False Myths versus Medical Facts: Ten Common Misconceptions Related to Dry Eye Disease
Abstract
1. Introduction
2. From False Myths to Medical Facts
2.1. False Myth #1: “Dry Eye Is Just a Disorder or a Dysfunction”
2.2. False Myth #2: “Dry Eye Is a Disease of the Cornea, Lids, Tears, etc…”
2.3. False Myth #3: “Dry Eye Is Caused by a Lack of Tears”
2.4. False Myth #4: “Corneal Pain without Stain: Is It Real? No”
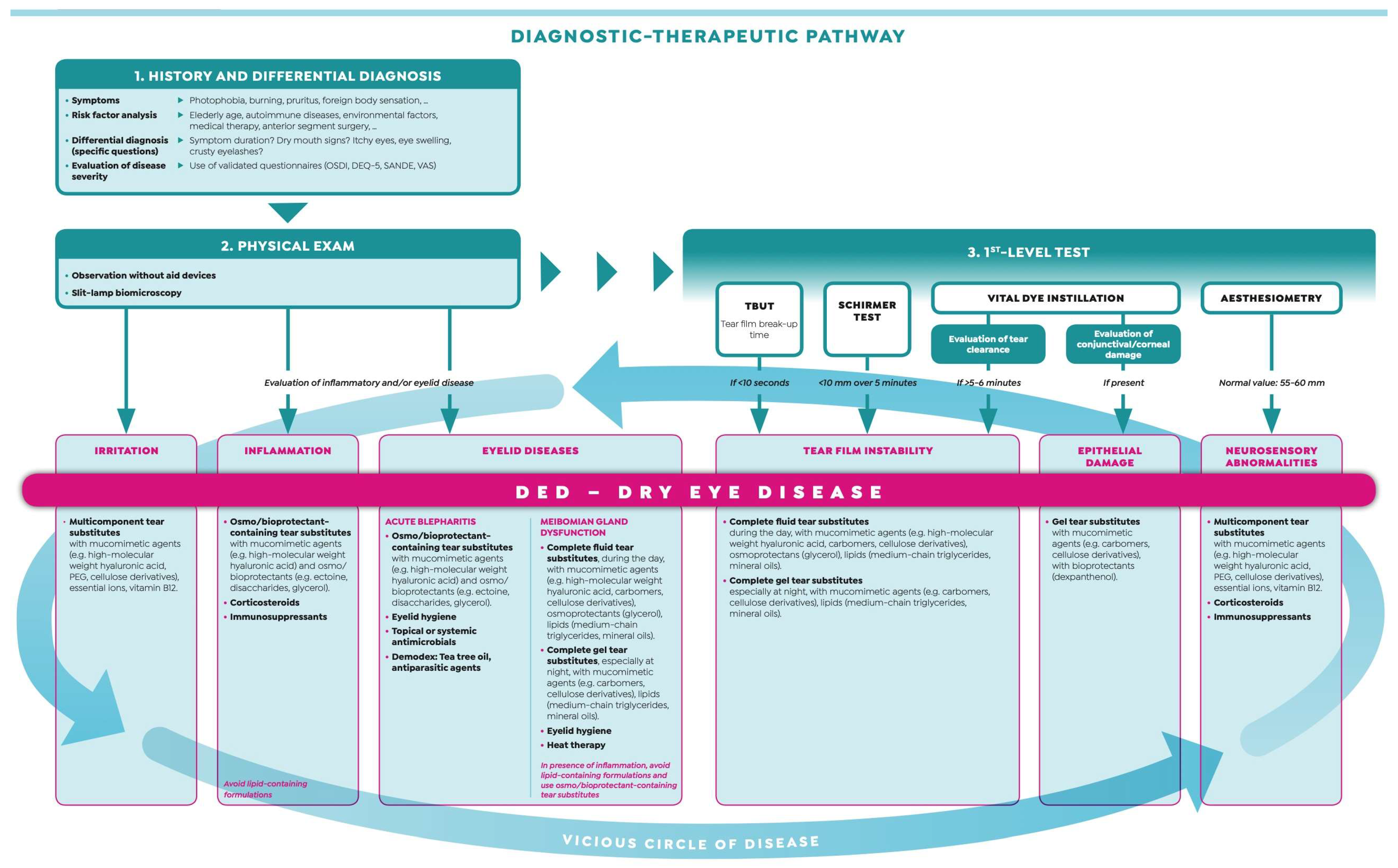
2.5. False Myth #5: “The Diagnosis of Dry Eye Is Time-Consuming and Requires High-Tech Equipment”
2.6. False Myth #6: “Dry Eye Patients without Corneal Damage Do Not Suffer from Visual Disturbance”
2.7. False Myth #7: “Dry Eye Disease does not Influence Surgical Outcomes”
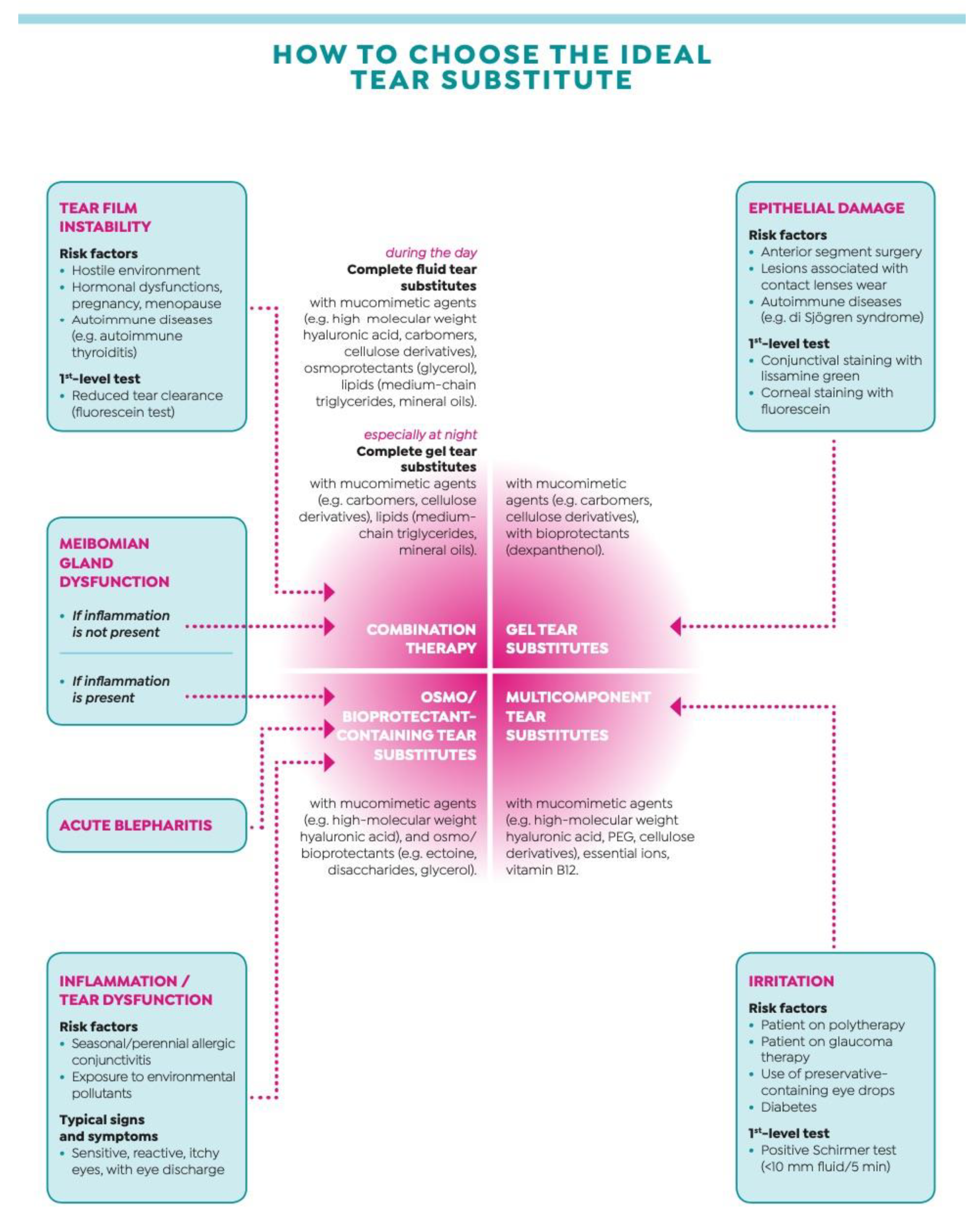
2.8. False Myth #8: “All Tear Substitutes Are the Same”
2.9. False Myth #9: “Tear Substitutes Can Be Used on As-Need Basis”
2.10. False Myth #10: “All Corticosteroids Are Equally Risky for Medium/Long Term Use in Severe Dry Eye”
3. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Shimazaki, J. Definition and diagnostic criteria of dry eye disease: Historical overview and future directions. Investig. Ophthalmol. Vis. Sci. 2018, 59, 7–12. [Google Scholar] [CrossRef]
- Tear Film & Ocular Surface Society. The definition and classification of dry eye disease: Report of the definition and classification subcommittee of the international dry eye workshop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Galor, A. Why Internists should care about dry eye disease. J. Clin. Med. 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Galor, A. What’s new in dry eye disease diagnosis? Current advances and challenges. F1000Research 2018, 7, 1952. [Google Scholar] [CrossRef]
- Thoft, R.A.; Friend, J. Biochemical transformation of regenerating ocular surface epithelium. Investig. Ophthalmol. Vis. Sci. 1977, 16, 14–20. [Google Scholar]
- Gipson, I.K. The ocular surface: The challenge to enable and protect vision. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4390–4398. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Giannaccare, G.; Vukatana, G.; Mulè, R.; Malavolta, N.; Campos, E.C. Predictive role of tear protein expression in the early diagnosis of Sjogren’s syndrome. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 55, 561–570. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Bernabei, F.; Scorcia, V.; Campos, E.C. Ocular surface system alterations in ocular graft-versus-host disease: All the pieces of the complex puzzle. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Schargus, M.; Geerling, G. The “wet” dry eye. Ophthalmologe 2009, 106, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Watanabe, H.; Dogru, M.; Kojima, T.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. A new perspective on dry eye classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 2020, 46, S2–S13. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.; Baran, I.; Jacobs, D.S. Corneal Pain without Stain: Is it Real? Ocul. Surf. 2009, 7, 28–40. [Google Scholar] [CrossRef]
- Belmonte, C.; Nichols, J.J.; Cox, S.M.; Brock, J.A.; Begley, C.G.; Bereiter, D.A.; Dartt, D.A.; Galor, A.; Hamrah, P.; Ivanusic, J.J.; et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017, 15, 404–437. [Google Scholar] [CrossRef]
- Galor, A.; Moein, H.R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef]
- Galor, A. Dry eye symptoms. A nerve problem or a tear problem? Ophthalmology 2019, 126, 648–651. [Google Scholar] [CrossRef]
- Yang, J.M.; Li, F.; Liu, Q.; Ruedi, M.; Wei, E.T.; Lentsman, M.; Lee, H.S.; Choi, W.; Kim, S.J.; Yoon, K.C. A novel TRPM8 agonist relieves dry eye discomfort. BMC Ophthalmol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Aggarwal, S.; Colon, C.; Kheirkhah, A.; Hamrah, P. Efficacy of autologous serum tears for the treatment of neuropathic corneal pain. Ocul. Surf. 2019, 17, 532–539. [Google Scholar] [CrossRef]
- Giannaccare, G.; Buzzi, M.; Fresina, M.; Velati, C.; Versura, P. Efficacy of 2-month treatment with cord blood serum eye drops in ocular surface disease: An in vivo confocal microscopy study. Cornea 2017, 36, 915–921. [Google Scholar] [CrossRef]
- Rolando, M.; Cantera, E.; Mencucci, R.; Rubino, P.; Aragona, P. The correct diagnosis and therapeutic management of tear dysfunction: Recommendations of the P.I.C.A.S.S.O. board. Int. Ophthalmol. 2017, 38, 875–895. [Google Scholar] [CrossRef]
- Yamanishi, R.; Uchino, M.; Kawashima, M.; Uchino, Y.; Yokoi, N.; Tsubota, K. Characteristics of individuals with dry eye symptoms without clinical diagnosis: Analysis of a web-based survey. J. Clin. Med. 2019, 8, 721. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Iester, M.; Macrì, A.; Calabria, G. Low spatial-contrast sensitivity in dry eyes. Cornea 1998, 17, 376–379. [Google Scholar] [CrossRef]
- Miljanovic, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of dry eye syndrome on vision-related quality of life. Am. J. Ophthalmol. 2007, 143, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired functional visual acuity of dry eye patients. Am. J. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Li, M.; Gong, L.; Chapin, W.J.; Zhu, M. Assessment of vision-related quality of life in dry eye patients. Investig. Opthalmol. Vis. Sci. 2012, 53, 5722–5727. [Google Scholar] [CrossRef] [PubMed]
- Koh, S. Mechanisms of visual disturbance in dry eye. Cornea 2016, 35, S83–S88. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Giannaccare, G.; Bernabei, F.; Pellegrini, M.; Guaraldi, F.; Turchi, F.; Torrazza, C.; Senni, C.; Scotto, R.; Sindaco, D.; Di Cello, L.; et al. Bilateral morphometric analysis of corneal sub-basal nerve plexus in patients undergoing unilateral cataract surgery: A preliminary in vivo confocal microscopy study. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Miyake, K.; Yokoi, N. Influence on ocular surface after cataract surgery and effect of topical diquafasol on postoperative dry eye: A multicenter prospective randomized study. Clin. Ophthalmol. 2017, 11, 529–540. [Google Scholar] [CrossRef]
- Hanyuda, A.; Ayaki, M.; Tsubota, K.; Negishi, K. Discrepancies in persistent dry eye signs and symptoms in bilateral pseudophakic patients. J. Clin. Med. 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Rousseau, A.; Baudouin, C. Management of dry eye disease to optimize cataract surgery outcomes: Two table for a daily clinical practice. J. Français Ophthalmol. 2019, 42, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vadoothker, S.; Munir, W.M.; Saeedi, M. Ocular surface disease and glaucoma medications: A clinical approach. Eye Contact Lens 2019, 45, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Tailor, R.; Mohamed, S. Ocular surface disease exacerbated glaucoma: Optimizing the ocular surface improves intraocular pressure control. J. Glaucoma 2014, 23, 56–60. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castilllo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Aragona, P.; Messmer, E.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Pucker, A.D.; Ng, S.M.; Nichols, J.J. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Swanson, M. Compliance with and typical usage of artificial tears in dry eye conditions. J. Am. Optom. Assoc. 1998, 69, 649–655. [Google Scholar] [PubMed]
- Holly, F.J.; Lemp, M.A. Tear physiology and dry eyes. Surv. Ophthalmol. 1977, 22, 69–87. [Google Scholar] [CrossRef]
- Asbell, P.; Vingrys, A.J.; Tan, J.; Ogundele, A.; Downie, L.E.; Jerkins, G.; Shettle, L. Clinical outcomes of fixed versus as-needed use of artificial tears in dry eye disease: A 6-week, observer-masked phase 4 clinical trial. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Keller, P.R. A pragmatic approach to the management of dry eye disease: Evidence into practice. Optom. Vis. Sci. 2015, 92, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Yokoi, N.; Kawashima, M.; Ryutaro, Y.; Uchino, Y.; Tsubota, K. Treatment trends in dry eye disease and factors associated with ophthalmic follow-up discontinuation in Japan. J. Clin. Med. 2019, 8, 1120. [Google Scholar] [CrossRef]
- Farid, M. Dry eye disease: Let’s start thinking outside the artificial box. Ophthalmology 2017, 124, 1–3. [Google Scholar] [CrossRef]
- Baudouin, C.; Irkec, M.; Messmer, E.M.; Benitez-del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, C.A.; Barabino, S.; Bonzano, C.; Traverso, C.E. The use of topical corticosteroids for treatment of dry eye syndrome. Ocul. Immunol. Inflamm. 2017, 27, 266–275. [Google Scholar] [CrossRef]
- Sall, K.; Stevenson, O.D.; Mundorf, T.K.; Reis, B.L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA phase 3 study group. Ophthalmology 2000, 107, 631–639. [Google Scholar] [CrossRef]
- Dogru, M.; Tsubota, K. Pharmacotherapy of dry eye. Expert Opin. Pharmacother. 2011, 12, 325–334. [Google Scholar] [CrossRef]
- Holland, E.J.; Luchs, J.; Karpecki, P.M.; Nichols, K.K.; Jackson, M.A.; Sall, K.; Tauber, J.; Roy, M.; Raychaudhuri, A.; Shojaei, A. Lifitegrast for the treatment of dry eye disease: Results of a phase III, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology 2017, 124, 53–60. [Google Scholar] [CrossRef]
- Aragona, P.; Stilo, A.; Ferreri, F.; Mobrici, M. Effects of the topical treatment with NSAIDs on corneal sensitivity and ocular surface of Sjögren’s syndrome patients. Eye 2005, 19, 535–539. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- McGhee, C.N.J.; Dean, S.; Danesh-Meyer, H. Locally administered ocular corticosteroids: Benefits and risks. Drug Saf. 2002, 25, 33–55. [Google Scholar] [CrossRef]
- Aragona, P.; Spinelli, R.; Rania, L.; Postorino, E.; Sommario, M.S.; Roszkowska, A.M.; Puzzolo, D. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren sydrome. Eur. J. Ophthalmol. 2013, 23, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, I.J.; Dickersin, K.; Hutfless, S.T.; Akpek, E.K. Gaps in current knowledge and priorities for future research in dry eye. Cornea 2017, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A. Dry eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Progress Retin. Eye Res. 2020. [Google Scholar] [CrossRef]
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423. [Google Scholar] [CrossRef]
- Siedlecki, A.N.; Smith, S.D.; Siedlecki, A.R.; Hayek, S.M.; Sayegh, R.R. Ocular pain response to treatment in dry eye patients. Ocul. Surf. 2020, 18, 305–311. [Google Scholar] [CrossRef]
- Yang, W.J.; Yang, Y.N.; Cao, J.; Man, Z.H.; Yuan, J.; Xiao, X.; Xing, Y.Q. Risk factors for dry eye syndrome: A retrospective case-control study. Optom. Vis. Sci. 2015, 92, 199–205. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannaccare, G.; Scorcia, V. False Myths versus Medical Facts: Ten Common Misconceptions Related to Dry Eye Disease. Biomedicines 2020, 8, 172. https://doi.org/10.3390/biomedicines8060172
Giannaccare G, Scorcia V. False Myths versus Medical Facts: Ten Common Misconceptions Related to Dry Eye Disease. Biomedicines. 2020; 8(6):172. https://doi.org/10.3390/biomedicines8060172
Chicago/Turabian StyleGiannaccare, Giuseppe, and Vincenzo Scorcia. 2020. "False Myths versus Medical Facts: Ten Common Misconceptions Related to Dry Eye Disease" Biomedicines 8, no. 6: 172. https://doi.org/10.3390/biomedicines8060172
APA StyleGiannaccare, G., & Scorcia, V. (2020). False Myths versus Medical Facts: Ten Common Misconceptions Related to Dry Eye Disease. Biomedicines, 8(6), 172. https://doi.org/10.3390/biomedicines8060172





