Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases
Abstract
:1. Introduction
2. Methods
3. NPs and Their Role in Dental Caries Control
4. NPs and Their Role in Root Canal Treatment
5. NPs and Their Role in Periodontitis and Peri-Implantitis
6. NPs and Their Role in Orthodontics Infection Control
7. NPs and Their Role in Denture Base Material
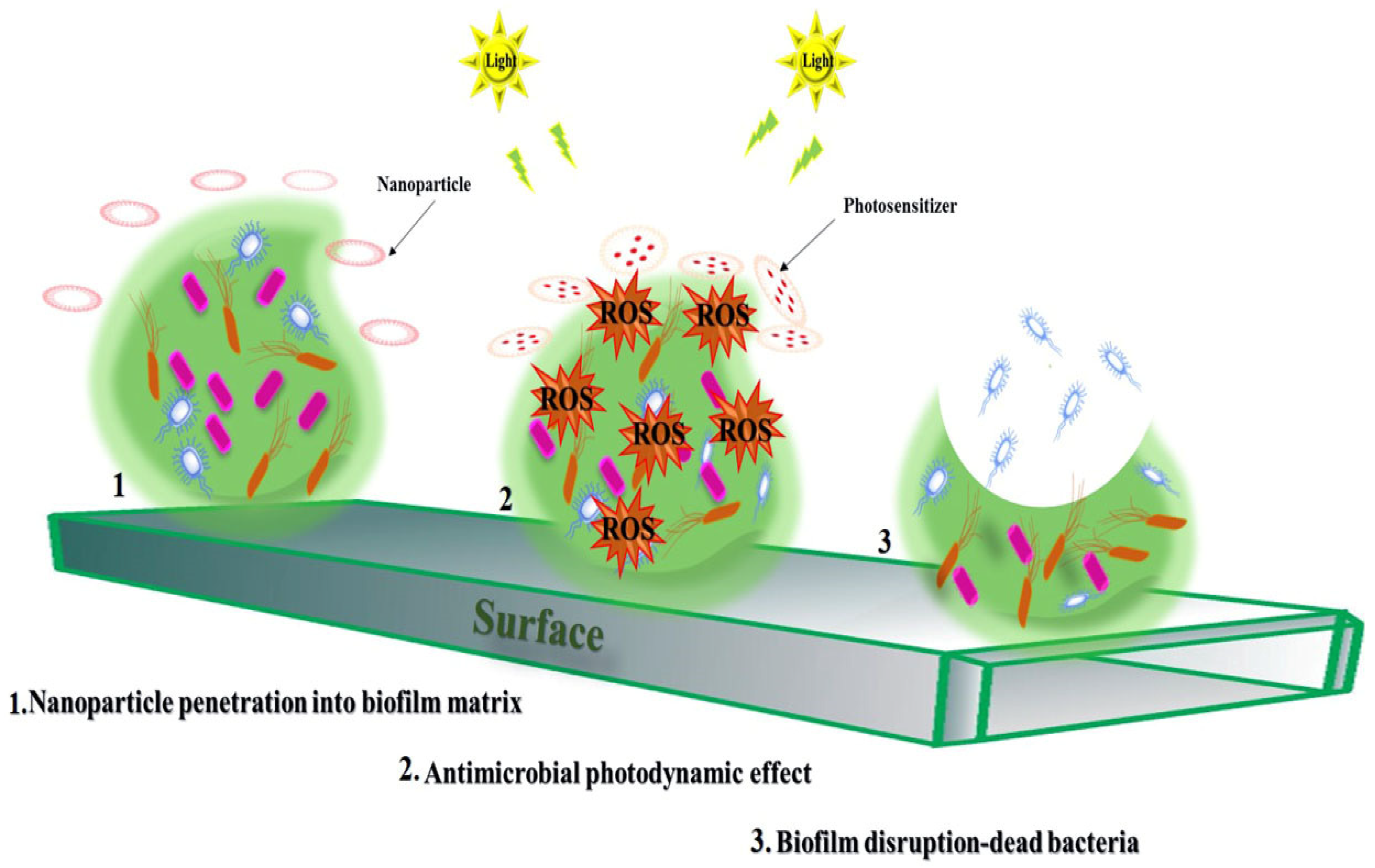
8. Applications of NPs in aPDT
9. Application of NPs in Drug Delivery Systems
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, O. Light therapy: Complementary antibacterial treatment of oral biofilm. Adv. Dent. Res. 2012, 24, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Klein, M.I.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Koo, H. The specific degree-of-polymerization of A-type proanthocyanidin oligomers impacts Streptococcus mutans glucan-mediated adhesion and transcriptome responses within biofilms. Biofouling 2013, 29, 629–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.T.; Baker, H.V.; Burne, R.A. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J. Bacteriol. 2006, 188, 2983–2992. [Google Scholar] [CrossRef] [Green Version]
- Browngardt, C.M.; Wen, Z.T.; Burne, R.A. RegM is required for optimal fructosyltransferase and glucosyltransferase gene expression in Streptococcus mutans. FEMS Microbiol. Lett. 2004, 240, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef] [Green Version]
- Decker, E.-M.; Klein, C.; Schwindt, D.; Von Ohle, C. Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int. J. Oral Sci. 2014, 6, 195–204. [Google Scholar] [CrossRef]
- Benoit, D.S.; Sims, K.R., Jr.; Fraser, D. Nanoparticles for Oral Biofilm Treatments. ACS Nano 2019, 13, 4869–4875. [Google Scholar] [CrossRef]
- Dosselli, R.; Millioni, R.; Puricelli, L.; Tessari, P.; Arrigoni, G.; Franchin, C.; Segallaa, A.; Teardoa, E.; Reddi, E. Molecular targets of antimicrobial photodynamic therapy identified by a proteomic approach. J. Proteom. 2012, 77, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Sahlan, M.; Ardiellaputri, A.P.; Hermasnyah, H.; Wijanarko, A.; Darwita, R.R. Anti oral biofilm mouthwash nanoemulsion containing extract propolis and curcumin. In Proceedings of the AIP Conference Proceedings, Bante, Indonesia, 1–2 November 2018; Volume 2024, p. 020059. [Google Scholar] [CrossRef]
- Sanchez, M.; Toledano-Osorio, M.; Bueno, J.; Figuero, E.; Toledano, M.; Medina-Castillo, A.; Osorio, R.; Herreraa, D.; Sanz, M. Antibacterial effects of polymeric PolymP-n Active nanoparticles. An in vitro biofilm study. Dent. Mater. 2019, 35, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Zou, T.; Lee, A.H.C.; Zhang, C. The Potential Translational Applications of Nanoparticles in Endodontics. Int. J. Nanomed. 2021, 16, 2087–2106. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 1–13. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Soliman, N.; Sol, V.; Ouk, T.-S.; Thomas, C.M.; Gasser, G. Encapsulation of a Ru (II) polypyridyl complex into polylactide nanoparticles for antimicrobial photodynamic therapy. Pharmaceutics 2020, 12, 961. [Google Scholar] [CrossRef]
- Maliszewska, I.; Wanarska, E.; Thompson, A.C.; Samuel, I.D.; Matczyszyn, K. Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy. Molecules 2021, 26, 623. [Google Scholar] [CrossRef]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendicke, F.; Dörfer, C.; Paris, S. Incomplete caries removal: A systematic review and meta-analysis. J. Dent. Res. 2013, 92, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Sionov, R.; Smoum, R.; Mechoulam, R.; Ginsburg, I.; Steinberg, D. Comparative Evaluation of Combinatory Interaction between Endocannabinoid System Compounds and Poly-L-lysine against Streptococcus mutans Growth and Biofilm Formation. Biomed. Res. Int. 2020, 2020, 7258380. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Bozec, L.; Perez, R.A.; Kim, H.-W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 371–394. [Google Scholar] [CrossRef] [Green Version]
- Croft, K.; Kervanto-Seppälä, S.; Stangvaltaite, L.; Kerosuo, E. Management of deep carious lesions and pulps exposed during carious tissue removal in adults: A questionnaire study among dentists in Finland. Clin. Oral Investig. 2019, 23, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, C.; Cohenca, N.; Lauridsen, E.; Flores, M.T.; O’Connell, A.C.; Day, P.F.; Tsilingaridis, G.; Abbott, P.V.; Fouad, A.F.; Hicks, L.; et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 1. Fractures and luxations. Dent. Traumatol. 2020, 36, 314–330. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Bahador, A.; Partoazar, A. Combinatorial therapy of chitosan hydrogel-based zinc oxide nanocomposite attenuates the virulence of Streptococcus mutans. BMC Microbiol. 2021, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Partoazar, A.; Bideskan, F.R.; Partoazar, M.; Talaei, N.; Dallal, M.M.S. Inhibition of Biofilm Formation of Staphylococcus aureus Strains Through ZnO/Zeolite Nanocomposite and Its Cytotoxicity Evaluation. BioNanoScience 2020, 10, 714–720. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; He, L.; Xu, H.H.; Weir, M.D.; Fan, M.; Yu, Z.; Zhang, M.; Zhou, X.; Liang, K.; Li, J. Dentin remineralization via adhesive containing amorphous calcium phosphate nanoparticles in a biofilm-challenged environment. J. Dent. 2019, 89, 103193. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Bermudez, J.; Dávila-Sánchez, A.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Hernández, M.; Astorga, J.; Reis, A.; Loguercio, A.D.; Farago, P.V.; et al. Zinc oxide and copper nanoparticles addition in universal adhesive systems improve interface stability on caries-affected dentin. J. Mech. Behav. Biomed. Mater. 2019, 100, 103366. [Google Scholar] [CrossRef]
- Pereira Prado, V.; Asquino, N.; Apellaniz, D.; Bueno Rossy, L.; Tapia, G.; Bologna Molina, R. Metalloproteinases (MMPs) of the extracellular matrix in dentistry. Odontoestomatologia 2016, 18, 19–28. [Google Scholar]
- Cao, W.; Zhang, Y.; Wang, X.; Chen, Y.; Li, Q.; Xing, X.; Xiao, Y.; Peng, X.; Ye, Z. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag+ ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J. Mater. Sci. Mater. Med. 2017, 28, 103. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Mousa, H.A.; Afifi, N.N. Preparation of PLGA-chitosan based nanocarriers for enhancing antibacterial effect of ciprofloxacin in root canal infection. Drug Deliv. 2020, 27, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic effect of newly introduced root canal medicaments; ozonated olive oil and chitosan nanoparticles, against persistent endodontic pathogens. Front Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef] [Green Version]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in endodontics—current status and future directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef]
- Parolia, A.; Kumar, H.; Ramamurthy, S.; Madheswaran, T.; Davamani, F.; Pichika, M.R.; Mak, K.K.; Fawzy, A.S.; Daood, U.; Pau, A.; et al. Effect of Propolis Nanoparticles against Enterococcus faecalis Biofilm in the Root Canal. Molecules 2021, 26, 715. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Shi, Z.; Shrestha, A.; Neoh, K.G. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J. Endod. 2008, 34, 1515–1520. [Google Scholar] [CrossRef]
- Baras, B.H.; Wang, S.; Melo, M.A.S.; Tay, F.; Fouad, A.F.; Arola, D.D.; Weir, M.D.; Xu, H.H. Novel bioactive root canal sealer with antibiofilm and remineralization properties. J. Dent. 2019, 83, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Stacchi, C.; Berton, F.; Perinetti, G.; Frassetto, A.; Lombardi, T.; Khoury, A.; Andolsek, F.; Di Lenarda, R. Risk factors for peri-implantitis: Effect of history of periodontal disease and smoking habits. A systematic review and meta-analysis. J. Oral Maxillofac. Res. 2016, 7, e3. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis–a review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Anchun, M.; Zhu, Z.; Quan, Y. Antibacterial titanium plate deposited by silver nanoparticles exhibits cell compatibility. Int. J. Nanomed. 2010, 5, 337–342. [Google Scholar] [PubMed] [Green Version]
- Chi, M.; Qi, M.; Wang, P.; Weir, M.D.; Melo, M.A.; Sun, X.; Dong, B.; Li, C.; Wu, J.; Wang, L.; et al. Novel bioactive and therapeutic dental polymeric materials to inhibit periodontal pathogens and biofilms. Int. J. Mol. Sci. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Lastra, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Gene expression of Porphyromonas gingivalis ATCC 33277 when growing in an in vitro multispecies biofilm. PLoS ONE 2019, 14, e0221234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamanaka, T.; Furukawa, T.; Matsumoto-Mashimo, C.; Yamane, K.; Sugimori, C.; Nambu, T.; Mori, N.; Nishikawa, H.; Walker, C.B.; Leung, K.-P.; et al. Gene expression profile and pathogenicity of biofilm-forming Prevotella intermedia strain 17. BMC Microbiol. 2009, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syahiran, S.; Taib, W.R.W.; Jaffar, N. Aggregatibacter actinomycetemcomitans: The virulence factors and relation to persistence biofilm formation. Biomedicine 2020, 40, 429–435. [Google Scholar] [CrossRef]
- Buchmann, R.; Müller, R.F.; Heinecke, A.; Lange, D.E. Actinobacillus actinomycetemcomitans in destructive periodontal disease. Three-year follow-up results. J. Periodontol. 2000, 71, 444–453. [Google Scholar] [CrossRef]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef]
- Liang, G.; Shi, H.; Qi, Y.; Li, J.; Jing, A.; Liu, Q.; Feng, W.; Li, G.; Gao, S. Specific Anti-biofilm Activity of Carbon Quantum Dots by Destroying, P. gingivalis Biofilm Related Genes. Int. J. Nanomed. 2020, 15, 5473–5489. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.; Tredwin, C.; Handy, R. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Aguayo, S.; Donos, N.; Spratt, D.; Bozec, L. Probing the nanoadhesion of Streptococcus sanguinis to titanium implant surfaces by atomic force microscopy. Int. J. Nanomed. 2016, 11, 1443–1450. [Google Scholar]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.; Xu, H.H.K. Novel dental cement to combat biofilms and reduce acids for orthodontic applications to avoid enamel demineralization. Materials 2016, 9, 413. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.-J.; Xing, D.; Wang, L.; Zhou, H.; Weir, M.D.; Bai, Y.-X.; Xu, H.H. Novel rechargeable calcium phosphate nanoparticle-containing orthodontic cement. Int. J. Oral Sci. 2017, 9, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Poosti, M.; Ramazanzadeh, B.; Zebarjad, M.; Javadzadeh, P.; Naderinasab, M.; Shakeri, M.T. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur. J. Orthod. 2013, 35, 676–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA denture base materials containing titanium dioxide nanoparticles: A literature review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef] [PubMed]
- Anehosur, G.V.; Kulkarni, R.; Naik, M.; Nadiger, R. Synthesis and determination of antimicrobial activity of visible light activated TiO2 nanoparticles with polymethyl methacrylate denture base resin against Staphylococcus aureus. J. Gerontol. Geriat. Res. 2012, 1, 1–8. [Google Scholar]
- Bacali, C.; Carpa, R.; Buduru, S.; Moldovan, M.L.; Baldea, I.; Constantin, A.; Moldovan, M.; Prodan, D.; Dascalu, M.L.; Lucaciu, O.; et al. Association of Graphene Silver Polymethyl Methacrylate (PMMA) with Photodynamic Therapy for Inactivation of Halitosis Responsible Bacteria in Denture Wearers. Nanomaterials 2021, 11, 1643. [Google Scholar] [CrossRef]
- Bhatt, L.; Chen, L.; Guo, J.; Klie, R.F.; Shi, J.; Pesavento, R.P. Hydrolyzed Ce (IV) salts limit sucrose-dependent biofilm formation by Streptococcus mutans. J. Inorg. Biochem. 2020, 206, 110997. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 2019, 5, e02568. [Google Scholar] [CrossRef] [Green Version]
- Shvero, D.K.; Davidi, M.P.; Weiss, E.I.; Srerer, N.; Beyth, N. Antibacterial effect of polyethyleneimine nanoparticles incorporated in provisional cements against Streptococcus mutans. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 367–371. [Google Scholar] [CrossRef]
- Chen, C.; Weir, M.D.; Cheng, L.; Lin, N.J.; Lin-Gibson, S.; Chow, L.C.; Zhou, X.; Xu, H.H. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Aati, S.; Ngo, H.; Fawzy, A. pH-dependent delivery of chlorhexidine from PGA grafted mesoporous silica nanoparticles at resin-dentin interface. J. Nanobiotechnol. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Partoazar, A.; Talaei, N.; Bahador, A.; Pourhajibagher, M.; Dehpour, S.; Sadati, M.; Bakhtiarian, A. Antibiofilm activity of natural zeolite supported NanoZnO: Inhibition of Esp gene expression of Enterococcus faecalis. Nanomedicine 2019, 14, 675–687. [Google Scholar] [CrossRef]
- Liu, T.; Aman, A.; Ainiwaer, M.; Ding, L.; Zhang, F.; Hu, Q.; Song, Y.; Ni, Y.; Tang, X. Evaluation of the anti-biofilm effect of poloxamer-based thermoreversible gel of silver nanoparticles as a potential medication for root canal therapy. Sci. Rep. 2021, 11, 1–16. [Google Scholar]
- Azad, A.; Rostamifar, S.; Modaresi, F.; Bazrafkan, A.; Rezaie, Z. Assessment of the Antibacterial Effects of Bismuth Nanoparticles against Enterococcus faecalis. Biomed Res. Int. 2020, 2020, 5465439. [Google Scholar] [CrossRef]
- Louwakul, P.; Saelo, A.; Khemaleelakul, S. Efficacy of calcium oxide and calcium hydroxide nanoparticles on the elimination of Enterococcus faecalis in human root dentin. Clin. Oral Investig. 2017, 21, 865–871. [Google Scholar] [CrossRef]
- Monzavi, A.; Eshraghi, S.; Hashemian, R.; Momen-Heravi, F. In vitro and ex vivo antimicrobial efficacy of nano-MgO in the elimination of endodontic pathogens. Clin. Oral Investig. 2015, 19, 349–356. [Google Scholar] [CrossRef]
- Del Carpio-Perochena, A.; Bramante, C.M.; Duarte, M.A.H.; de Moura, M.R.; Aouada, F.A.; Kishen, A. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor. Dent. Endod. 2015, 40, 195–201. [Google Scholar] [CrossRef]
- Leng, D.; Li, Y.; Zhu, J.; Liang, R.; Zhang, C.; Zhou, Y.; Li, M.; Wang, Y.; Rong, D.; Wu, D.; et al. The Antibiofilm activity and mechanism of nanosilver-and nanozinc-incorporated mesoporous calcium-silicate nanoparticles. Int. J. Nanomed. 2020, 15, 3921–3936. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Kim, D.; Liu, Y.; Karabucak, B.; Koo, H. Novel endodontic disinfection approach using catalytic nanoparticles. J. Endod. 2018, 44, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Siddalingam, R.P.; Yuen, K.H.; Ambu, S.P.; Davamani, F. Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilms. PLoS ONE 2017, 12, e0174888. [Google Scholar]
- Miglani, S.; Tani-Ishii, N. Biosynthesized selenium nanoparticles: Characterization, antimicrobial, and antibiofilm activity against Enterococcus faecalis. PeerJ 2021, 9, e11653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhan, L.; Zhang, X.; Wu, R.; Liao, L.; Wei, J. Silver Nanoparticles Coated Poly (L-Lactide) Electrospun Membrane for Implant Associated Infections Prevention. Front Pharmacol. 2020, 11, 431. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Lee, S.J.; Moon, J.-H.; Kim, J.H.; Heo, D.N.; Bang, J.B.; Lim, H.-N.; Kwon, I.K. Preparation of antibacterial chitosan membranes containing silver nanoparticles for dental barrier membrane applications. J. Ind. Eng. Chem. 2018, 66, 196–202. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 146–153. [Google Scholar] [CrossRef]
- Vega-Jiménez, A.; Almaguer-Flores, A.; Flores-Castañeda, M.; Camps, E.; Uribe-Ramírez, M.; Aztatzi-Aguilar, O.; De Vizcaya-Ruiz, A. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology 2017, 28, 435101. [Google Scholar] [CrossRef] [Green Version]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ying, D.; Qi, M.; Li, X.; Fu, L.; Sun, X.; Wang, L.; Zhou, Y. Anti-biofilm property of bioactive upconversion nanocomposites containing chlorin e6 against periodontal pathogens. Molecules 2019, 24, 2692. [Google Scholar] [CrossRef] [Green Version]
- Esteban Florez, F.L.; Mendonca de Oliveira, M.R.; de Oliveira Junior, O.B.; Hiers, R.D.; Khajotia, S.S.; Pretel, H. Bioluminescence analysis of antibacterial photodynamic therapy using methylene blue mediated by low-intensity level laser against cariogenic biofilms. Photomed. Laser Surg. 2018, 36, 258–265. [Google Scholar] [CrossRef]
- De Carvalho Goulart, R.; Thedei, G., Jr.; Souza, S.L.; Tedesco, A.C.; Ciancaglini, P. Comparative study of methylene blue and erythrosine dyes employed in photodynamic therapy for inactivation of planktonic and biofilm-cultivated Aggregatibacter actinomycetemcomitans. Photomed. Laser Surg. 2010, 28 (Suppl. S1), S85–S90. [Google Scholar] [CrossRef] [PubMed]
- Street, C.N.; Pedigo, L.A.; Loebel, N.G. Energy dose parameters affect antimicrobial photodynamic therapy–mediated eradication of periopathogenic biofilm and planktonic cultures. Photomed. Laser Surg. 2010, 28 (Suppl S1), S61–S66. [Google Scholar] [CrossRef]
- López-Jiménez, L.; Fusté, E.; Martínez-Garriga, B.; Arnabat-Domínguez, J.; Vinuesa, T.; Viñas, M. Effects of photodynamic therapy on Enterococcus faecalis biofilms. Lasers Med. Sci. 2015, 30, 1519–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, A.L.P.; Di Filippo, L.D.; Besegato, J.F.; de Annunzio, S.R.; de Camargo, B.A.F.; de Melo, P.B.G.; Rastelli, A.N.d.S.; Fontana, C.R.; Chorilli, M. Current applications of drug delivery nanosystems associated with antimicrobial photodynamic therapy for oral infections. Int. J. Pharm. 2020, 592, 120078. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Bahador, A. Propolis nanoparticle enhances the potency of antimicrobial photodynamic therapy against Streptococcus mutans in a synergistic manner. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Klepac-Ceraj, V.; Patel, N.; Song, X.; Holewa, C.; Patel, C.; Kent, R.; Amiji, M.M.; Soukos, N.S. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg. Med. 2011, 43, 600–606. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, L.M.; Calixto, G.M.F.; Chorilli, M.; Giusti, J.S.M.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric nanoparticle-based photodynamic therapy for chronic periodontitis in vivo. Int. J. Mol. Sci. 2016, 17, 769. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hayashi, J.I.; Fujimura, T.; Iwamura, Y.; Yamamoto, G.; Nishida, E.; Ohno, T.; Okada, K.; Yamamoto, H.; Kikuchi, T.; et al. New irradiation method with indocyanine green-loaded nanospheres for inactivating periodontal pathogens. Int. J. Mol. Sci. 2017, 18, 154. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Lynch, C.D.; Sun, X.; Li, X.; Qi, M.; Ma, C.; Li, C.; Dong, B.; Zhou, Y.; et al. Nanoparticles having amphiphilic silane containing Chlorin e6 with strong anti-biofilm activity against periodontitis-related pathogens. J. Dent. 2019, 81, 70–84. [Google Scholar] [CrossRef]
- Okamoto, I.; Miyaji, H.; Miyata, S.; Shitomi, K.; Sugaya, T.; Ushijima, N.; Akasaka, T.; Enya, S.; Saita, S.; Kawasaki, H. Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates. ACS Omega 2021, 6, 9279–9290. [Google Scholar] [CrossRef]
- Misba, L.; Kulshrestha, S.; Khan, A.U. Antibiofilm action of a toluidine blue O-silver nanoparticle conjugate on Streptococcus mutans: A mechanism of type I photodynamic therapy. Biofouling 2016, 32, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.-C.; Hsieh, C.-M.; Chen, C.-P.; Tsai, T.; Chen, C.-T. Assessment of photodynamic inactivation against periodontal bacteria mediated by a chitosan hydrogel in a 3D gingival model. Int. J. Mol. Sci. 2016, 17, 1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossmann, A.; Kranz, S.; Andre, G.; Völpel, A.; Albrecht, V.; Fahr, A.; Sigusch, B.W. Photodynamic killing of Enterococcus faecalis in dentinal tubules using mTHPC incorporated in liposomes and invasomes. Clin. Oral Investig. 2015, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, E.; Barras, A.; Liu, J.; Fraire, J.C.; Lajunen, T.; Xiong, R.; Forier, K.; Li, C.; Urtti, A.; Boukherroub, R.; et al. Exploring light-sensitive nanocarriers for simultaneous triggered antibiotic release and disruption of biofilms upon generation of laser-induced vapor nanobubbles. Pharmaceutics 2019, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging applications of drug delivery systems in oral infectious diseases prevention and treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashi, T.S.J.; Eskandarion, S.; Esfandyari-Manesh, M.; Marashi, S.M.A.; Samadi, N.; Fatemi, S.M.; Atyabi, F.; Eshraghi, S.; Dinarvand, R. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int. J. Nanomed. 2012, 7, 221–234. [Google Scholar]
- Sun, L.; Xu, J.; Sun, Z.; Zheng, F.; Liu, C.; Wang, C.; Hu, X.; Xia, L.; Liu, Z.; Xia, R. Decreased Porphyromonas gingivalis adhesion and improved biocompatibility on tetracycline-loaded TiO2 nanotubes: An in vitro study. Int. J. Nanomed. 2018, 13, 6769–6777. [Google Scholar] [CrossRef] [Green Version]
- Hegge, A.B.; Bruzell, E.; Kristensen, S.; Tønnesen, H. Photoinactivation of Staphylococcus epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin–effect of selected nanocarrier: Studies on curcumin and curcuminoides XLVII. Eur. J. Pharm. Sci. 2012, 47, 65–74. [Google Scholar] [CrossRef]
- Gholibegloo, E.; Karbasi, A.; Pourhajibagher, M.; Chiniforush, N.; Ramazani, A.; Akbari, T.; Bahador, A.; Khoobi, M. Carnosine-graphene oxide conjugates decorated with hydroxyapatite as promising nanocarrier for ICG loading with enhanced antibacterial effects in photodynamic therapy against Streptococcus mutans. J. Photochem. Photobiol. B 2018, 181, 14–22. [Google Scholar] [CrossRef]
- Di Giulio, M.; Di Lodovico, S.; Fontana, A.; Traini, T.; Di Campli, E.; Pilato, S.; D’Ercole, S.; Cellini, L. Graphene Oxide affects Staphylococcus aureus and Pseudomonas aeruginosa dual species biofilm in Lubbock Chronic Wound Biofilm model. Sci Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci Rep. 2020, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kiarostami, K.; Mahmoudi Khatir, N.; Rezania, S.; Muhamad, I.I. Green synthesis of Mg0. 99 Zn0. 01O nanoparticles for the fabrication of κ-Carrageenan/NaCMC hydrogel in order to deliver catechin. Polymers 2020, 12, 861. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]

| Nanoparticles | Size (nm) | Microorganism | Nanoparticle Therapeutic Effect | Ref. |
|---|---|---|---|---|
| Cerium oxide | - | S. mutans | - Reduces adherent bacteria | [60] |
| Magnesium oxide | ~20 | S. mutans S. sobrinus | - Antibacterial and anti-biofilm activity | [61] |
| Polyethyleneimine | - | S. mutans | - Prevents caries and inflammation | [62] |
| NACP | ~116 | Total streptococci | - Increases the Ca and P ion release at cariogenic pH - Inhibits biofilms and remineralizes tooth lesions - Reduces metabolic activity and lactic acid of biofilm | [63] |
| ZnO and silver | ~50 and 20 | S. mutans Lactobacillus | - Inhibits bacterial growth | [64] |
| Graphene | - | S. mutans | - Inhibits S. mutans adhesion and growth - Anti-biofilm effects - Application as filler in dental adhesives | [65] |
| MSN-PLGA | ~107 | S. mutans | - Anti-biofilm effects - Application as adhesive and restorative dentistry | [66] |
| ZnO/Z | ~30 | E. faecalis | - Anti-biofilm effects - Decreases esp gene expression | [67] |
| AgNPs-PL | ~21.62 | E. faecalis | - Increases their permeability - Destabilizes bacterial membranes - Anti-biofilm activity | [68] |
| Bismuth NPs | ~40 | E. faecalis | - Antibacterial activity | [69] |
| CO and CH | - | E. faecalis | - Antibacterial activity | [70] |
| MgO | ~70–150 | E. faecalis | - Antibacterial activity | [71] |
| Chitosan | ~85–221 | E. faecalis | - Anti-biofilm activity - Inhibition of bacterial recolonization | [72] |
| Ag/Zn-MCSNs | ~200–250 | E. faecalis | - Anti-biofilm activity - Destroys cell membranes | [73] |
| Iron oxide | - | E. faecalis | - Improves catalytic activity - Enhances antibacterial activity on root canal surfaces | [74] |
| Chitosan-propolis | ~247–512 | E. faecalis | - Disrupts the biofilm structure - Alters the expression of biofilm-associated genes | [75] |
| Selenium | ~40–150 | E. faecalis | - Anti-biofilm activity | [76] |
| PLLA@Ag | - | S. aureus | - Antibacterial activity - Good biocompatibility - Implant-associated infections prevention | [77] |
| AgCSP | ~300 | P. gingivalis | - Antibacterial activity - Good biocompatibility - Tissue regeneration | [78] |
| AgNP/NSC | - | A. actinomycetemcomitans | - Kills the adherent bacteria - Anti-biofilm activity - Control of peri-implantitis | [79] |
| Bismuth subsalicylate | ~4–22 | P. gingivalis A. actinomycetemcomitans | - Antibacterial activity - Application in dental materials | [80] |
| Nanoparticles | Size (nm) | Photosensitizer | Light Source | Microorganism | Therapeutic Effects | Ref. |
|---|---|---|---|---|---|---|
| Nano-c | - | ICG | 810 nm light at an energy density of 4–24 J/cm2 | P. gingivalis | - Bactericidal effects | [93] |
| Chitosan | ~60 | Rose bengal | 260 nm light at an energy density of 4–60 J/cm2 | S. oralis, P. intermedia, A. naeslundii | - Increased affinity to bacterial cell membrane - Anti-biofilm effects against multispecies bacterial biofilms | [94] |
| Fe3O4 | ~122.4 | Chlorin e6 | 630 nm light at an energy density of 100 J/cm2 | S. sanguinis F. nucleatum P. gingivalis | - Anti-biofilm activity - Inhibits the occurrence and progression of periodontitis | [95] |
| Lys-Au | - | Rose bengal | LED | S. mutans | - Bacterial inactivation - Anti-biofilm activity | [96] |
| Silver | ~18 | TBO | 630 nm light at an energy density of 100 J/cm2 | S. mutans | - Anti-biofilm activity - Anti metabolic activity | [97] |
| HPMC | - | TBO | 630 nm light at an energy density of 32.4 J/cm2 | S. aureus A. actinomycetemcomitans P. gingivalis | - Anti-biofilm activity | [98] |
| UCNPs | ~25 | Chlorin e6 | 980 nm light at an energy density of 750 J/cm2 | P. gingivalis P. intermedia F.nucleatum | - Anti-biofilm effects | [83] |
| Liposomes | ~100–150 | tetra-m-hydroxyphenyl chlorin | 652 nm light at an energy density of 100 J/cm2 | E. faecalis | - Antibacterial activity | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrasiabi, S.; Chiniforush, N.; Barikani, H.R.; Partoazar, A.; Goudarzi, R. Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases. Biomedicines 2021, 9, 1435. https://doi.org/10.3390/biomedicines9101435
Afrasiabi S, Chiniforush N, Barikani HR, Partoazar A, Goudarzi R. Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases. Biomedicines. 2021; 9(10):1435. https://doi.org/10.3390/biomedicines9101435
Chicago/Turabian StyleAfrasiabi, Shima, Nasim Chiniforush, Hamid Reza Barikani, Alireza Partoazar, and Ramin Goudarzi. 2021. "Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases" Biomedicines 9, no. 10: 1435. https://doi.org/10.3390/biomedicines9101435
APA StyleAfrasiabi, S., Chiniforush, N., Barikani, H. R., Partoazar, A., & Goudarzi, R. (2021). Nanostructures as Targeted Therapeutics for Combating Oral Bacterial Diseases. Biomedicines, 9(10), 1435. https://doi.org/10.3390/biomedicines9101435






