Corneal Nerve Abnormalities in Painful Dry Eye Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
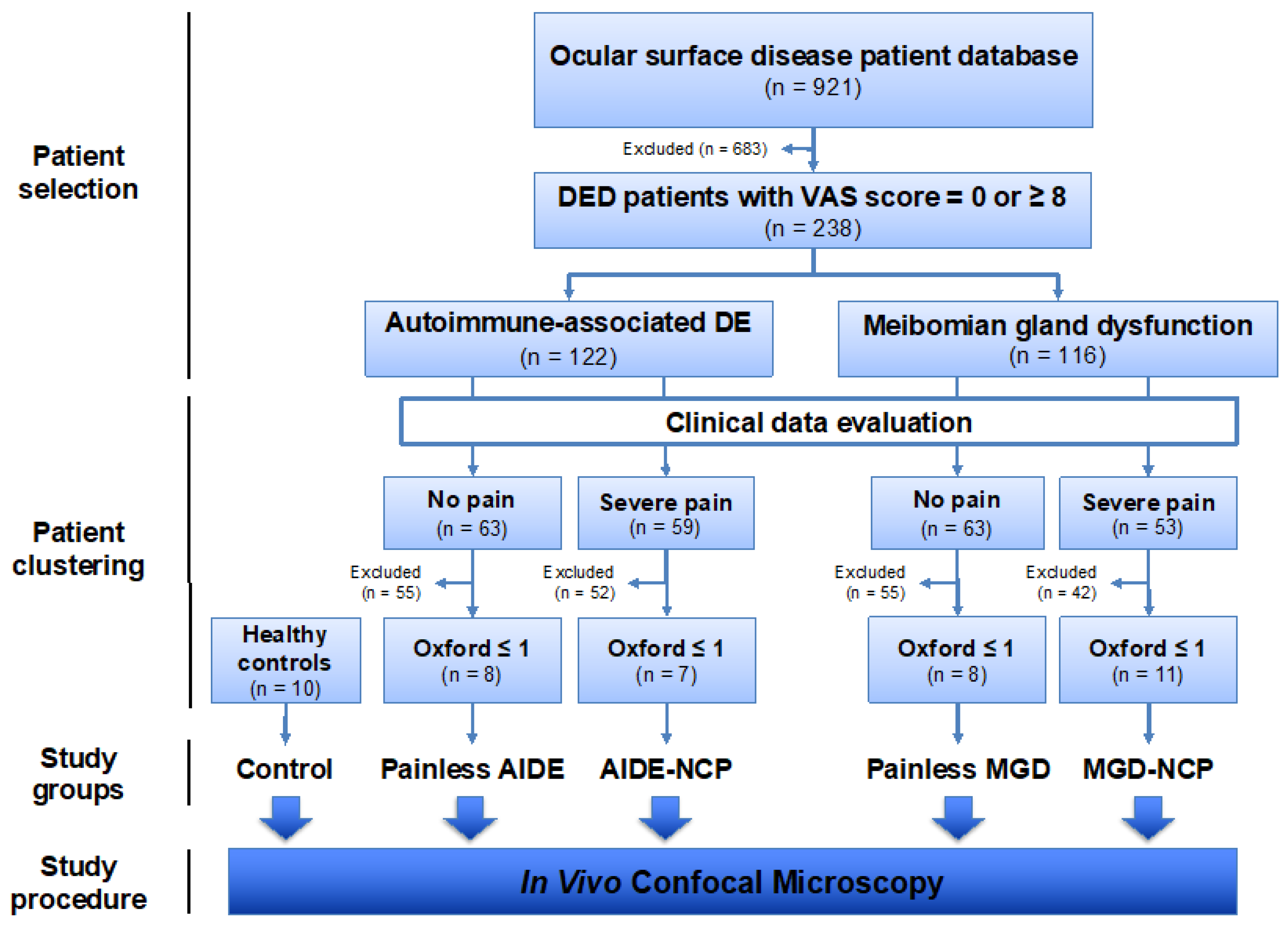
2.2. Patient Cohorts
2.2.1. Patient Selection for Clinical Features Study
2.2.2. Patient Clustering for Ultrastructural IVCM Study of the Cornea
Dry-Eye Disease-Related Neuropathic Corneal Pain Diagnostic Criteria
Painless Dry-Eye Disease Diagnostic Criteria
Study Conditions for IVCM Study
Study Controls
2.3. Clinical Data Evaluation
2.3.1. General Data
2.3.2. Patient History
2.3.3. Ocular Examination
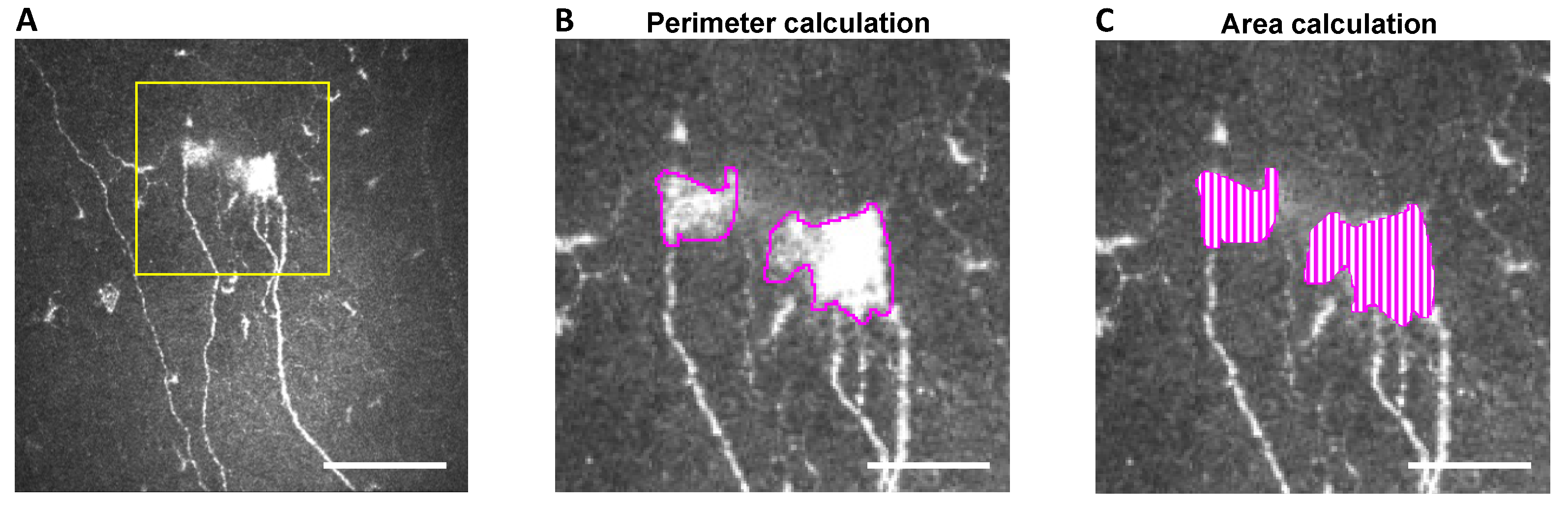
2.4. In Vivo Confocal Microscopy: Image Selection and Analysis
Image Selection and Analysis
2.5. Statistical Analyses
3. Results
3.1. Clinical Variable Comparisons between Painful and Painless DED Patients
3.2. Higher Symptoms and Signs in Painful DED Patients Compared with Their Respective Painless Counterparts
3.3. Higher Symptoms and Signs in Painful AIDE Patients Compared with Painful MGD
3.4. Higher Symptoms and Signs in Painless AIDE Compared with Painless MGD
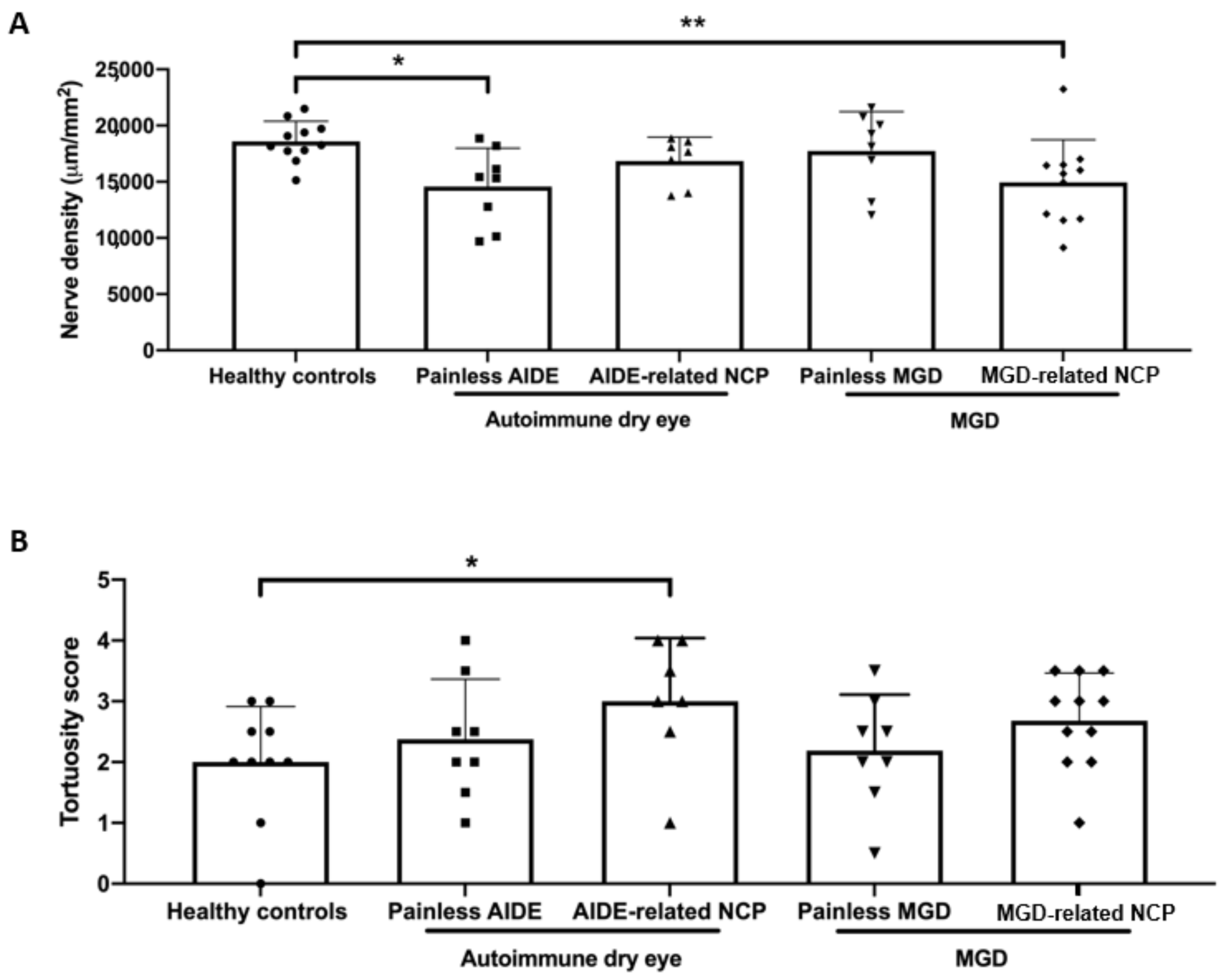
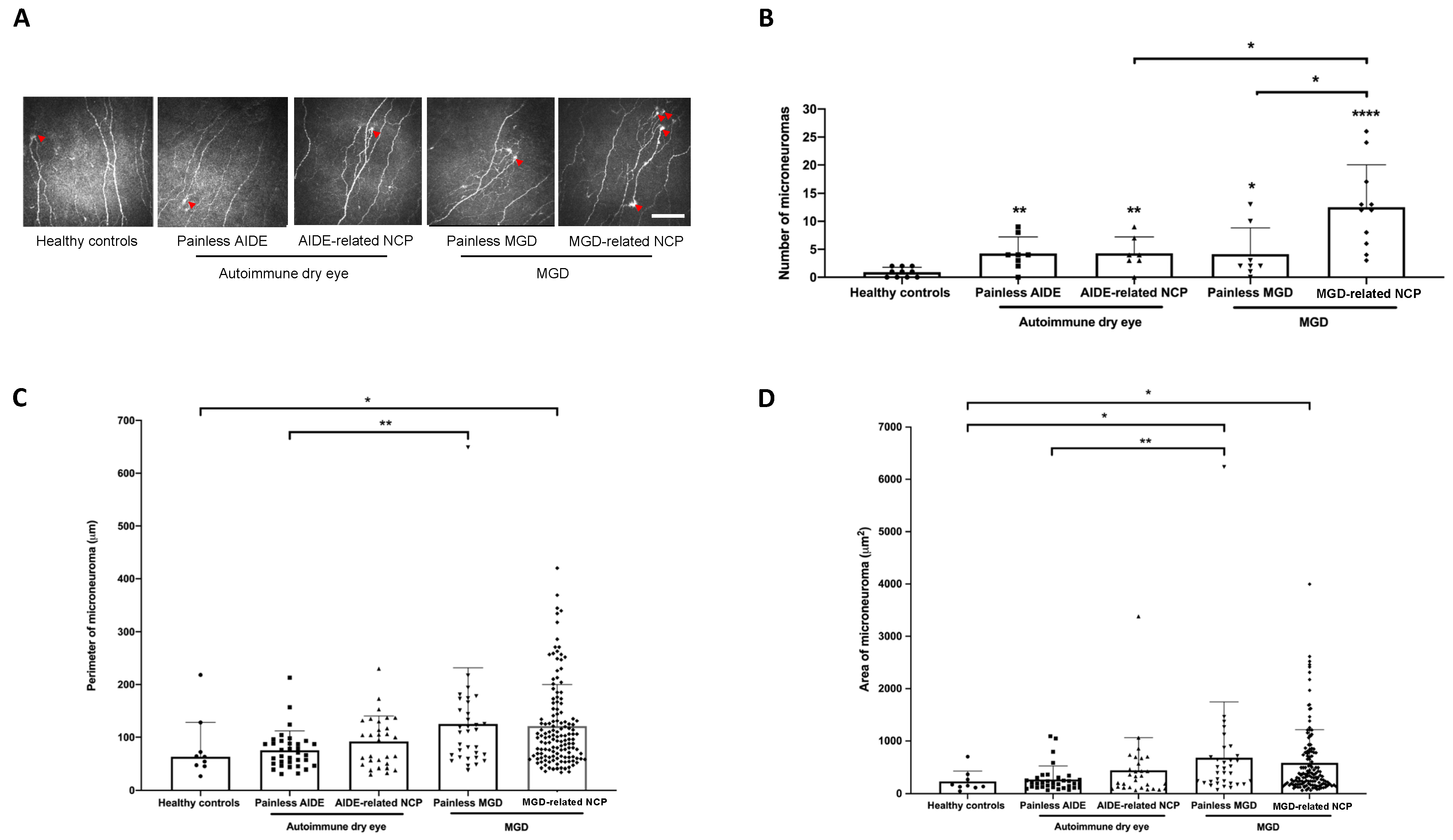
3.5. IVCM Image Analysis
3.6. DED-Related NCP Patients Have Significant IVCM-Identified Corneal Nerve Alterations Compared with Healthy Controls
3.7. MGD-Related NCP Patients Had Higher Microneuromas Compared with Painless MGD and AIDE-Related NCP Patients
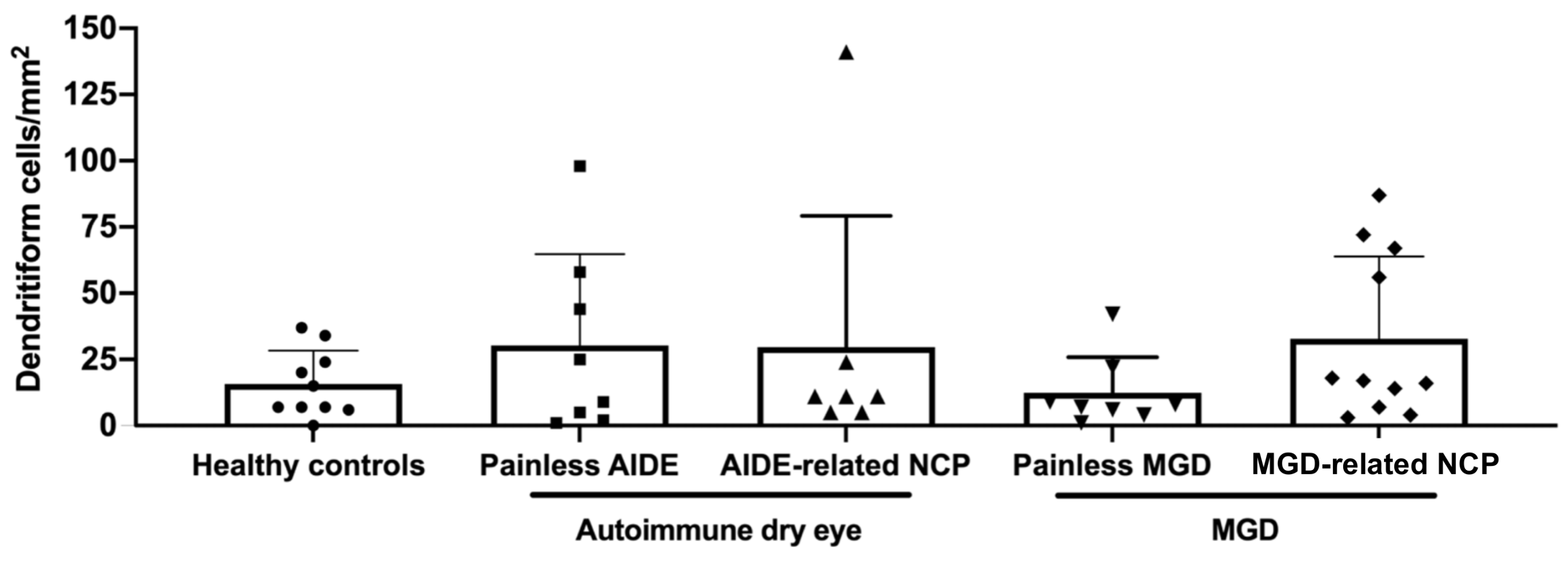
3.8. Higher Microneuroma Found in Painless DED Patients Compared with Healthy Controls
3.9. Structural Differences in Microneuromas between Painless MGD Patients and AIDE Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic Corneal Pain. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef]
- Galor, A.; Levitt, R.C.; Felix, E.; Martin, E.R.; Sarantopoulos, C. Neuropathic ocular pain: An important yet underevaluated feature of dry eye. Eye 2015, 29, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Moein, H.-R.; Lee, C.; Rodriguez, A.; Felix, E.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Fakih, D.; Zhao, Z.; Nicolle, P.; Reboussin, E.; Joubert, F.; Luzu, J.; Labbé, A.; Rostène, W.; Baudouin, C.; Parsadaniantz, S.M.; et al. Chronic dry eye induced corneal hypersensitivity, neuroinflammatory responses, and synaptic plasticity in the mouse trigeminal brainstem. J. Neuroinflamm. 2019, 16, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Moreno, A.; Baudouin, C.; Parsadaniantz, S.M.; Goazigo, A.R.-L. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef]
- Mcmonnies, C.W. The potential role of neuropathic mechanisms in dry eye syndromes. J. Optom. 2017, 10, 5–13. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Van Setten, G.; Rolando, M.; Irkeç, M.; Del Castillo, J.B.; Geerling, G.; Labetoulle, M.; Bonini, S.; ODISSEY European Consensus Group Members. Diagnosing the severity of dry eye: A clear and practical algorithm. Br. J. Ophthalmol. 2014, 98, 1168–1176. [Google Scholar] [CrossRef]
- Rosenthal, P.; Baran, I.; Jacobs, D.S. Corneal Pain without Stain: Is it Real? Ocul. Surf. 2009, 7, 28–40. [Google Scholar] [CrossRef]
- Levitt, E.A.; Galor, A.; Chowdhury, A.R.; Felix, E.; Sarantopoulos, C.D.; Zhuang, G.Y.; Patin, D.; Maixner, W.; Smith, S.B.; Martin, E.R.; et al. Evidence that dry eye represents a chronic overlapping pain condition. Mol. Pain 2017, 13, 1744806917729306. [Google Scholar] [CrossRef]
- Goyal, S.; Hamrah, P. Understanding Neuropathic Corneal Pain—Gaps and Current Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 59–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.-J.; Kim, J.; Yoon, K.C. Treatment Response to Gabapentin in Neuropathic Ocular Pain Associated with Dry Eye. J. Clin. Med. 2020, 9, 3765. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Feuer, W.; Lee, D.J.; Florez, H.; Faler, A.L.; Zann, K.L.; Perez, V.L. Depression, Post-traumatic Stress Disorder, and Dry Eye Syndrome: A Study Utilizing the National United States Veterans Affairs Administrative Database. Am. J. Ophthalmol. 2012, 154, 340–346.e2. [Google Scholar] [CrossRef] [PubMed]
- Qazi, Y.; Hurwitz, S.; Khan, S.; Jurkunas, U.V.; Dana, R.; Hamrah, P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology 2016, 123, 1458–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, A.M.; Feuer, W.; Felix, E.; Levitt, R.C.; McClellan, A.L.; Sarantopoulos, K.D.; Galor, A. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: Incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br. J. Ophthalmol. 2017, 101, 1238–1243. [Google Scholar] [CrossRef]
- Bayraktutar, B.N.; Ozmen, M.C.; Muzaaya, N.; Dieckmann, G.; Koseoglu, N.D.; Müller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Hamrah, P. Comparison of clinical characteristics of post-refractive surgery-related and post-herpetic neuropathic corneal pain. Ocul. Surf. 2020, 18, 641–650. [Google Scholar] [CrossRef]
- Cruzat, A.; Qazi, Y.; Hamrah, P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. Ocul. Surf. 2017, 15, 15–47. [Google Scholar] [CrossRef] [Green Version]
- Labbé, A.; Liang, Q.; Wang, Z.; Zhang, Y.; Xu, L.; Baudouin, C.; Sun, X. Corneal Nerve Structure and Function in Patients With Non-Sjögren Dry Eye: Clinical Correlations. Investig. Opthalmol. Vis. Sci. 2013, 54, 5144–5150. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Randon, M.; Michee, S.; Tahiri, R.; Labbe, A.; Baudouin, C. In vivo confocal microscopy evaluation of ocular and cutaneous alterations in patients with rosacea. Br. J. Ophthalmol. 2016, 101, 268–274. [Google Scholar] [CrossRef]
- Ross, A.R.; Al-Aqaba, M.A.; Almaazmi, A.; Messina, M.; Nubile, M.; Mastropasqua, L.; Dua, H.S.; Said, D.G. Clinical and in vivo confocal microscopic features of neuropathic corneal pain. Br. J. Ophthalmol. 2019, 104, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef] [PubMed]
- Goazigo, A.R.-L.; Guerrero-Moreno, A.; Fakih, D.; Parsadaniantz, S.M. How does chronic dry eye shape peripheral and central nociceptive systems? Neural Regen. Res. 2021, 16, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Hwang, J.; Mehra, D.; Galor, A. Corneal Nerve Abnormalities in Ocular and Systemic Diseases. Exp. Eye Res. 2021, 202, 108284. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; Rajan, R.; Jiao, H.; Wu, M.; Zhang, A.C.; De Silva, M.E.H.; Makrai, E.; Stepp, M.A.; Di Girolamo, N.; Downie, L.E. Identification of presumed corneal neuromas and microneuromas using laser-scanning in vivo confocal microscopy: A systematic review. Br. J. Ophthalmol. 2021, 10, 1136. [Google Scholar] [CrossRef]
- Labbé, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The Relationship between Subbasal Nerve Morphology and Corneal Sensation in Ocular Surface Disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 4926–4931. [Google Scholar] [CrossRef]
- Nicolle, P.; Liang, H.; Reboussin, E.; Rabut, G.; Warcoin, E.; Brignole-Baudouin, F.; Melik-Parsadaniantz, S.; Baudouin, C.; Labbe, A.; Goazigo, A.R.-L. Proinflammatory Markers, Chemokines, and Enkephalin in Patients Suffering from Dry Eye Disease. Int. J. Mol. Sci. 2018, 19, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tervo, T.M.; Moilanen, J.A.O.; Rosenberg, M.E.; Tuominen, I.S.J.; Valle, T.; Vesaluoma, M.H. In Vivo Confocal Microscopy for Studying Corneal Diseases and Conditions Associated with Corneal Nerve Damage. Adv. Exp. Med. Biol. 2002, 506, 657–665. [Google Scholar] [CrossRef]
- Moein, H.-R.; Akhlaq, A.; Dieckmann, G.; Abbouda, A.; Pondelis, N.; Salem, Z.; Müller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; et al. Visualization of microneuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain? Ocul. Surf. 2020, 18, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Wilhelm, S.; Schacht, A.; Perrot, S.; Kosek, E.; Cruccu, G.; Freynhagen, R.; Tesfaye, S.; Lledó, A.; Choy, E.; et al. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: Data from the randomized, double-blind, COMBO-DN study. Pain 2014, 155, 2171–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Kheirkhah, A.; Cavalcanti, B.M.; Cruzat, A.; Jamali, A.; Hamrah, P. Correlation of corneal immune cell changes with clinical severity in dry eye disease: An in vivo confocal microscopy study. Ocul. Surf. 2021, 19, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.S. Diagnosis and Treatment of Ocular Pain: The Ophthalmologist’s Perspective. Curr. Ophthalmol. Rep. 2017, 5, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dermer, H.; Hwang, J.; Mittal, R.; Cohen, A.K.; Galor, A. Corneal sub-basal nerve plexus microneuromas in individuals with and without dry eye. Br. J. Ophthalmol. 2021, 10, 1136. [Google Scholar] [CrossRef]
- Stepp, M.A.; Pal-Ghosh, S.; Downie, L.E.; Zhang, A.C.; Chinnery, H.R.; Machet, J.; Di Girolamo, N. Corneal Epithelial “Neuromas”: A Case of Mistaken Identity? Cornea 2020, 39, 930–934. [Google Scholar] [CrossRef]
- van Hecke, O.; Kamerman, P.R.; Attal, N.; Baron, R.; Bjornsdottir, G.; Bennett, D.L.; Bennett, M.I.; Bouhassira, D.; Diatchenko, L.; Freeman, R.; et al. Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies. Pain 2015, 156, 2337–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollert, J.; Maier, C.; Attal, N.; Bennett, D.L.; Bouhassira, D.; Enax-Krumova, E.K.; Finnerup, N.B.; Freynhagen, R.; Gierthmühlen, J.; Haanpää, M.; et al. Stratifying patients with peripheral neuropathic pain based on sensory profiles: Algorithm and sample size recommendations. Pain 2017, 158, 1446–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallinikos, P.; Berhanu, M.; O’Donnell, C.; Boulton, A.J.M.; Efron, N.; Malik, R. Corneal Nerve Tortuosity in Diabetic Patients with Neuropathy. Investig. Opthalmol. Vis. Sci. 2004, 45, 418–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Yoon, H.J.; You, I.C.; Ko, B.Y.; Yoon, K.C. Clinical characteristics of dry eye with ocular neuropathic pain features: Comparison according to the types of sensitization based on the Ocular Pain Assessment Survey. BMC Ophthalmol. 2020, 20, 1–7. [Google Scholar] [CrossRef]
| Autoimmune Dry Eye (AIDE) | Meibomian Gland Dysfunction (MGD) | |||||
|---|---|---|---|---|---|---|
| All Patients in the Study | Painless | Painful | Painless | Painful | ||
| VAS | - | 0 | 8–10 | 0 | 8–10 | |
| Number of patients (%) | 238 (100%) | 63 (27%) | 59 (25%) | 63 (27%) | 53 (22%) | |
| Age (mean ± SD) | 56.8 ± 15.4 | 58.1 ± 13.8 | 57.0 ± 15.0 | 55.4 ± 16.0 | 56.8 ± 16.9 | |
| Gender (n, %) | Male | 40 (17%) | 6 (10%) | 4 (7%) | 16 (25%) | 14 (26%) |
| Female | 198 (83%) | 57 (91%) † | 55 (93%) ‡‡ | 47 (75%) | 39 (74%) | |
| Autoimmune Dry Eye (AIDE) | Meibomian Gland Dysfunction (MGD) | |||||
|---|---|---|---|---|---|---|
| All Patients in the Study | Painless | Painful | Painless | Painful | ||
| OSDI score (mean ± SD) | 62.5 ± 24.8 | 57.5 ± 25.7 † | 76.8 ± 15.1 #### | 46.6 ± 24.1 | 70.8 ± 21.0 ●●●● | |
| Itching (n, %) | Never | 115 (49%) | 38 (62%) | 20 (34%) | 35 (56%) | 22 (42%) |
| Rarely | 44 (19%) | 10 (16%) | 8 (14%) | 17 (27%) | 9 (17%) | |
| Sometimes | 51 (22%) | 10 (16%) | 21 (36%) | 8 (13%) | 12 (23%) | |
| Always | 26 (11%) | 3 (5%) | 10 (17%) ### | 3 (5%) | 10 (19%) ●●● | |
| Burning (n, %) | Never | 66 (28%) | 22 (35%) | 4 (7%) | 35 (57%) | 5 (9%) |
| Rarely | 39 (16%) | 18 (29%) | 6 (10%) | 9 (14%) | 6 (11%) | |
| Sometimes | 86 (36%) | 18 (29%) | 31 (52%) | 15 (24%) | 22 (41%) | |
| Always | 46 (19%) | 5 (8%) † | 18(30%) #### | 3(5%) | 20 (38%) ●● | |
| Foreign body sensation (n, %) | Never | 73 (31%) | 21 (33%) | 5 (8%) | 32 (52%) | 15 (28%) |
| Rarely | 38 (16%) | 15 (24%) | 5 (8%) | 11 (18%) | 7 (13%) | |
| Sometimes | 84 (35%) | 17 (27%) | 33 (56%) | 13 (21%) | 21 (40%) | |
| Always | 42 (18%) | 10 (16%) † | 16 (27%) ####, ‡ | 6 (10%) | 10 (19%) ●●●● | |
| Dryness sensation (n, %) | Never | 94 (39%) | 28 (44%) | 11 (19%) | 33 (52%) | 22 (42%) |
| Rarely | 32 (13%) | 14 (22%) | 6 (10%) | 6 (10%) | 6 (11%) | |
| Sometimes | 71 (30%) | 15 (24%) | 23 (39%) | 19 (30%) | 14 (26%) | |
| Always | 41 (17%) | 6 (10%) | 19 (32%) ####, ‡ | 5 (8%) | 11 (21%) | |
| Autoimmune Dry Eye (AIDE) | Meibomian Gland Dysfunction (MGD) | ||||
|---|---|---|---|---|---|
| All Patients in the Study | Painless | Painful | Painless | Painful | |
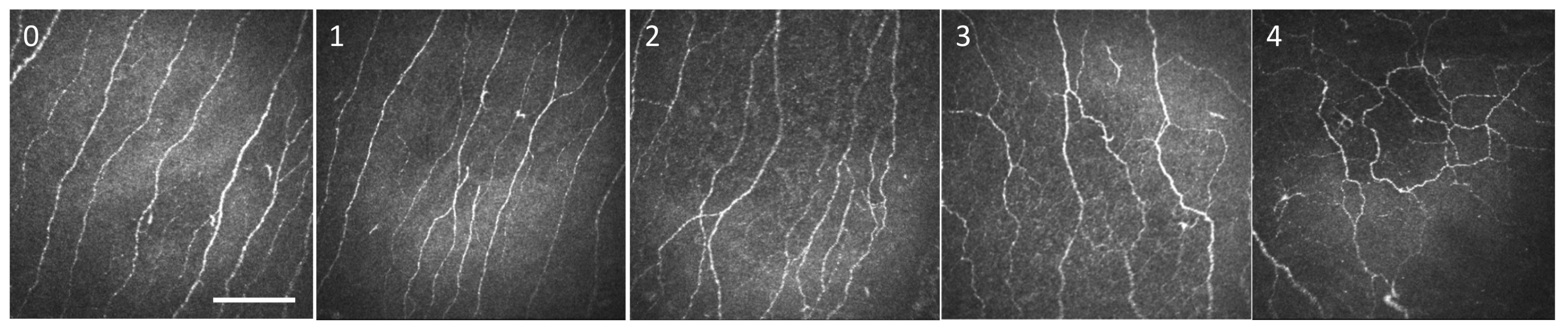
Oxford score | 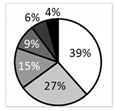 | ††††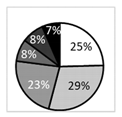 | ‡‡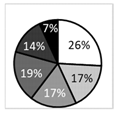 | 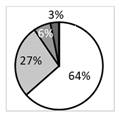 | ●●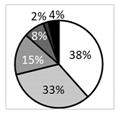 |
| BUT (s, mean ± SD) | 4.94 ± 0.18 | 4.82 ± 0.35 | 4.19 ± 0.31 | 5.70 ± 0.36 | 5.00 ± 0.39 ‡ |
| NIKBUT (first, mean ± SD) | 5.06 ± 5.35 | 4.08 ± 4.77 | 3.55 ± 3.65 | 5.83 ± 5.14 | 6.66 ± 7.45 |
| NIKBUT (average, mean ± SD) | 7.78 ± 6.90 | 6.8 ± 6.52 | 5.10 ± 5.41 | 9.24 ± 6.87 | 9.27 ± 8.18 ‡ |
| Schirmer (mm/5 min, mean ± SD) | 10.9 ± 10.0 | 10.1 ± 10.1 | 8.8 ± 9.1 | 12.8 ± 10.6 | 12.0 ± 9.8 |
| Tear meniscus (mm, mean ± SD) | 0.209 ± 0.188 | 0.172 ± 0.198 | 0.224 ± 0.193 | 0.235 ± 0.200 | 0.196 ± 0.141 |
| Osmolarity (mOsmol/L, mean ± SD) | 309 ± 17 | 307 ± 15 | 311 ± 19 | 306 ± 16 | 310 ± 19 |
| IVCM-Assessed Corneal Ultrastructural Characteristics (vs. Healthy Controls) | |||||||
|---|---|---|---|---|---|---|---|
| ↘ Nerve Density | ↗ Nerve Tortortuosity | ↗ Number of Dendritic Cells | ↗ Number of MN | ↗ Perimeter of MN | ↗ Area of MN | ||
| MGD | MGD-NCP | ✓ | ns | ns | ✓ | ✓ | ✓ |
| Painless | ns | ns | ns | ✓ | ns | ✓ | |
| AIDE | AIDE-NCP | ns | ✓ | ns | ✓ | ns | ns |
| Painless | ✓ | ns | ns | ✓ | ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Moreno, A.; Liang, H.; Moreau, N.; Luzu, J.; Rabut, G.; Melik Parsadaniantz, S.; Labbé, A.; Baudouin, C.; Réaux-Le Goazigo, A. Corneal Nerve Abnormalities in Painful Dry Eye Disease Patients. Biomedicines 2021, 9, 1424. https://doi.org/10.3390/biomedicines9101424
Guerrero-Moreno A, Liang H, Moreau N, Luzu J, Rabut G, Melik Parsadaniantz S, Labbé A, Baudouin C, Réaux-Le Goazigo A. Corneal Nerve Abnormalities in Painful Dry Eye Disease Patients. Biomedicines. 2021; 9(10):1424. https://doi.org/10.3390/biomedicines9101424
Chicago/Turabian StyleGuerrero-Moreno, Adrian, Hong Liang, Nathan Moreau, Jade Luzu, Ghislaine Rabut, Stéphane Melik Parsadaniantz, Antoine Labbé, Christophe Baudouin, and Annabelle Réaux-Le Goazigo. 2021. "Corneal Nerve Abnormalities in Painful Dry Eye Disease Patients" Biomedicines 9, no. 10: 1424. https://doi.org/10.3390/biomedicines9101424
APA StyleGuerrero-Moreno, A., Liang, H., Moreau, N., Luzu, J., Rabut, G., Melik Parsadaniantz, S., Labbé, A., Baudouin, C., & Réaux-Le Goazigo, A. (2021). Corneal Nerve Abnormalities in Painful Dry Eye Disease Patients. Biomedicines, 9(10), 1424. https://doi.org/10.3390/biomedicines9101424






