1. Introduction
Glioblastoma (GBM) is the deadliest adult brain cancer. Among possible cellular sources of GBM are neural stem cells (NSC), oligodendrocyte progenitor cells and astrocytes [
1]. GBMs exhibit a highly heterogeneous molecular makeup and are characterized by genomic instability and high tendency for infiltration. GBM exists in a variety of molecular phenotypes, including isocitrate dehydrogenase wild type, mutant type and some others [
2]. Molecular heterogeneity greatly reduces chances of finding a highly potent and universally useful drug against any one specific molecular target for this type of cancer.
The global standard of care, known as the Stupp protocol [
3], consists of surgical resection followed by administration of the alkylating agent temozolomide (TMZ) in combination with radio-therapy. However, the Stupp protocol only extends median survival to ~14 months from diagnosis compared to 12 months when using radio-therapy alone [
3]. This treatment protocol and the resultant survival prognosis have not significantly changed for the last 15 years.
Considering that GBM does not metastatically spread around the body and that primary tumors are often reasonably well localized, it is surprising that we are still making so little progress in improving treatment outcomes. The key reason for this is that, despite maximal surgical resection, the tumor inevitably reoccurs. Interestingly, most secondary tumors arise within <2 cm of the resection edge [
4]. These recurrences originate from the infiltrating GBM cells which spread from the leading edge into non-neoplastic tissue parenchyma. Today, the macroscopic boundaries of the primary tumor are usually identified by neurosurgeons using specific staining with 5-aminolevulinic acid (5ALA, trade name Gliolan
®) which was approved by the Food and Drug Administration in 2017 for the optical detection of GBM. 5ALA is fairly selectively converted into protoporphyrin IX in cancer cells [
5]. Thus, by illuminating the tumor by near-UV blue light and monitoring resultant red fluorescence, surgeons are able to detect macroscopic boundaries of GBM. However, this does not allow visualization of the microscopic infiltrations. Moreover, in many cases, even though surgeons suspect infiltrations in certain areas, they are unable to remove residual disease due to the risk of severe neurological deficits.
Poor prognosis for patients with GBM necessitates research into alternative approaches for the treatment of this disease. One such approach is photodynamic therapy (PDT), as we have recently reviewed [
6]. PDT is based on a photochemical reaction triggered by the absorption of photons of light by the molecules of a photosensitizer. Singlet oxygen and reactive oxygen species released by this reaction damage cellular macromolecules and eventually kill the cells. Even though quite a few molecules could theoretically be used as photosensitizers, only 5ALA has been extensively explored as a photosensitizer for GBM therapy, including in clinical trials [
7]. The motivation for working with 5ALA is mainly the selective accumulation of fluorescent protoporphyrin IX in cancer cells which may intuitively suggest that PDT should only damage the malignant cells and not the healthy tissue. However, so far, experimental and clinical applications of 5ALA as a PDT agent have not been particularly successful [
6] as 5ALA appears to be a poor photosensitizer, unable to generate sufficient amounts of free radicals to induce a powerful effect. High-power red light at approximately ~630 nm was used for its photoactivation in the published trials [
8,
9,
10]. This contrasts with the peak absorption of protoporphyrin IX which is near 420 nm [
8,
9,
10,
11,
12] This was largely motivated by the much better penetration of red light through brain tissue, but clearly, it cannot be efficient in terms of triggering the required photochemistry.
Over the past 10 years, the application of light to the brains of living animals has become a major tool in experimental neuroscience (a technology known as “optogenetics”), and a wealth of information is now available from these experiments. Hundreds of studies have used light to control cells, which are induced to express light-sensitive proteins. The wavelengths used for excitation are typically below 550 nM (blue-green-yellow). Despite the accepted notion that infra-red light (~700 nm and above) penetrates deeper into the tissue, which could be an advantage in the case of PDT, the vast experience accumulated with optogenetics unequivocally demonstrates that large quantities of light energy are damaging for a healthy brain. Moreover, light, especially the longer-wave red and infra-red light, easily releases heat which destroys brain cells (for further discussion, see [
6]).
These considerations led us to investigate whether we might be more successful using another photosensitizer with a different principle of action and selectivity.
By serendipity, we discovered that one of the dyes routinely used to image mitochondrial membrane potential (MMP), tetramethylrhodamine (TMRM), acts as an efficient photosensitizer in patient-derived primary GBM cell lines and that it is possible to achieve at least partial selectivity over non-malignant primary rat astrocytes (RA). TMRM, which is a rhodamine derivative driven into the mitochondria by their negative membrane potential has been in routine laboratory use as a research reagent but never tested as a potential therapeutic. After brief (<1 min) illumination with a green light of moderate intensity, TMRM causes the rapid and irreversible depolarization of GBM mitochondria, which ultimately leads to the apoptosis-mediated death of these cells. Here, we explored the effectiveness of TMRM as a photosensitizer for PDT (TMRM-PDT). We also attempted to increase the efficacy of TMRM-PDT using the cAMP-elevating compound NKH477 (a water-soluble analogue of forskolin) and a glycolysis inhibitor clotrimazole.
2. Materials and Methods
2.1. Primary Cultures of RA
Primary cultures of RA were prepared from the cerebral cortices, cerebellum and brainstem of Wistar rat pups (P2) as previously described [
13]. Briefly, the brains of terminally anesthetized Wistar P2 pups were dissected out, crudely cross-chopped and incubated with agitation at 37 °C for 15 min in a solution containing HBSS, DNase I (0.04 mg/mL), trypsin from bovine pancreas (0.25 mg/mL) and BSA (3 mg/mL). Trypsinization was terminated by the addition of equal volumes of culture media comprised of DMEM, 10% heat-inactivated FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin and the suspension was then centrifuged at 2000 rpm, at room temperature (RT) for 10 min. The supernatant was aspirated, and the remaining pellet was resuspended in 15 mL HBSS containing BSA (3 mg/mL) and DNase I (0.04 mg/mL) and gently triturated. After the cell debris settled, the cell suspension was filtered through a 40 μm cell strainer (BD Falcon, BD Biosciences, Franklin Lakes, NJ, USA) and cells were collected after centrifugation. Cells were seeded in a T75 flask containing the culture media (see above) and maintained at 37 °C with 5% CO
2. Once the cultures reached confluence and 1 week later, the flasks were mildly shaken overnight to remove microglia and oligodendrocytes.
2.2. GBM Cell Lines
UP007 and UP029 were kindly provided by Prof. J. Pilkington (University of Portsmouth) and maintained using standard laboratory protocols in media containing 10% serum and 1% penicillin/streptomycin (0.1 mg/mL penicillin, 100 units/mL streptomycin). In some experiments, we also used primary GBM cell lines specifically derived from the infiltrative edge of surgically removed tumors as described in Smith et al. [
6]. These are designated as glioblastoma invasive margin (GIN) cell lines. Their culturing conditions and handling were the same as those for UP cell lines. All of the cell lines used were of the IDH-wildtype genetic background.
2.3. Measurement of Cell Viability
Cytotoxicity was assessed by lactate dehydrogenase (LDH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and PrestoBlueTM (Invitrogen, Paisley, UK) assays.
2.3.1. LDH Assay
LDH is an intracellular enzyme that is released from cells upon the disruption of the cell membrane or cell lysis. Thermo Scientific™ Pierce™ LDH Cytotoxicity Assay (cat no. 88954) was used to determine the toxicity of TMRM in the absence of light illumination. This is a colorimetric assay where the amount of LDH in a sample is proportional to the amount of red formazan product produced by the consumption of NADH generated by the LDH-mediated conversion of L-lactate. After adding reaction buffers to the sample culture media, color intensity in wells was measured using an Infinite® 200 PRO microplate reader.
2.3.2. MTT Assay
The MTT assay was used to assess the potential detrimental effects of prolonged TMRM loading on cells. RA and GBM cell lines were seeded in a 96-well culture plate at 1 × 10
4 cells/mL in 10% FBS culture media at 37 °C in 5% CO
2 atmosphere and allowed to attach overnight. After 24 h, the culture media were replaced with fresh media containing different concentrations of TMRM (50 nM, 100 nM, 300 nM, 800 nM) and the plates were incubated for 48 h protected from light. Tests were done in triplicates. After incubation, the MTT reagent was added into the media at a final concentration of 0.5 mg/mL and incubated for 2 h 37 °C under low light conditions. Then, the MTT reagent was removed and 100 µL DMSO in each well was added and the cells were incubated at 37 °C for 30 min to dissolve the formazan crystal precipitates. Absorbance was measured at 570 nm. Cell viability was calculated by the following formula:
2.3.3. PrestoBlueTM Assay
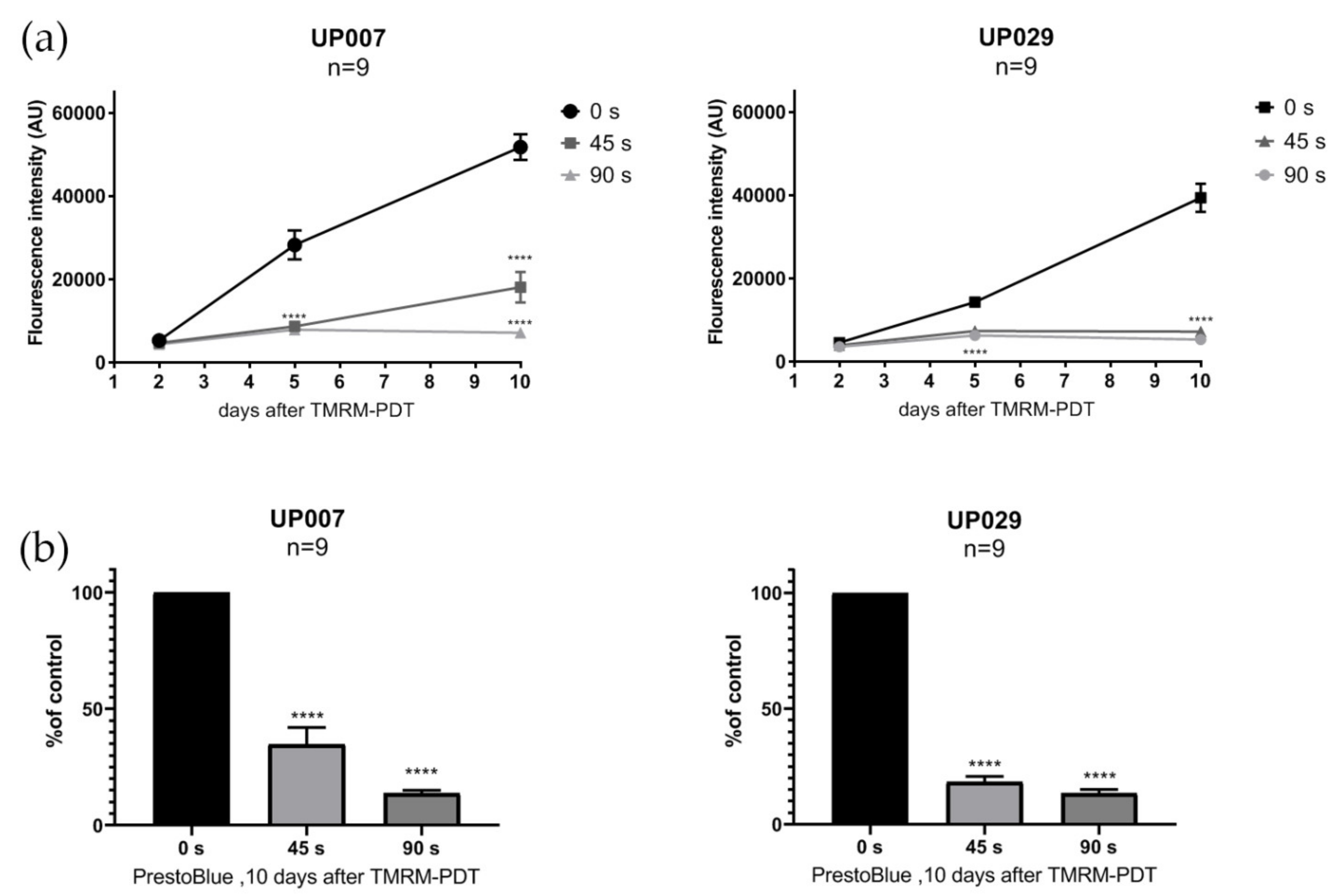
To assess the viability of the cells after photoactivation with different illumination durations, we performed the PrestoBlueTM cell viability assay (Invitrogen) on UP007, UP029 and GIN8 cell lines. This assay does not require the fixation of cells and can be performed on the same set of cells several times. Cells were seeded in 96-well plates using the technique described above. Twenty-four hours later, cell lines were loaded with TMRM (300 nM × 40 min) and photoactivated for 45 s and 90 s (1.06 mW/mm2). PrestoBlue assay was performed on day 2, 5 and 10 after the photoactivation of TMRM, following the manufacturer’s instructions. Briefly, 10 µL of PrestoBlue reagent was added to each well and incubated for 20 min. Fluorescence was measured at 560 nM, thus reflecting the amount of the fluorescent product of the conversion of the reagent.
2.4. Assessment of Basal Mitochondrial Membrane Potential (MMP) Using Potential-Driven Dye TMRM
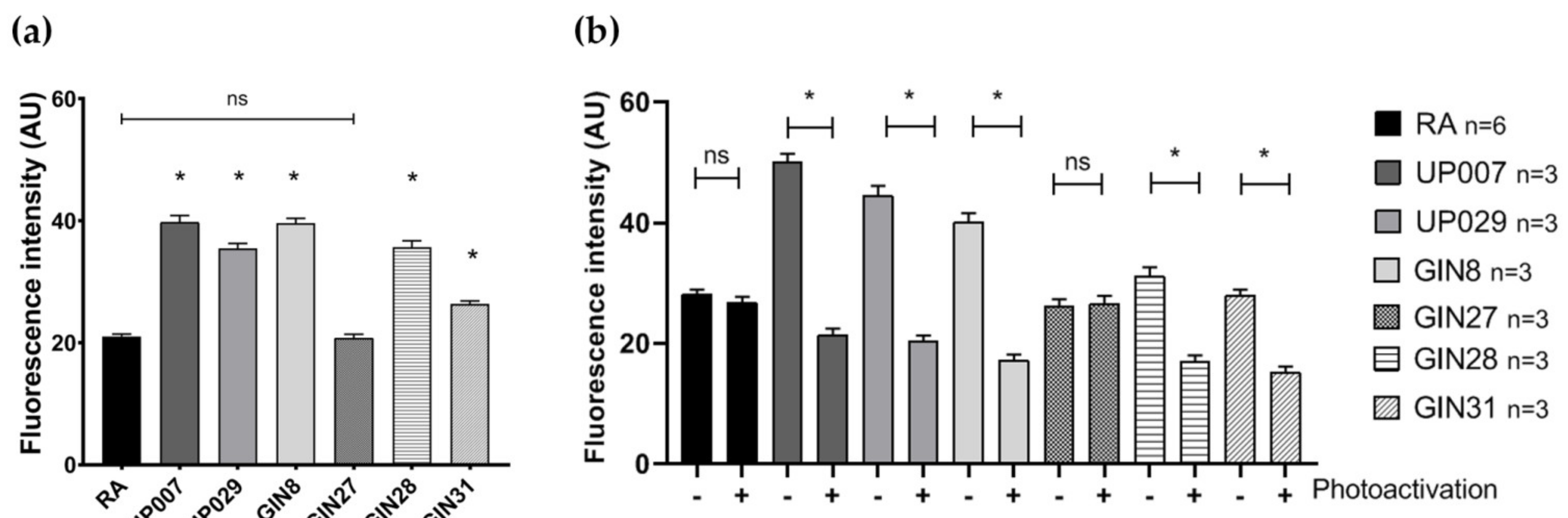
Cells were plated in 96-well plates and allowed to attach overnight. The following day, cells were loaded with 200 nM TMRM for 1 h. Images were taken using a ZOE (Bio-Rad, Watford, UK) fluorescent cell imager. Fluorescence intensity (as an estimate for MMP) was measured using the Fiji image processing tool and compared across cell types. Image acquisition parameters were fixed across all measurements.
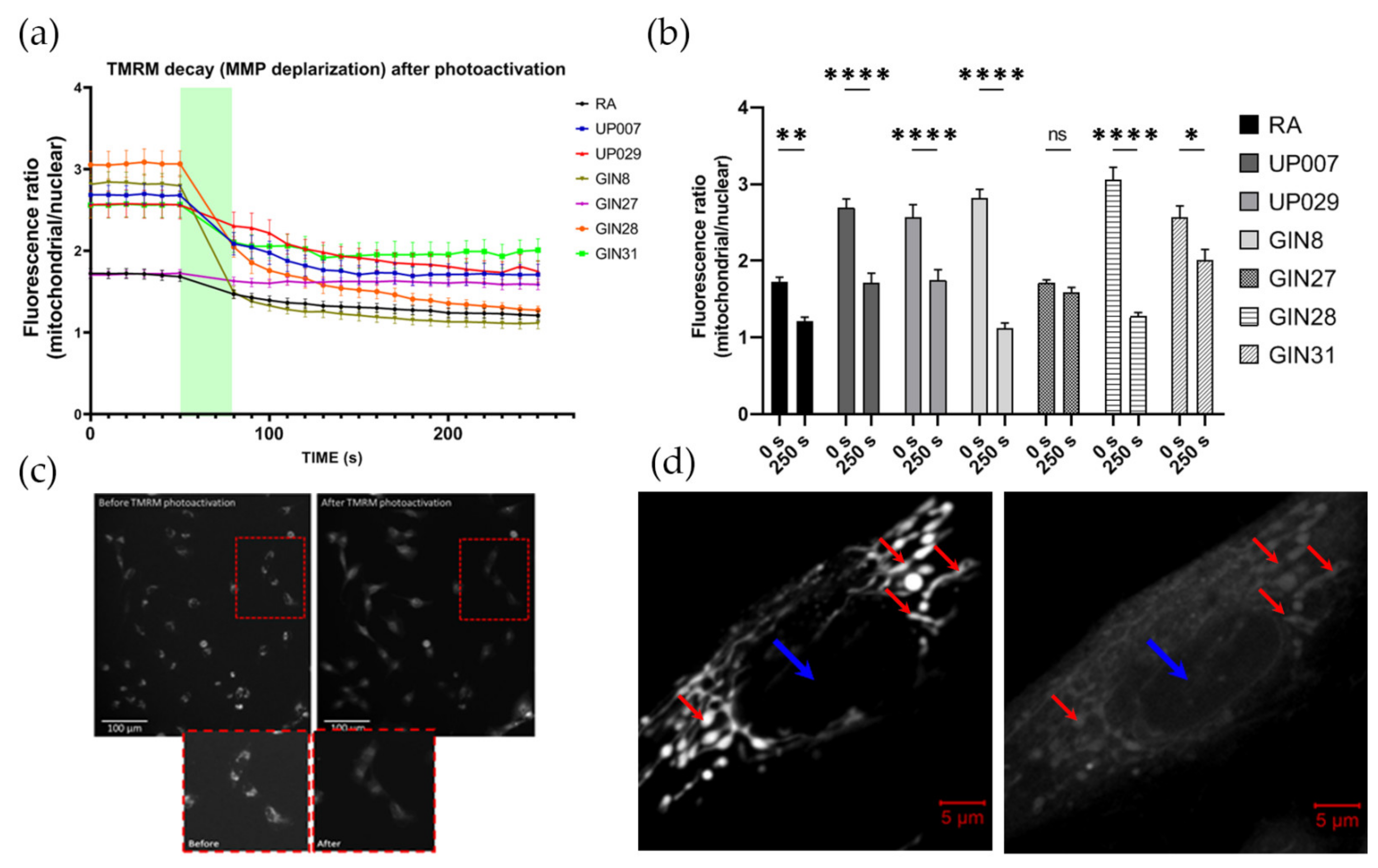
2.5. Measurements of Mitochondrial Depolarization Dynamics Caused by TMRM
TMRM decay dynamics was tested using the following protocol. The cells were plated onto glass cover slips coated with type 1 rat tail collagen at a concentration of 0.25 mg/mL to enhance the attachment of the cells. Cover slips were placed inside small corning dishes at a density of 5 × 104 cells/mL. Dishes were incubated overnight in standard culture conditions. The next day, cells were loaded with 200 nM TMRM for 1 h. Before the photoactivation of TMRM, baseline images using a standard rhodamine filter block of Leica microscopes (excitation 515 nm–560 nm, emission-high pass filter 580 nm) were obtained as a sequence of six images, one every 10 s, for a total of one minute. This was followed by constant illumination with same green light for 30 s (photoactivation) followed by a series of 20 images every 10 s, for a total of 3 min. Imaging was conducted using a Leica DMIRB Inverted florescent microscope connected to a R6 Retiga digital camera and controlled by the Micromanager software. Imaging parameters such as exposure time and light intensity (1.4 mW/mm2, 10× objective) were fixed throughout all imaging sessions. ImageJ software was used to process the images.
2.6. Assessment of MMP Recovery after TMRM-PDT
In this and all other series, we used ×5 objectives (unless specifically indicated) to illuminate large areas with numerous cells to achieve a uniform biological outcome across the whole pool of cells in an individual dish. Since two different makes of microscopes were used (Zeiss and Leica) the light power density was slightly different between some datasets (1.4 and 1.06 mW/mm2 for Leica vs. 1.4 mW/mm2 for Zeiss); however, this did not qualitatively affect the outcomes.
To ensure that all cells were evenly illuminated, we used a special plating technique which ensured that they were localized in the center of the well. This was important because in the preliminary experiments, we found that the cells located at the margins of the wells and their walls do not receive sufficient quantities of light and therefore do not react to PDT.
To this end, cells were plated as 3 µL drops, containing an average of ~300–350 cells/drop at the centers of the wells in a 96-well plate. After 45 min, when cells had attached to the bottom, wells were filled with 100 µL of fresh media and incubated overnight. This plating approach was used for all experiments involving TMRM-PDT. The next day, cells were incubated with 300 nM TMRM for 40 min, and TMRM was photoactivated for 40 s using a ×5 objective (1.06 mW/mm2). Media in the wells were replaced and cells were returned into the incubator. Mitochondrial potential was measured 24 h later using TMRM.
2.7. TMRM-PDT
2.7.1. Evaluating the Efficacy of TMRM as a Photosensitizer: Effect of TMRM Photoactivation on Cell Viability
Cells were plated in the centers of the well as described above and loaded with 300 nM TMRM for 45 min. Photoactivation of TMRM was carried out for 40 s (1.06 mW/mm2). Green light was used using standard filter blocks of Leica microscopes with a pass-band of ~520 nm–540 nm. Media were then replaced and the cells were incubated for 72 h. Fixation, nuclear staining, imaging and analysis were carried out as indicated above in this and further experiments in this section.
2.7.2. Concentration-Response Test for PDT with TMRM-PDT
On day 1, cells were plated following the previously described plating protocol, and were then allowed to grow in a cell culture incubator overnight. On day 2, media were removed, and cells were loaded with TMRM (100 nM, 300 nM, 800 nM) for 40 min. The center of each well was then illuminated by green light (~530 nm–580 nm) using a LSM780 ZEISS confocal microscope with a ×5 objective at 1.4 mW/mm2 for 30 s. After the photoactivation, the media were exchanged and cells were incubated for 72 h. After 3 days incubation, the cells were fixed in 4% paraformaldehyde (PFA) for 15 min, washed in PBS three times and stained with 1 μg/mL DAPI for 10 min. Images were taken using a confocal microscope with objective power x5 to include all DAPI positive cells in one image for each well.
2.7.3. Exposure-Dependence of TMRM-PDT
Cells were seeded as described above and loaded with TMRM (100 nM) for 40 min. Photoactivation was carried out for 30 s, 60 s or 90 s at 1.4 mW/mm2. Media were replaced and cells were then incubated for 72 h.
2.7.4. Retention of TMRM in Mitochondria of RA and GBM Cells
Cells were seeded as described above. Cells were loaded with different concentrations of TMRM for 40 min. After 40 min, TMRM was removed and fresh media was added. After 24 h, photoactivation was performed for 30 s at 1.4 mW/mm2. Media were replaced with fresh media and the cells were incubated for 72 h.
2.7.5. Assessment of the Effect of Green Light Alone Using Presto Blue Viability Assay
UP007, UP029 and RA were plated in the centers of the wells in a 96-well plate as described above. The next day, the cells were irradiated with green light without TMRM staining for 60 s or 120 s (1.4 mW/mm2). Note that the strength of this stimulus considerably exceeded all illumination regimes applied in other tests. After 3 days, cell viability was assessed with PrestoBlue assay following the manufacturer protocol.
2.7.6. Incubation with NKH477, Clotrimazole and Photoactivation
Cells were seeded in 96 well-plates using the technique described above. Milder TMRM-PDT conditions were used in these experiments in order to more easily reveal any additive or synergistic effects of the combined treatment. Specifically, 200 nM TMRM was used to load the cells instead of 300 nM. A Leica EC3 florescent microscope was used to illuminate the cells with a ×5 objective lens, light dose of 1.06 mW/mm2 and the duration of illumination of 17 s only. To test whether NKH477 can enhance TMRM-PDT, GBM cells were pre-incubated with 10 µM NKH477 for 24 h before TMRM-PDT was conducted. The PDT outcome was tested on day 3 as previously described. We also tested whether we could potentiate the outcome of PDT with clotrimazole (a glycolysis inhibitor). Immediately after TMRM-PDT, 10 µM of the drug was also added to the wells. Cells were incubated with clotrimazole for three days before evaluating the outcome by the nuclear count as described above.
2.8. Assessment of Caspase Activation after PDT
To confirm that GBM cells affected by PDT undergo apoptosis, we used a genetic reporter of apoptosis CA-GFP (Caspase Activated Green fluorescent protein) as described in [
14]. During apoptosis, caspase is activated via proteolytic cleavage. In CA-GFP, GFP fluorescence is completely quenched by a quenching peptide attached via the four amino acid caspase-7 cleavage motif Asp–Glu–Val–Asp. After the initiation of apoptosis, proteolytic removal of the quenching peptide by caspase-8 and caspase-9 results in restored GFP fluorescence. In order to stably express the reporter in dividing GBM cells, we generated a lentiviral vector where CA-GFP was expressed under control of the EF1α promoter, which is highly active and stable in GBM (own unpublished observation). GBM cells were loaded with TMRM (300 nM × 40 min) and photoactivated for 90 s (1.06 mW/mm
2). The plates were kept for 10 days and the surviving cells were then fixed, stained with DAPI and imaged using a ZOE imager.
2.9. Lentiviral Production
The full protocol was described in our previous study [
15]. Briefly, for lentiviral production, Lenti-X™293 T Cell Line (Clontech, San Francisco, CA, USA) was transfected with plasmids pNHP (7.5 μg), pHEF-VSVG (3.1 μg), pCEP4-tat (0.7 μg) and pTYF-EF1α-CA-GFP (3.9 μg). Cells were then placed in an incubator under standard cell culture conditions. Culture media were collected after approximately 30 and 48 h after transfection and stored at 4 °C. Then, the media were filtrated and centrifuged in 20% sucrose at 74,000×
g for 2 h. The supernatant was aspirated and 25 µL PBS was added. The following day, the lentiviral vector pellet was resuspended, aliquoted and frozen at −80 °C.
2.10. Statistical Analysis
The data were shown as mean ± SEM; the numbers of independent experiments are indicated on the figures and in the text. Statistical analysis was performed using one-way or two-way ANOVA using Prism software version 8.00. Differences were considered statistically significant at p < 0.05.
4. Discussion
We here re-evaluated the potential of PDT to therapeutically target the infiltration of residual GBM disease within the brain parenchyma adjacent to the area of surgical resection, with the aim of reducing the number and viability of surviving tumor cells, and thus, improve patient prognosis. Controversies surround the use of 5ALA as a photosensitizer for PDT. First, the main peak of its excitation is 405 nm–420 nm, but this wavelength essentially does not spread in brain tissue. Furthermore, the wavelengths > 600 nm which were attempted for PDT with 5ALA may penetrate deeper into the tissues, but they are not efficient for 5ALA excitation. Second, 5ALA has a low ROS yield, meaning that it generates a small amount of free radicals upon photoactivation, and this becomes a major issue when long wavelength light is used [
6].
In this study, we demonstrated the application of the MMP-driven dye TMRM as a photosensitizer for PDT targeting GBM cells. TMRM is a member of the rhodamine family and is commonly used to measure MMP in cells [
17]. Changes of MMP directly correlate with changes in TMRM florescence intensity [
18]. TMRM is highly mobile and instantly leaves the mitochondria if not retained by the MMP. It has also been reported to have minimal non-specific (non-mitochondrial) accumulation and interference with mitochondrial respiration compared to other commonly used rhodamine derivatives [
19]. Moreover, TMRM has peak excitation/emission wavelengths of 548 nm/573 nm, respectively. Thus, TMRM can be effectively excited by a light of green/yellow spectrum. In comparison with the 405 nm–420 nm peak for 5ALA, this must increase the efficiency of PDT due to the better tissue penetration of these wavelengths.
We found that TMRM-PDT is effective in compromising the viability/survival of the GBM cells, as shown in
Figure 3. Unfortunately, the effect was also seen on normal RA. However, by carefully tweaking the protocol in terms of TMRM loading concentration and illumination times, we were able to achieve significant cytotoxic selectivity to GBM cells over RA (
Figure 4). Possibly, using lower concentrations of TMRM in vivo might help to achieve a selective suppressant effect on GBM infiltrating cells. The ability of GBM mitochondria to accumulate more TMRM and better retain it matches with the generally known tendency of tumor cells to have hyperpolarized mitochondria [
20,
21]. We believe that greater selectivity can be achieved with the protocol where photoactivation takes place after the cells are allowed to dissipate the TMRM initially loaded into them. In our experiment, this was demonstrated using 48 h delay (
Figure 6). We assume that the selective suppression of the GBM cells was due to the retention of the dye in their mitochondria because of the hyperpolarized MMP. This could and should make the clearance of the dye out of normal cells more efficient than in tumor cells. From a clinical perspective, this protocol would require a preloading of the tissue with TMRM prior to light application which should follow after a delay. One needs to take into account that, unless the molecules are actively retained in brain cells in vivo, they are going to be very quickly washed away into the general circulation. Thus, in the living brain, it might only take 1–2 h for the healthy cells to release TMRM while the GBM cell could still have it concentrated in their mitochondria.
In this work, we did not study the potential impact of PDT on neurons, but this is a particularly difficult task in vitro. Essentially all experiments on cultured rodent neurons employ cells from embryos which have a completely different metabolic profile compared to the mature neurons in the brain in vivo. We do not believe that such cultures would be a suitable model for this type of work. Instead, it may be better to test whether TMRM strongly affects neurons in rodent studies in vivo, as we hope to do in future studies.
We were also able to show that TMRM is not toxic without light illumination at concentrations up to 3.2 µM, which is consistent with previous data [
18]. Further, it requires comparatively low power to elicit specific cytotoxicity which decreases the chances for light-related tissue damage (see [
6] for further discussion).
We attempted to enhance the efficacy of TMRM-PDT by employing a sub-lethal PDT regimen combined with NKH477 or clotrimazole. Clotrimazole is an inhibitor of phosphofructokinase, one of the key enzymes in glycolysis [
22]. It has previously been tested on GBM cells and has resulted in the blockade of the cell cycle and consequently cell death. We reasoned that TMRM-PDT would perturb mitochondrial energy production and the addition of a glycolysis inhibitor would further compromise cellular energy sources and induce cell death. Unfortunately, clotrimazole at the low concentration we used (10 µM) was enough to affect RA. Moreover, no additive effect was detected on GBM cells (
Figure 10). However, a combination with NKH477 has shown some promising results. NKH477 is a water-soluble forskolin hydrochloride derivative that can directly stimulate adenylate cyclase, the generator of cAMP [
23]. cAMP elevation is known to cause detrimental effects on GBM cells [
24,
25,
26]. Moreover, it has been documented that an elevated cAMP level in GBM leads to hyperpolarization of mitochondria [
25] and would theoretically lead to the greater accumulation of TMRM in GBM cells and enhance the PDT effect. Interestingly, NHK477 significantly potentiated the effect of TMRM-PDT only for UP007 and GIN27 GBM cells (
Figure S4). A trend was noted with other cell lines, but differences were not statistically significant (
Figure 10). Heterogenous responses of GBM lines to this approach are not surprising because these tumors are characterized by a non-uniform molecular makeup and biological behavior. A hypothetical explanation for the effect of NKH477 is that it changes gene expression in GBM cells and via yet poorly defined mechanisms leads to the further hyperpolarization of their mitochondria, as illustrated by
Supplementary Figure S5.
5. Conclusions
There is an urgent need for more innovative and less invasive therapeutic modalities to treat GBM. The major source for GBM recurrences is that of the infiltrating GBM cells that are left after surgical resection for which local therapy modalities could be used to efficaciously target residual disease cells.
Its proposed topical application of TMRM may raise some of the usual concerns, such as liver or kidney toxicity, but might be less problematic because the overall dose delivered into the brain will be fairly small, especially because only a periphery of the postoperative cavity needs to be impregnated with it. As a first step, one could start by testing the consequences of injecting TMRM into the brain of experimental animals and then checking for signs of pathology and inflammation.
We envision a treatment protocol for GBM where, following surgery, the walls of the cavity and ~2 cm of the surrounding parenchyma are infiltrated with TMRM, and after a delay to allows healthy brain cells to expel the photosensitizer, light is directly delivered into the brain parenchyma where disease infiltrations are suspected.
Light delivery systems for this type of surgery are already being developed [
27] and our early findings encourage continued enthusiasm in this research area.