A Gadolinium DO3A Amide m-Phenyl Boronic Acid MRI Probe for Targeted Imaging of Sialated Solid Tumors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Chemistry
2.3. Cell Culture and Animals
2.4. Relaxivity Measurement
2.5. NMRD Measurements
2.6. Cytotoxicity Studies
2.7. In Vitro Studies
2.8. In Vivo MRI
2.9. Hematoxylin and Eosin Staining
3. Results and Discussion
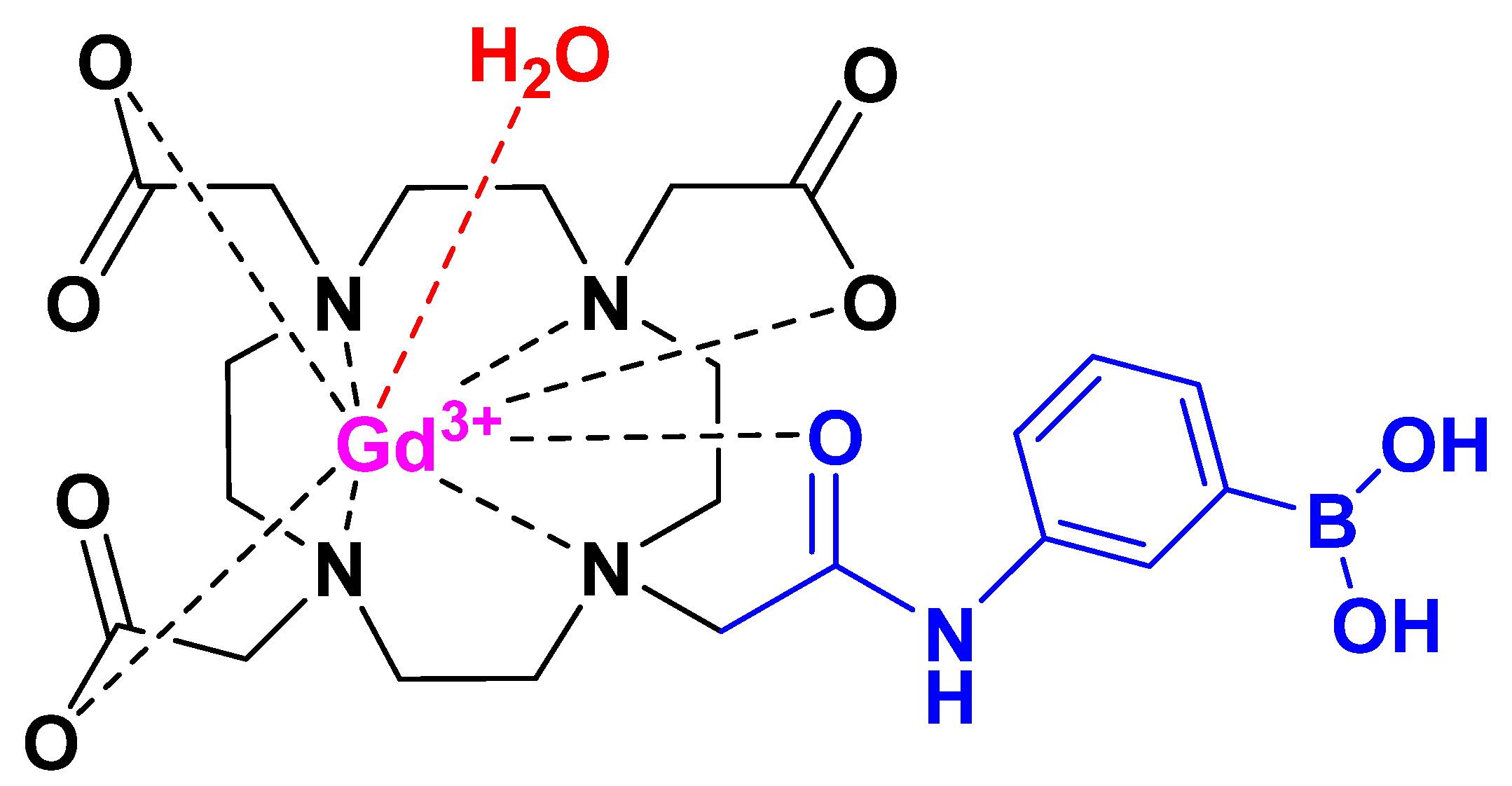
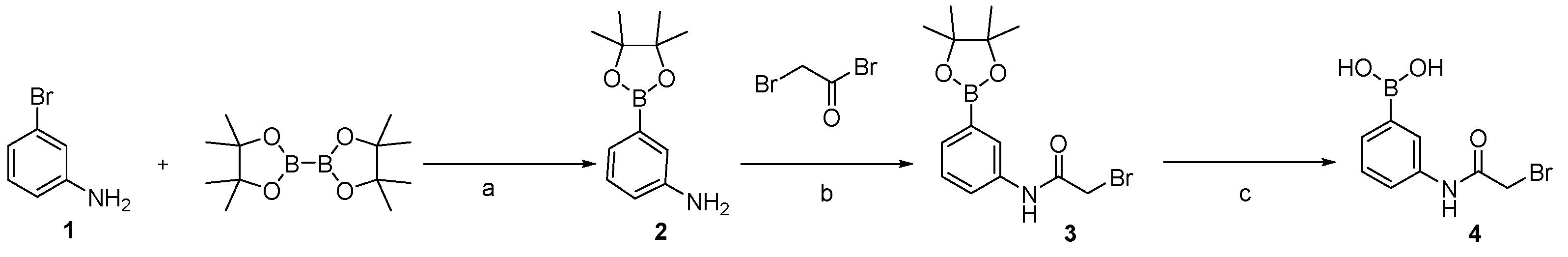
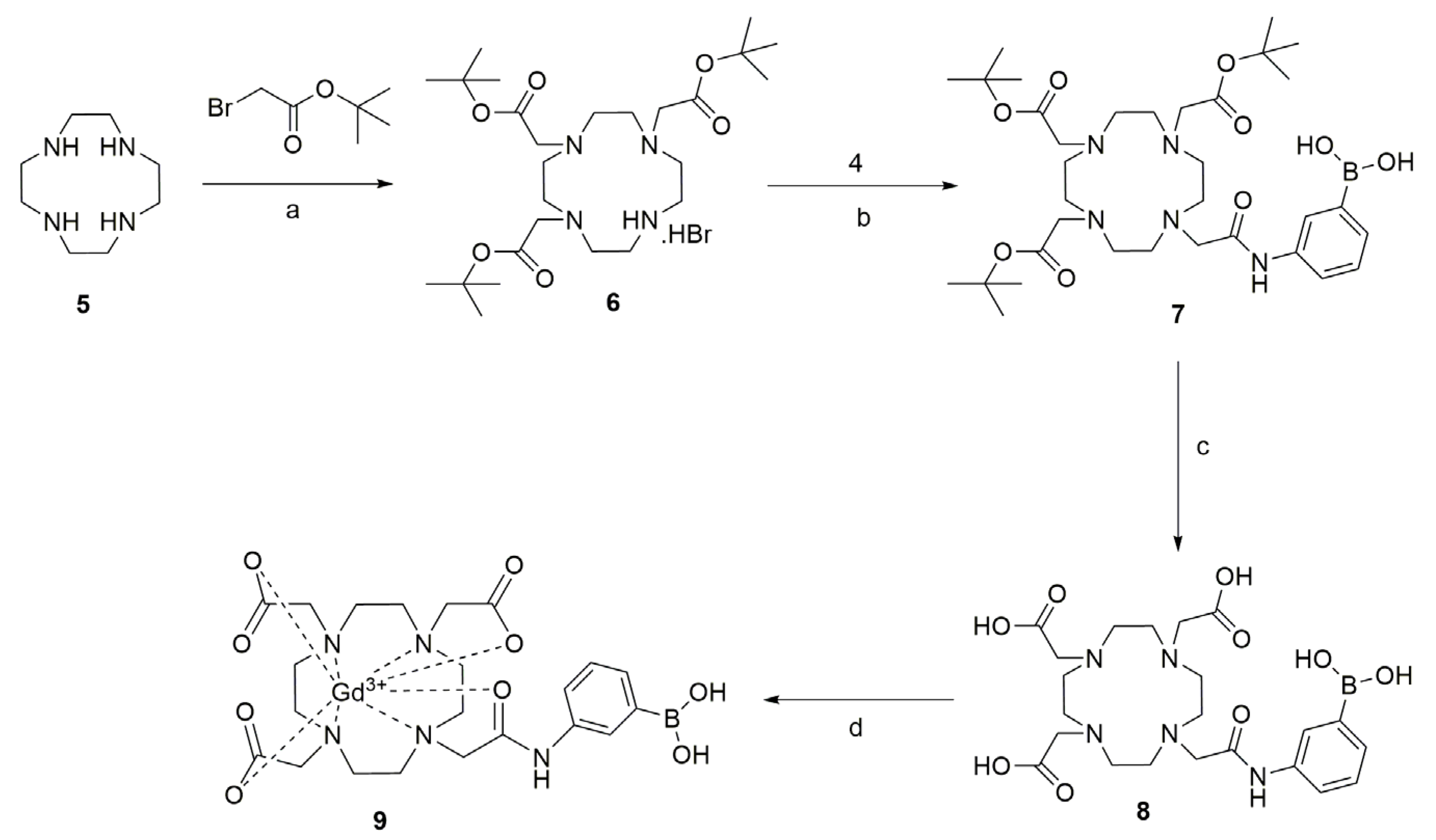
3.1. Sythesis of Gd-DO3A-Am-PBA
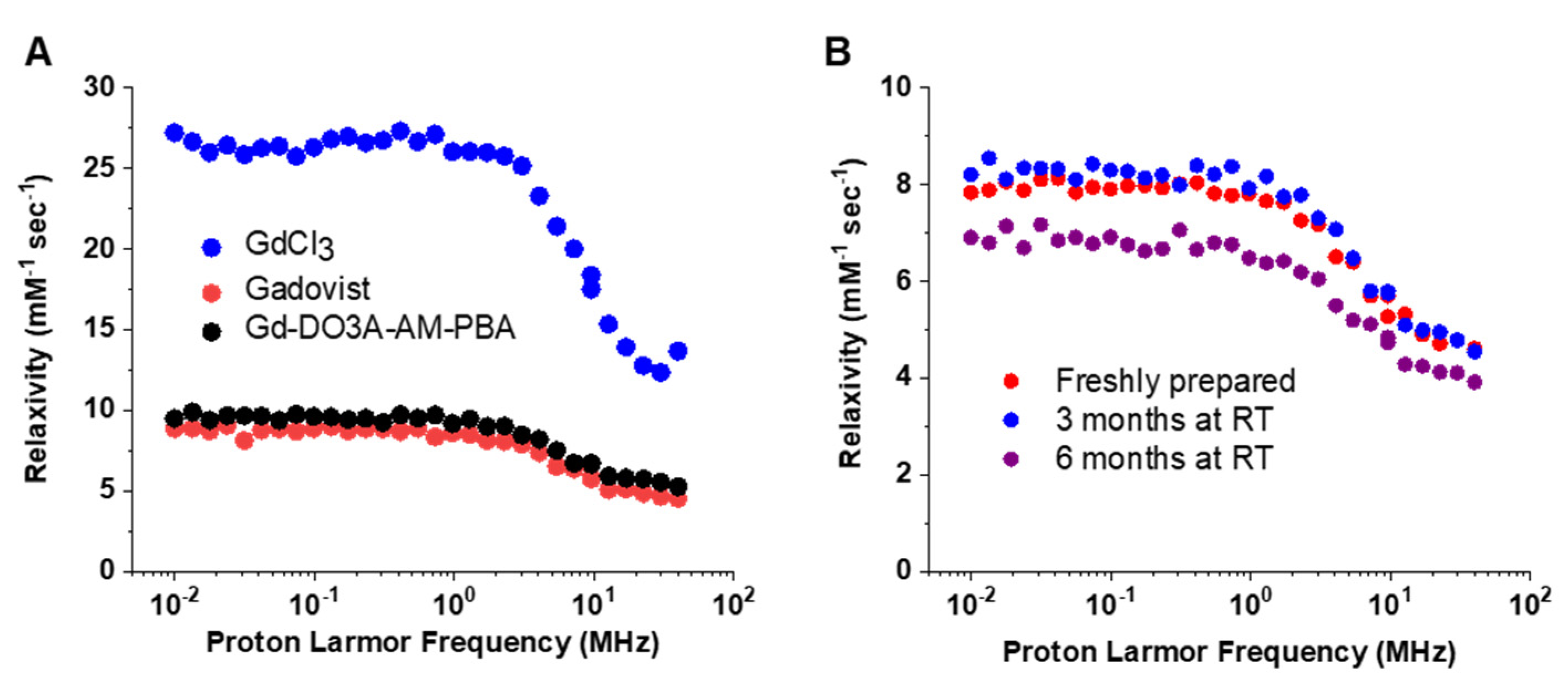
3.2. Measurements of Relaxation Rate
3.3. Measurement of Relaxivity and Stability
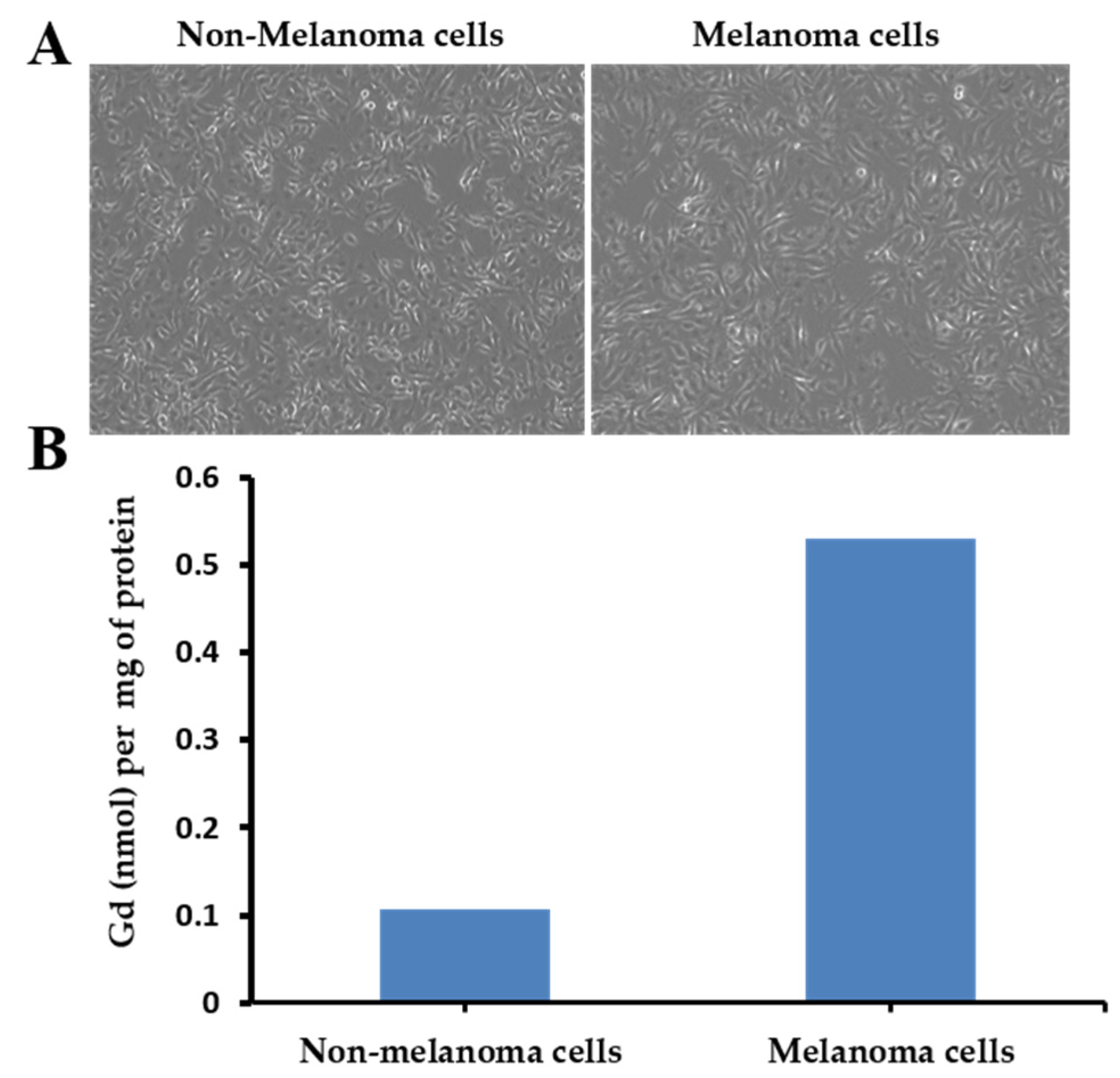
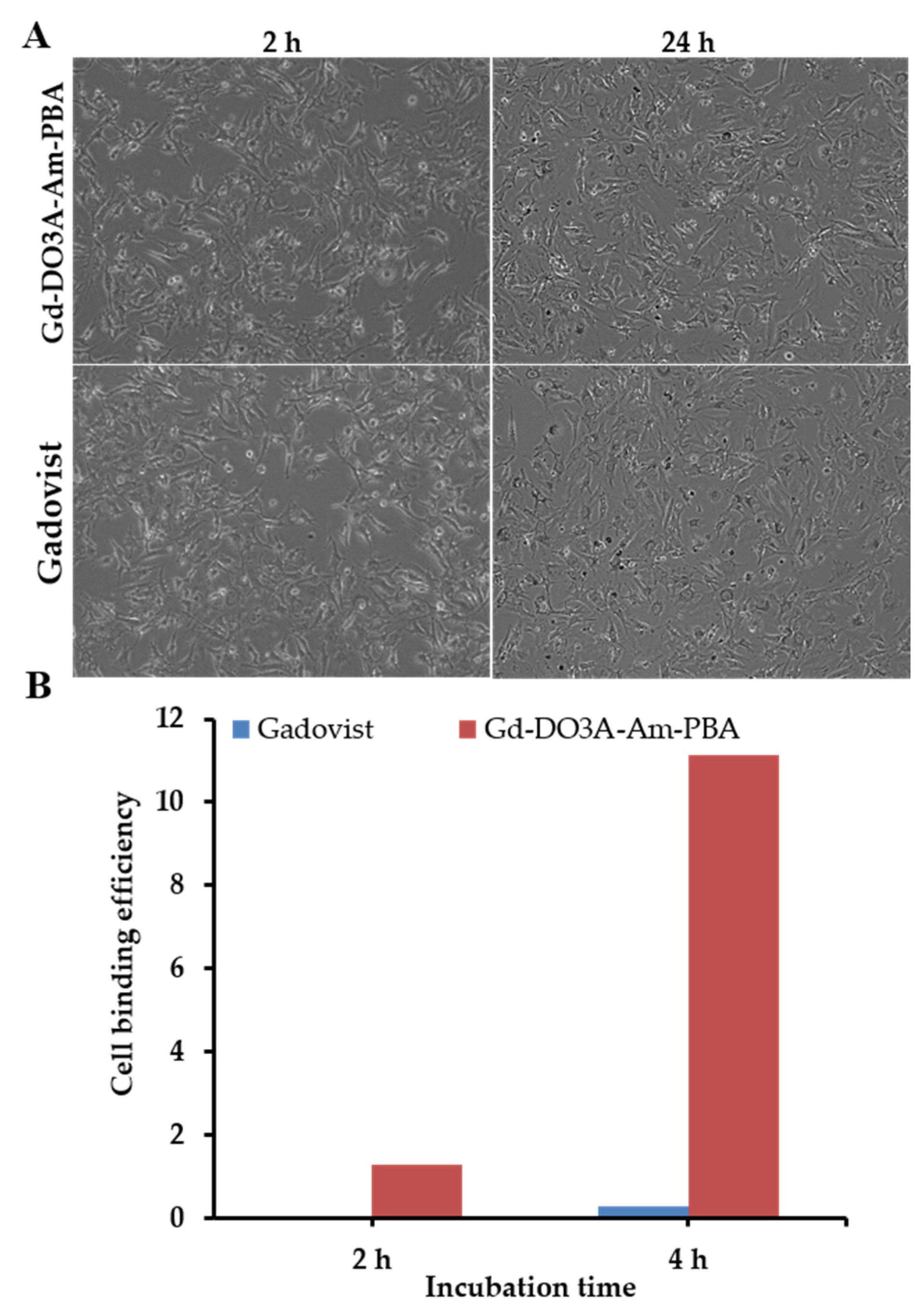
3.4. Binding Efficiency
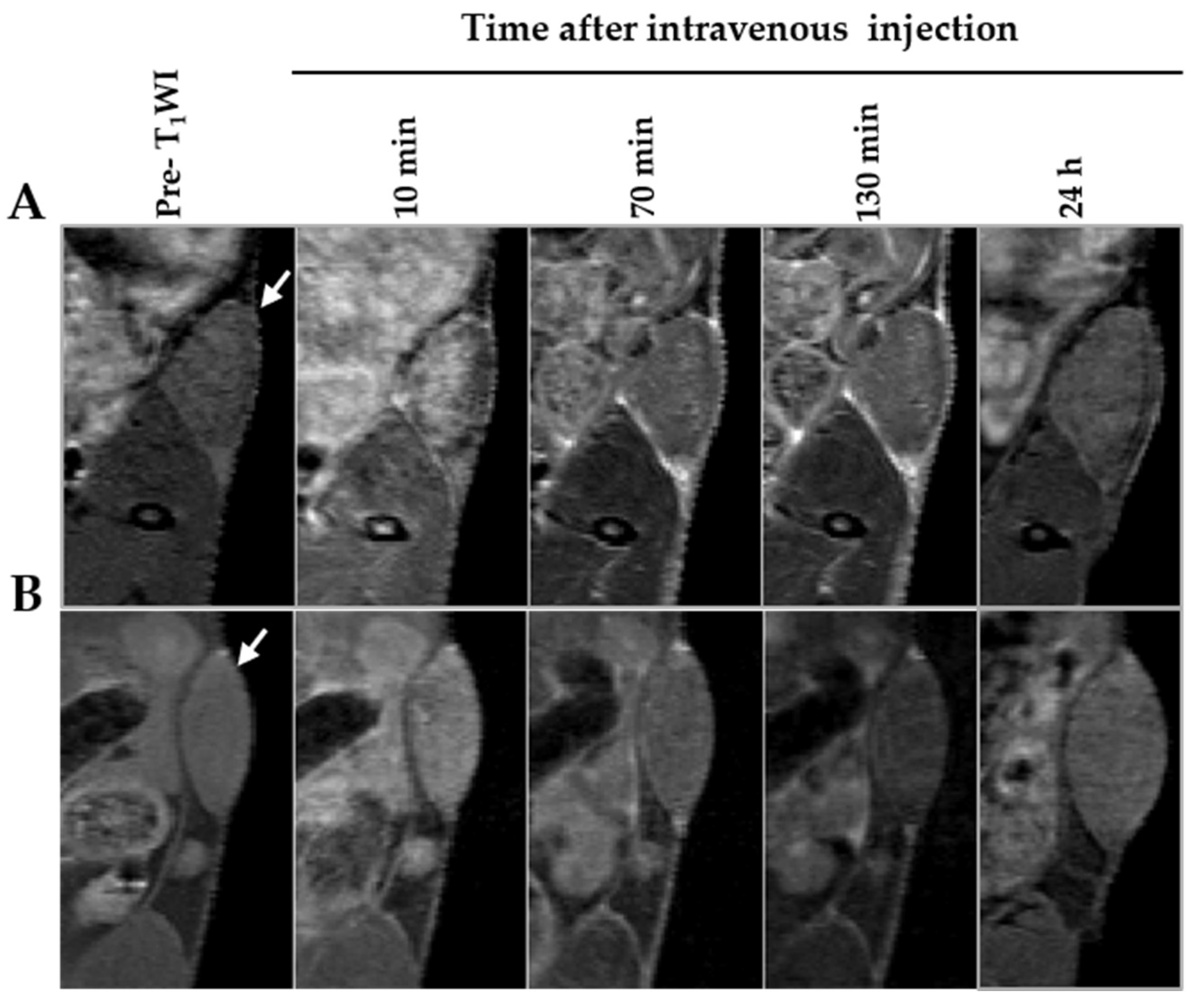
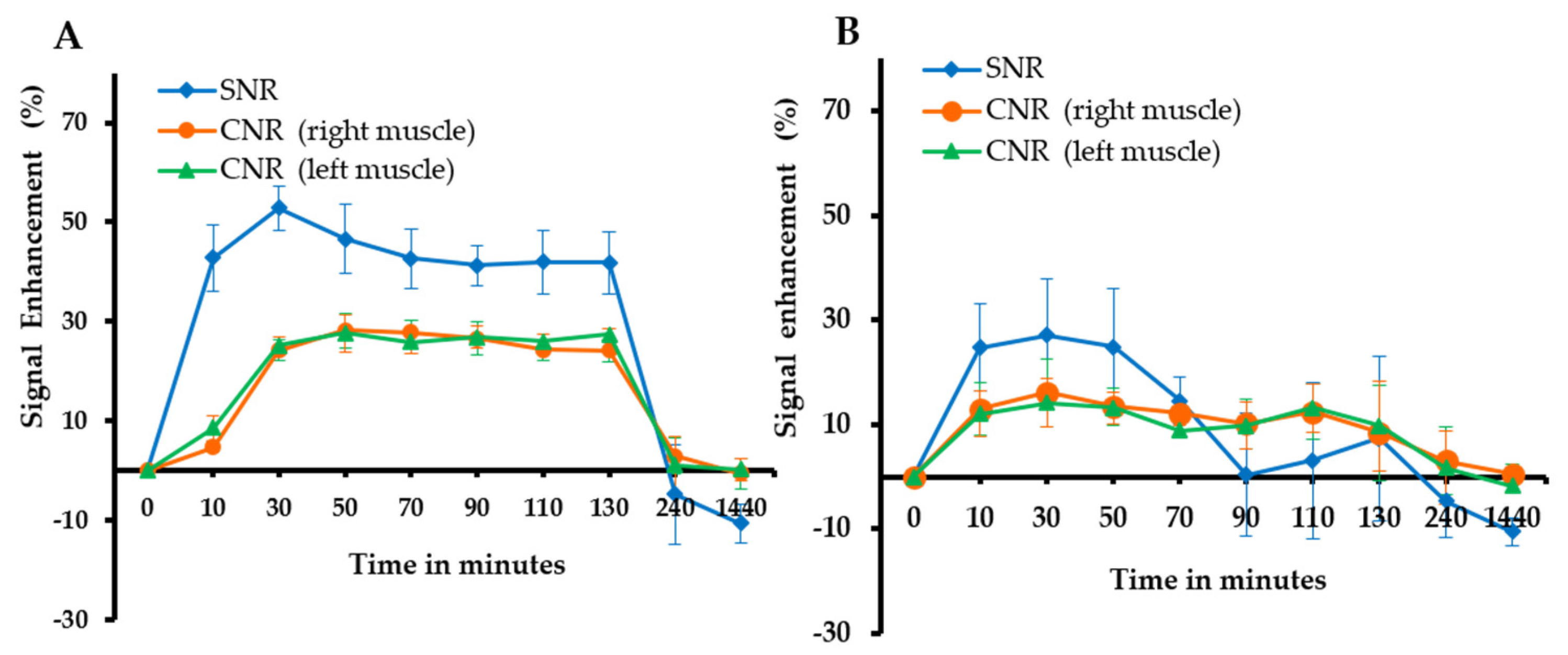
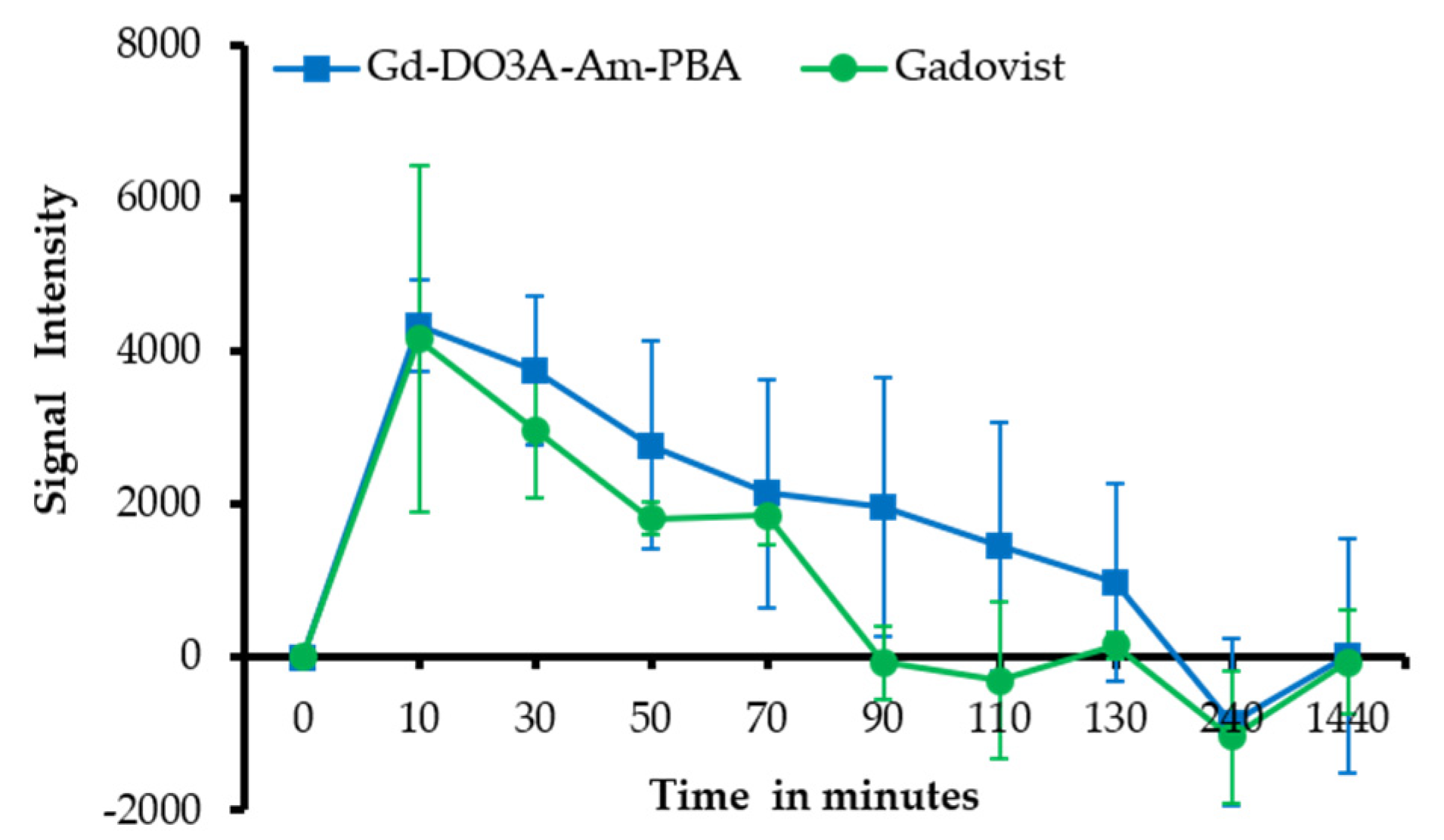
3.5. In Vivo MRI of Tumor Model Mice
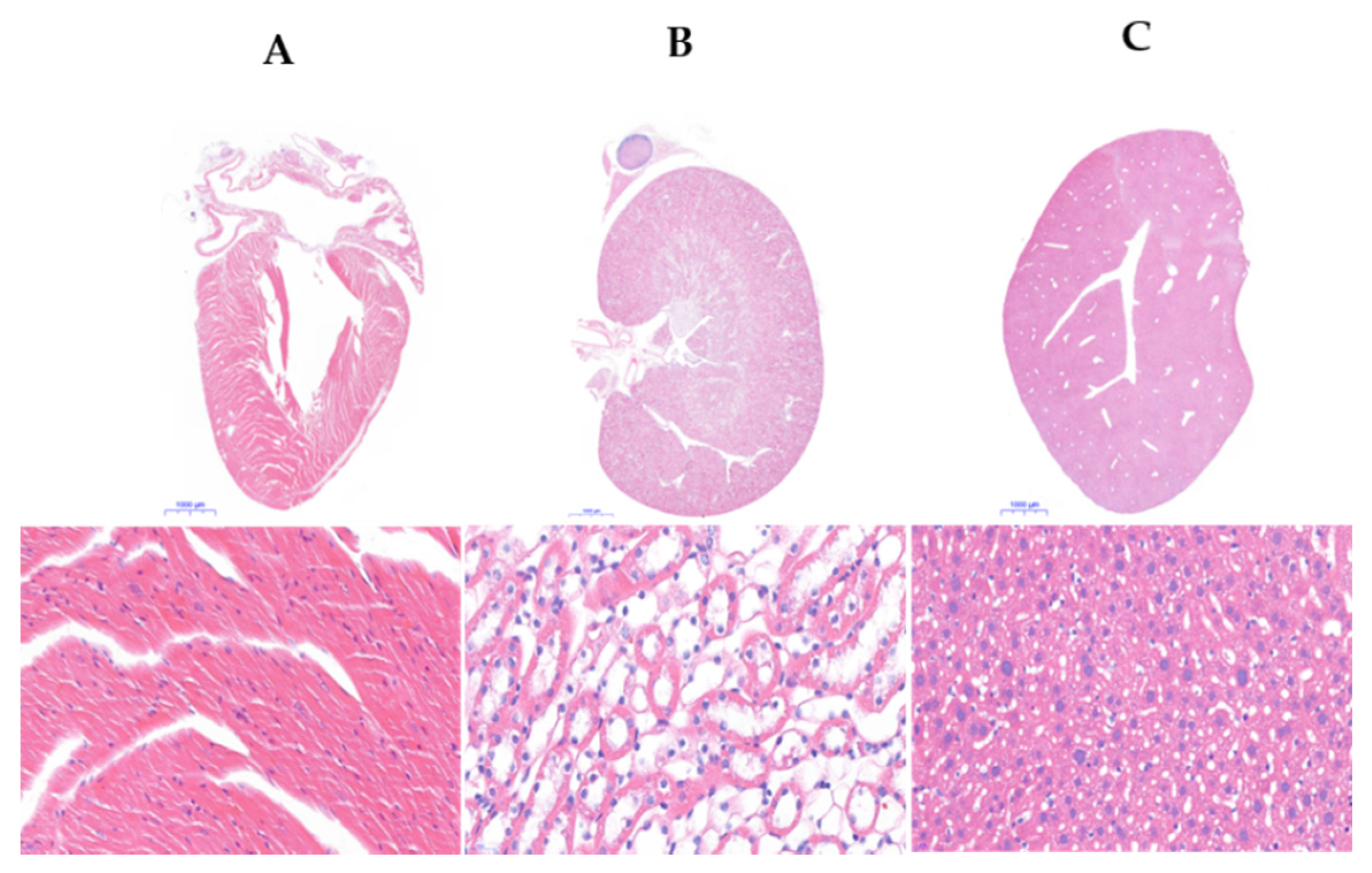
3.6. Histological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Carr, D.H.; Brown, J.; Bydder, G.M.; Weinmann, H.J.; Speck, U.; Thomas, D.J.; Young, I.R. Intravenous Chelated Gadolinium as a Contrast Agent in NMR Imaging of Cerebral-Tumors. Lancet 1984, 323, 484–486. [Google Scholar] [CrossRef]
- Merbach, A.E.; Helm, L.; Toth, E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Morse, S.V.; Boltersdorf, T.; Harriss, B.I.; Chan, T.G.; Baxan, N.; Jung, H.S.; Pouliopoulos, A.N.; Choi, J.J.; Long, N.J. Neuron labeling with rhodamine-conjugated Gd-based MRI contrast agents delivered to the brain via focused ultrasound. Theranostics 2020, 10, 2659–2674. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meade, T.J. Molecular Magnetic Resonance Imaging with Gd (III)-Based Contrast Agents: Challenges and Key Advances. J. Am. Chem. Soc. 2019, 141, 17025–17041. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.K.; Thomson, P.C.; Kingsmore, D.B.; Blessing, K.; Daly, C.D.; Cowper, S.E.; Roditi, G.H. Diagnosing nephrogenic systemic fibrosis in the post-FDA restriction era. J. Magn. Reson. Imaging 2015, 41, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S.; Morcos, S.K.; Almén, T.; Bellin, M.-F.; Bertolotto, M.; Bongartz, G.; Clement, O.; Leander, P.; Heinz-Peer, G.; Reimer, P.; et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: Updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2013, 23, 307–318. [Google Scholar] [CrossRef]
- Clough, T.J.; Jiang, L.; Wong, K.-L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-A.; Lee, J.-J.; Jung, J.-C.; Yu, D.-Y.; Oh, C.; Ha, S.; Kim, T.-J.; Chang, Y. Gd-DOTA Conjugate of RGD as a Potential Tumor-Targeting MRI Contrast Agent. ChemBioChem 2008, 9, 2811–2813. [Google Scholar] [CrossRef]
- Menchise, V.; Digilio, G.; Gianolio, E.; Cittadino, E.; Catanzaro, V.; Carrera, C.; Aime, S. In Vivo Labeling of B16 Melanoma Tumor Xenograft with a Thiol-Reactive Gadolinium Based MRI Contrast Agent. Mol. Pharm. 2011, 8, 1750–1756. [Google Scholar] [CrossRef]
- Jeong, Y.; Na, K. Synthesis of a gadolinium based-macrocyclic MRI contrast agent for effective cancer diagnosis. Biomater. Res. 2018, 22, 17. [Google Scholar] [CrossRef] [Green Version]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, H. Synthesis and Applications of Boronate Affinity Materials: From Class Selectivity to Biomimetic Specificity. Acc. Chem. Res. 2017, 50, 2185–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, A.; Cabral, H.; Sato, N.; Kataoka, K.; Miyahara, Y. Assessment of Tumor Metastasis by the Direct Determination of Cell-Membrane Sialic Acid Expression. Angew. Chem. Int. Ed. 2010, 49, 5494–5497. [Google Scholar] [CrossRef] [PubMed]
- Sanjoh, M.; Miyahara, Y.; Kataoka, K.; Matsumoto, A. Phenylboronic Acids-based Diagnostic and Therapeutic Applications. Anal. Sci. 2014, 30, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, H.; Uchimura, E.; Koshino, H.; Okano, T.; Kataoka, K. Anomalous Binding Profile of Phenylboronic Acid with N-Acetylneuraminic Acid (Neu5Ac) in Aqueous Solution with Varying pH. J. Am. Chem. Soc. 2003, 125, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Djanashvili, K.; Frullano, L.; Peters, J.A. Molecular Recognition of Sialic Acid End Groups by Phenylboronates. Chem. Eur. J. 2005, 11, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Sato, N.; Kataoka, K.; Miyahara, Y. Noninvasive Sialic Acid Detection at Cell Membrane by Using Phenylboronic Acid Modified Self-Assembled Monolayer Gold Electrode. J. Am. Chem. Soc. 2009, 131, 12022–12023. [Google Scholar] [CrossRef]
- Cao, J.-T.; Zhang, P.-H.; Liu, Y.-M.; Abdel-Halim, E.S.; Zhu, J.-J. Versatile Microfluidic Platform for the Assessment of Sialic Acid Expression on Cancer Cells Using Quantum Dots with Phenylboronic Acid Tags. ACS Appl. Mater. Interfaces 2015, 7, 14878–14884. [Google Scholar] [CrossRef]
- Lim, J.; Lee, J.; Jung, S.; Kim, W.J. Phenylboronic-acid-based nanocomplex as a feasible delivery platform of immune checkpoint inhibitor for potent cancer immunotherapy. J. Control. Release 2021, 330, 1168–1177. [Google Scholar] [CrossRef]
- Yoshito, K.; Seiji, S.; Takuji, T. Cellular Sialic Acid Level and Phenotypic Expression in B16 Melanoma Cells: Comparison of Spontaneous Variations and Bromodeoxyuridine and Theophylline Induced Changes. Cell Struct. Funct. 1989, 14, 35–43. [Google Scholar]
- Andreea, V.D.L.; Maria, I.I.G.; Rodica, M.A.; Daniela, A.G. The role of the sialic acid in monitoring the evolution of malignant melanoma. From murine models to human research. Rev. Română Med. Lab. 2013, 21, 2–4. [Google Scholar]
- Djanashvili, K.; Koning, G.A.; van der Meer, A.J.G.M.; Wolterbeek, H.T.; Peters, J.A. Phenylboronate 160Tb complexes for molecular recognition of glycoproteins expressed on tumor cells. Contrast Media Mol. Imaging 2007, 2, 35–41. [Google Scholar] [CrossRef]
- Frullano, L.; Rohovec, J.; Aime, S.; Maschmeyer, T.; Prata, M.I.; de Lima, J.J.P.; Geraldes, C.F.G.C.; Peters, J.A. Towards Targeted MRI: New MRI Contrast Agents for Sialic Acid Detection. Chem. Eur. J. 2004, 10, 5205–5217. [Google Scholar] [CrossRef] [Green Version]
- Uchimura, E.; Otsuka, H.; Okano, T.; Sakurai, Y.; Kataoka, K. Totally synthetic polymer with lectin-like function: Induction of killer cells by the copolymer of 3-acrylamidophenylboronic acid with N,N-dimethylacrylamide. Biotechnol. Bioeng. 2001, 72, 307–314. [Google Scholar] [CrossRef]
- Van Duin, M.; Peters, J.A.; Kieboom, A.P.G.; Van Bekkum, H. Studies on borate esters 1: The ph dependence of the stability of esters of boric acid and borate in aqueous medium as studied by 11B NMR. Tetrahedron 1984, 40, 2901–2911. [Google Scholar] [CrossRef]
- Geninatti Crich, S.; Alberti, D.; Szabo, I.; Aime, S.; Djanashvili, K. MRI Visualization of Melanoma Cells by Targeting Overexpressed Sialic Acid with a GdIII-dota-en-pba Imaging Reporter. Angew. Chem. Int. Ed. 2013, 52, 1161–1164. [Google Scholar] [CrossRef]
- Martinelli, J.; Tei, L.; Geninatti Crich, S.; Alberti, D.; Djanashvili, K. Towards Enhanced MRI Performance of Tumor-Specific Dimeric Phenylboronic Contrast Agents. Molecules 2021, 26, 1730. [Google Scholar] [CrossRef]
- Xu, D.; Sun, D.; Wang, W.; Peng, X.; Zhan, Z.; Ji, Y.; Shen, Y.; Geng, M.; Ai, J.; Duan, W. Discovery of pyrrolo[2,3-d]pyrimidine derivatives as potent Axl inhibitors: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 220, 113497. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.Y.; Lee, S.H. Self-assembly of pyrene boronic acid-based chemodosimeters for highly efficient mercury (II) ion detection. Tetrahedron Lett. 2019, 60, 151048. [Google Scholar] [CrossRef]
- Sun, C.; Lin, H.; Gong, X.; Yang, Z.; Mo, Y.; Chen, X.; Gao, J. DOTA-Branched Organic Frameworks as Giant and Potent Metal Chelators. J. Am. Chem. Soc. 2020, 142, 198–206. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, Z.-R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, I.; Crich, S.G.; Alberti, D.; Kálmán, F.K.; Aime, S. Mn loaded apoferritin as an MRI sensor of melanin formation in melanoma cells. Chem. Commun. 2012, 48, 2436–2438. [Google Scholar] [CrossRef] [PubMed]


| (mM−1sec−1) | R1 | R2 | R1/R2 |
|---|---|---|---|
| Gd-DO3A-Am-PBA | 3.295 | 4.1749 | 1.2670 |
| Gadovist | 4.9172 | 6.3266 | 1.2866 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajan, C.; Seema, J.; Chen, Y.-W.; Chen, T.-C.; Lin, M.-H.; Lin, C.-H.; Hwang, D.W.-H. A Gadolinium DO3A Amide m-Phenyl Boronic Acid MRI Probe for Targeted Imaging of Sialated Solid Tumors. Biomedicines 2021, 9, 1459. https://doi.org/10.3390/biomedicines9101459
Rajan C, Seema J, Chen Y-W, Chen T-C, Lin M-H, Lin C-H, Hwang DW-H. A Gadolinium DO3A Amide m-Phenyl Boronic Acid MRI Probe for Targeted Imaging of Sialated Solid Tumors. Biomedicines. 2021; 9(10):1459. https://doi.org/10.3390/biomedicines9101459
Chicago/Turabian StyleRajan, Christu, Jaya Seema, Yu-Wen Chen, Tsai-Chen Chen, Ming-Huang Lin, Chia-Huei Lin, and Dennis Wen-Han Hwang. 2021. "A Gadolinium DO3A Amide m-Phenyl Boronic Acid MRI Probe for Targeted Imaging of Sialated Solid Tumors" Biomedicines 9, no. 10: 1459. https://doi.org/10.3390/biomedicines9101459
APA StyleRajan, C., Seema, J., Chen, Y.-W., Chen, T.-C., Lin, M.-H., Lin, C.-H., & Hwang, D. W.-H. (2021). A Gadolinium DO3A Amide m-Phenyl Boronic Acid MRI Probe for Targeted Imaging of Sialated Solid Tumors. Biomedicines, 9(10), 1459. https://doi.org/10.3390/biomedicines9101459






