on CD4+ | 26.3 ± 11.8 | 24.1 ± 9.7 | 0.114 |
| CTLA-4 on CD4+ | 17.8 ± 7.4 | 12.9 ± 8.9 | 0.017 |
| CD8+ cells in lymphocytes | 23.5 ± 9.4 | 20.9 ± 11.4 | 0.120 |
| TIM3 on CD8+ | 16.0 ± 7.7 | 13.7 ± 8.9 | 0.138 |
| PD-1 on CD8+ | 31.1 ± 12.9 | 29.7 ± 11.4 | 0.265 |
| CTLA-4 on CD8+ | 27.7 ± 11.4 | 22.8 ± 12.9 | 0.056 |
| TIM3 in monocytes | 44.0 ± 19.7 | 31.8 ± 23.1 | 0.023 |
| PD-1 on monocytes | 16.3 ± 8.7 | 16.5 ± 10.7 | 0.952 |
| CLTA-4 on monocytes | 6.6 ± 4.8 | 5.6 ± 5.6 | 0.448 |
| CD14+ on monocytes | 53.9 ± 21.0 | 39.3 ± 23.3 | 0.008 |
| PD-L1 on CD14+ cells | 8.4 ± 14.6 | 8.2 ± 17.4 | 0.809 |
| PD-L2 on CD14+ cells | 45.6 ± 21.9 | 31.7 ± 22.3 | 0.025 |
| Galectin-9 on CD14+ cells | 12.3 ± 17.8 | 9.3 ± 16.8 | 0.519 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-H.; Wu, M.-F.; Hsu, C.-Y.; Lin, S.-Y.; Chang, Y.-N.; Lee, H.-S.; Wei, Y.-F.; Shu, C.-C. The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection. Biomedicines 2021, 9, 1479. https://doi.org/10.3390/biomedicines9101479
Wang P-H, Wu M-F, Hsu C-Y, Lin S-Y, Chang Y-N, Lee H-S, Wei Y-F, Shu C-C. The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection. Biomedicines. 2021; 9(10):1479. https://doi.org/10.3390/biomedicines9101479
Chicago/Turabian StyleWang, Ping-Huai, Ming-Fang Wu, Chi-Yu Hsu, Shu-Yung Lin, Ya-Nan Chang, Ho-Shen Lee, Yu-Feng Wei, and Chin-Chung Shu. 2021. "The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection" Biomedicines 9, no. 10: 1479. https://doi.org/10.3390/biomedicines9101479
APA StyleWang, P.-H., Wu, M.-F., Hsu, C.-Y., Lin, S.-Y., Chang, Y.-N., Lee, H.-S., Wei, Y.-F., & Shu, C.-C. (2021). The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection. Biomedicines, 9(10), 1479. https://doi.org/10.3390/biomedicines9101479






