Impact of Local High Doses of Radiation by Neutron Activated Mn Dioxide Powder in Rat Lungs: Protracted Pathologic Damage Initiated by Internal Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Radiations
2.2. Animals and Treatment
2.3. Pathology
2.4. SR-XRF-XANES Analysis
2.5. Mn particle Dose Rate
2.6. Statistical Analysis
3. Results
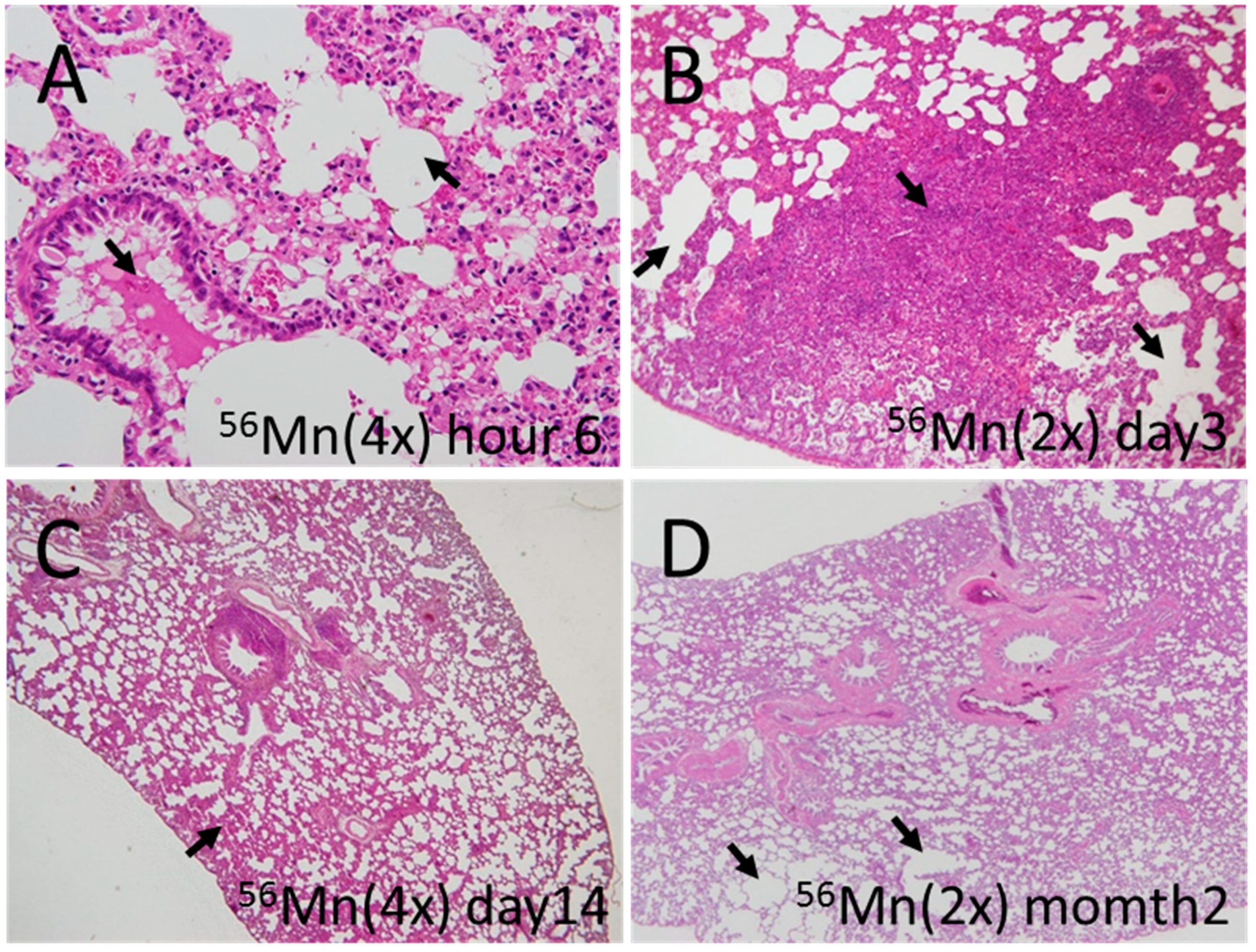
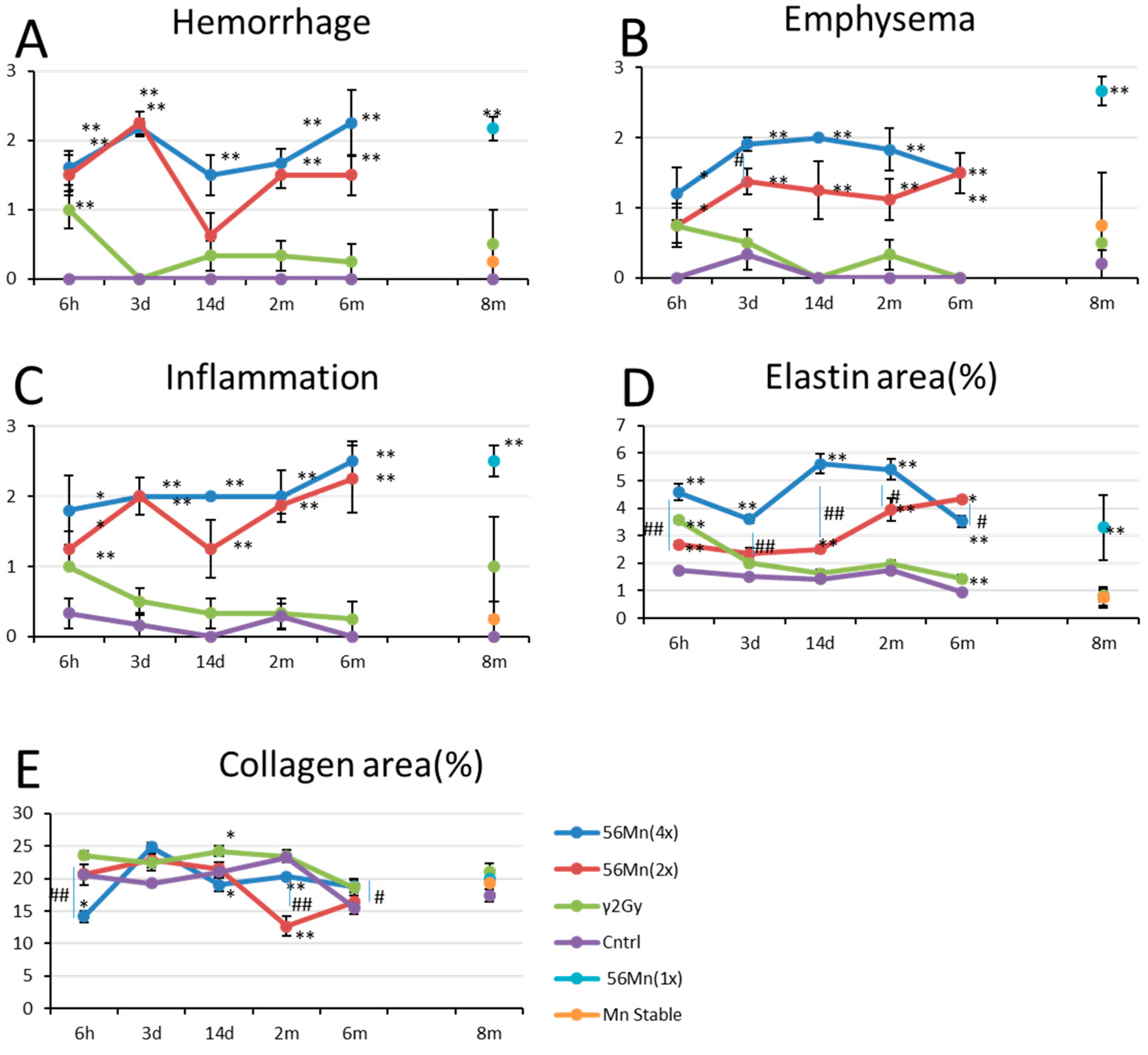
3.1. Early Event Damage to Lung Tissue for Internal Exposure Is Uncharacteristically Rapid and Severe
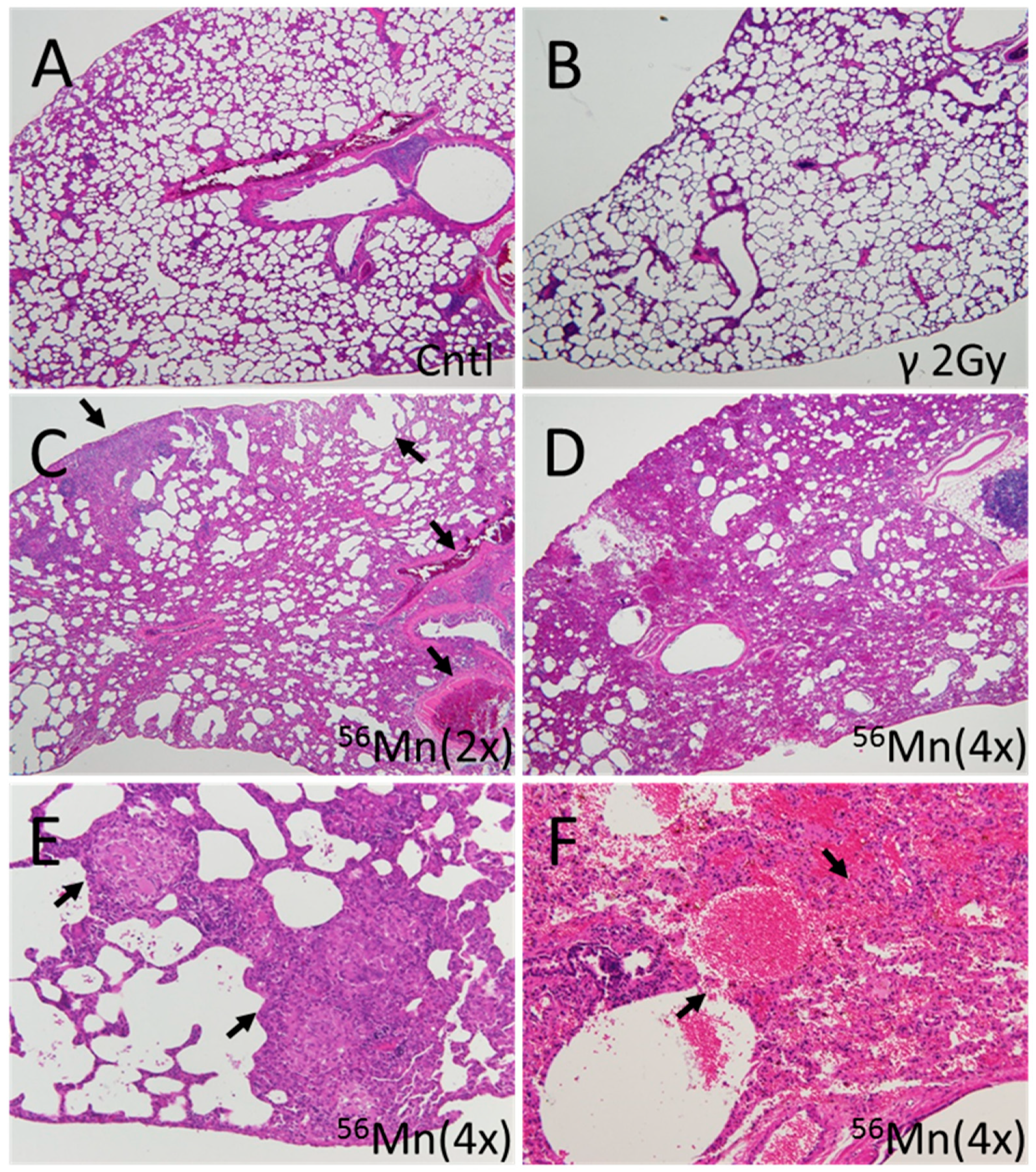
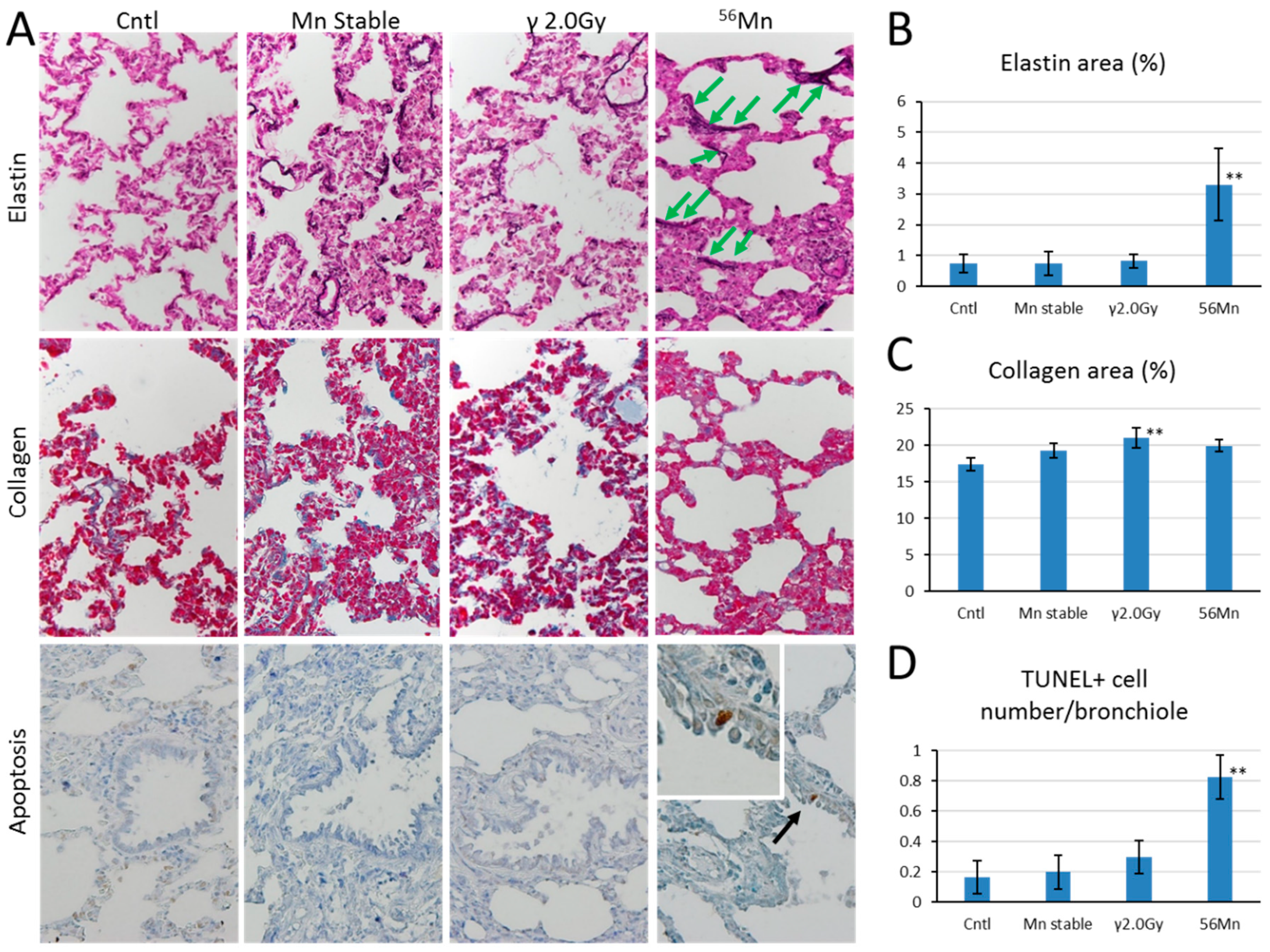
3.2. Late Histology with Prominent Atelectasis and Pneumonitis for both 2× and 4×56Mn Exposure, Granuloma and Severe Hemorrhage
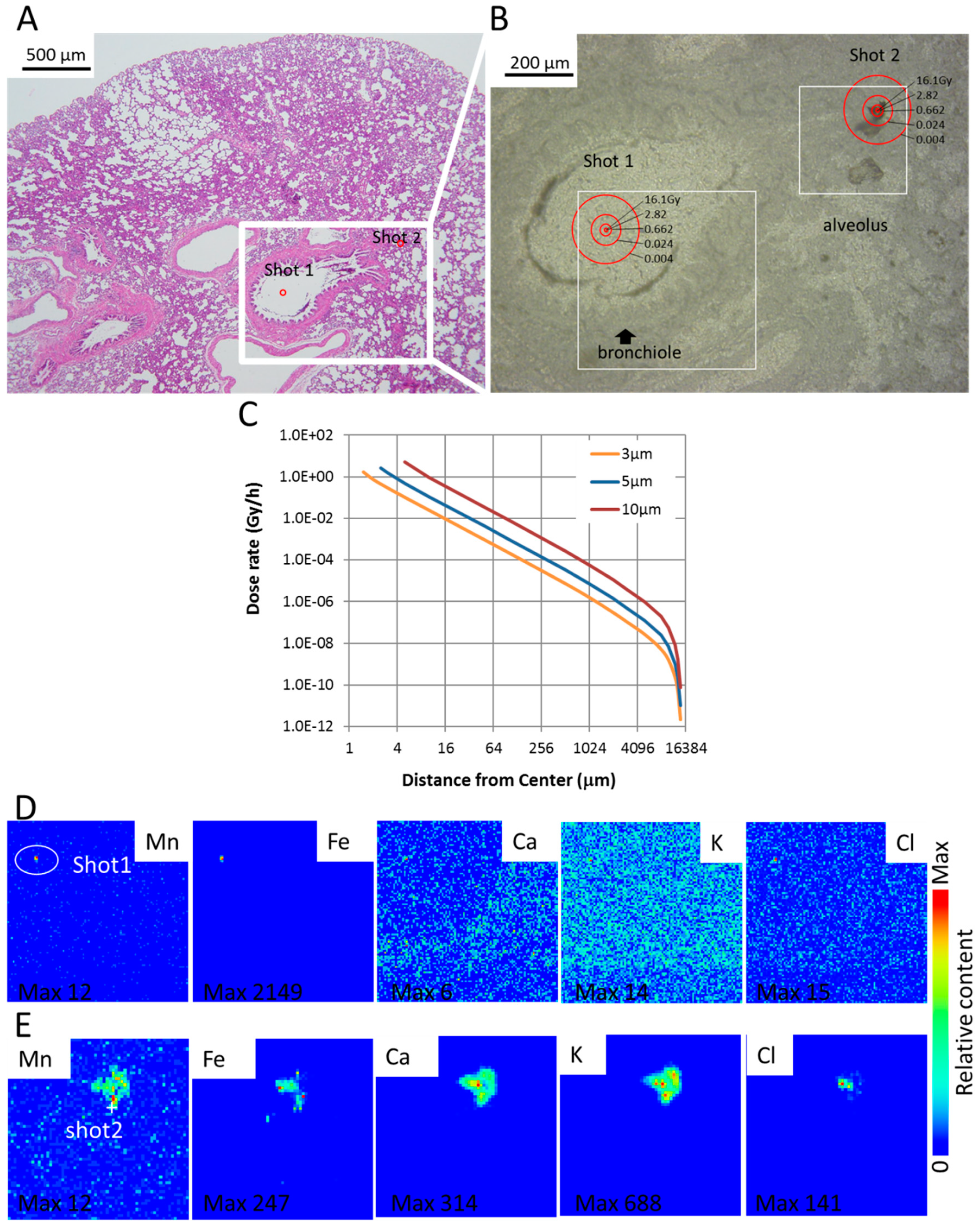
3.3. SR-XRF Analysis Revealed that Masses of Manganese, Iron and Calcium Attached to Damaged Tissue
3.4. Dose Rate and Accumulated Dose Are Functions of Diameter and Distance
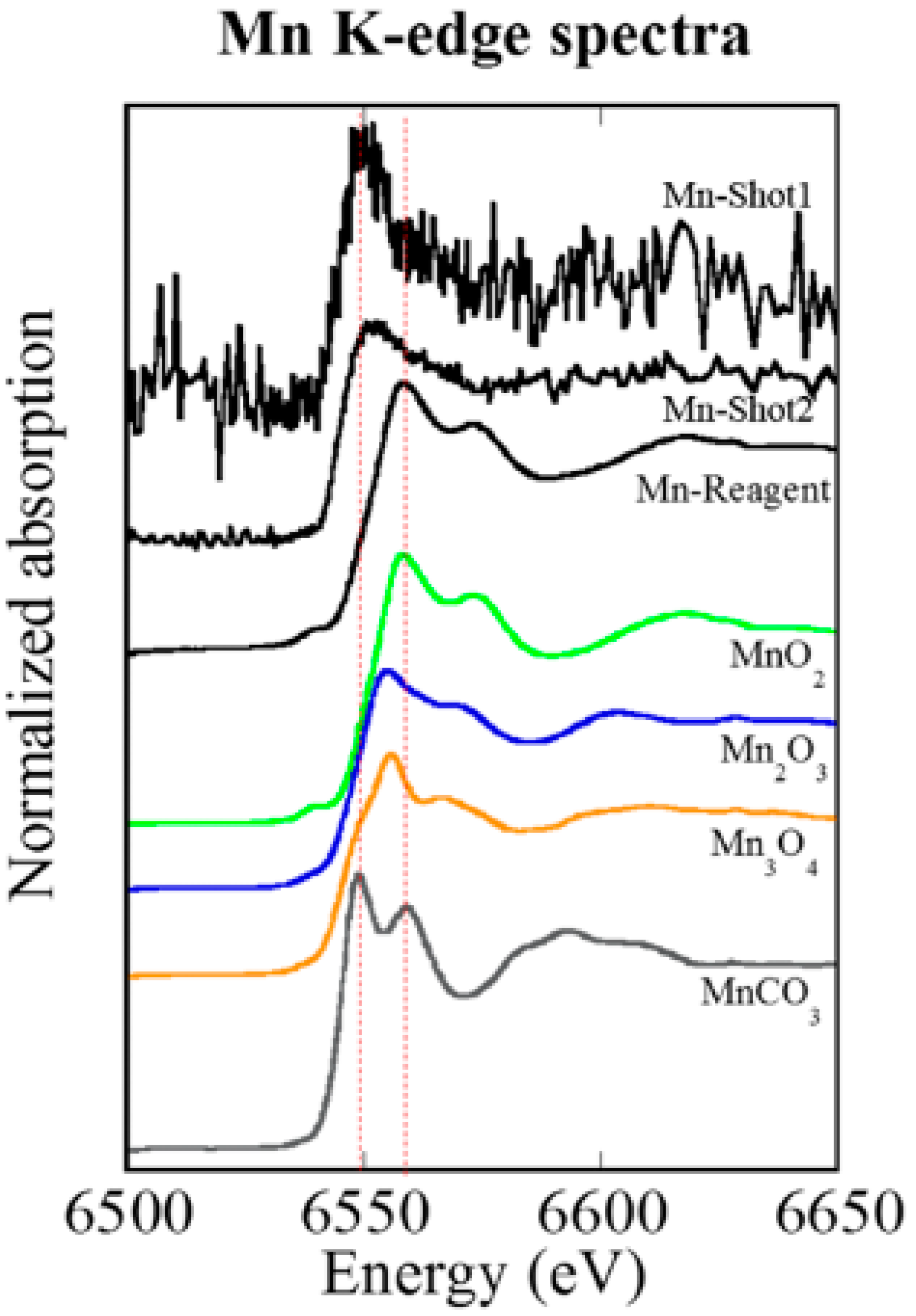
3.5. XANES Spectroscopy for the Analysis of Particles Embedded in Lung Tissue Samples (Shot1 and Shot2), They Were Determined to Be Mn
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shichijo, K.; Takatsuji, T.; Fukumoto, M.; Nakashima, M.; Matsuyama, M.M.; Sekine, I. Autoradiographic analysis of internal plutonium radiation exposure in Nagasaki atomic bomb victims. Heliyon 2018, 4, e00666. [Google Scholar] [CrossRef] [PubMed]
- Okajima, S.; Fujita, S. Radiation doses from residual radioactivity. In U.S. Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki; Final Report; Roesch, W., Ed.; Radiation Effects Research Foundation: Hiroshima, Japan, 1987; Volume 1, pp. 205–226. [Google Scholar]
- Shichijo, K.; Fujimoto, N.; Uzbekov, D.; Kairkhanova, Y.; Saimova, A.; Chaizhunusova, N.; Sayakenov, N.; Shabdarbaeva, D.; Aukenov, N.; Azimkhanov, A.; et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats-Part 2: Pathological effects. Radiat. Environ. Biophys. 2017, 56, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, K.; Fujimoto, N.; Uzbekov, D.; Kairkhanova, Y.; Saimova, A.; Chaizhunusova, N.; Sayakenov, N.; Shabdarbaeva, D.; Aukenov, N.; Azimkhanov, A.; et al. Erratum to: Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats-Part 2: Pathological effects. Radiat. Environ. Biophys. 2017, 56, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, V.; Rakhypbekov, T.; Otani, K.; Endo, S.; Satoh, K.; Kawano, N.; Shichijo, K.; Nakashima, M.; Takatsuji, T.; Sakaguchi, A.; et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats: Part 1: Dosimetry. Radiat. Environ. Biophys. 2017, 56, 47–54. [Google Scholar] [CrossRef]
- Lanin, A. Nuclear Rocket Engine Reactor; Springer: Berlin/Heidelberg, Germany, 2013; p. 110. [Google Scholar]
- Matsuu-Matsuyama, M.; Nakashima, M.; Shichijo, K.; Okaichi, K.; Nakayama, T.; Sekine, I. Basic fibroblast growth factor suppresses radiation-induced apoptosis and TP53 pathway in rat small intestine. Radiat. Res. 2010, 174, 52–61. [Google Scholar] [CrossRef]
- Takahashi, Y.; Manceau, A.; Geoffroy, N.; Marcus, M.A.; Usui, A. Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganase oxides. Geochim. Cosmochim. Acta 2007, 71, 984–1008. [Google Scholar] [CrossRef]
- Sugiyama, T.; Uo, M.; Wada, T.; Omagari, D.; Komiyama, K.; Miyazaki, S.; Numako, C.; Noguchi, T.; Jinbu, Y.; Kusama, M.; et al. Detection of trace metallic elements in oral lichenoid contact lesions using SR-XRF, PIXE, and XAFS. Sci. Rep. 2015, 5, 10672. [Google Scholar] [CrossRef]
- RADAR. The Decay Data. Available online: http://www.doseinfo-radar.com/RADARDecay.html (accessed on 12 December 2019).
- Berger, M.J.; Seltzer, S.M. Tables of energy-losses and ranges of electrons and positrons. In Studies in Penetration of Charged Particles in Matter; Nuclear Science Series Report Number 39; Committee on Nuclear Science: Washington, DC, USA, 1964; pp. 205–268. [Google Scholar]
- Manceau, A.; Marucus, M.A.; Grangeon, S. Determination of Mn valence states in mixed-valent manganates by XANES spectroscopy. Am. Mineral. 2012, 97, 816–827. [Google Scholar] [CrossRef]
- MacVittie, T.J.; Gibbs, A.; Farese, A.M.; Barrow, K.; Bennett, A.; Taylor-Howell, C.; Kazi, A.; Prado, K.; Parker, G.; Jackson, W. AEOL 10150 Mitigates Radiation-Induced Lung Injury in the Nonhuman Primate: Morbidity and Mortality are Administration Schedule-Dependent. Radiat. Res. 2017, 187, 298–318. [Google Scholar] [CrossRef]
- Hagiyama, M.; Yoneshige, A.; Inoue, T.; Sato, Y.; Mimae, T.; Okada, M.; Ito, A. The intracellular domain of cell adhesion molecule 1 is present in emphysematous lungs and induces lung epithelial cell apoptosis. J. Biomed. Sci. 2015, 22, 67. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Pietrofesa, R.A.; Arguiri, E.; Schweitzer, K.S.; Berdyshev, E.V.; McCarthy, M.; Corbitt, A.; Alwood, J.S.; Yu, Y.; Globus, R.K.; et al. Space radiation-associated lung injury in a murine model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L416–L428. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Petrache, I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Investig. 2012, 122, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Diab, K.J.; Adamowicz, J.J.; Kamocki, K.; Rush, N.I.; Garrison, J.; Gu, Y.; Schweitzer, K.S.; Skobeleva, A.; Rajashekhar, G.; Hubbard, W.C.; et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am. J. Respir. Crit. Care Med. 2010, 181, 344–352. [Google Scholar] [CrossRef]
- Petrache, I.; Natarajan, V.; Zhen, L.; Medler, T.R.; Richter, A.T.; Cho, C.; Hubbard, W.C.; Berdyshev, E.V.; Tuder, R.M. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat. Med. 2005, 11, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R.; Fuks, Z. Radiation and ceramide-induced apoptosis. Oncogene 2003, 22, 5897–5906. [Google Scholar] [CrossRef]
- Mathew, B.; Jacobson, J.R.; Berdyshev, E.; Huang, Y.; Sun, X.; Zhao, Y.; Gerhold, L.M.; Siegler, J.; Evenoski, C.; Wang, T.; et al. Role of sphingolipids in murine radiation-induced lung injury: Protection by sphingosine 1-phosphate analogs. FASEB J. 2011, 25, 3388–3400. [Google Scholar] [CrossRef] [PubMed]
- Homma-Takeda, S.; Kokubo, T.; Terada, Y.; Suzuki, K.; Ueno, S.; Hayao, T.; Inoue, T.; Kitahara, K.; Blyth, B.J.; Nishimura, M.; et al. Uranium dynamics and developmental sensitivity in rat kidney. J. Appl. Toxicol. 2013, 33, 685–694. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Kitahara, K.; Suzuki, K.; Blyth, B.J.; Suya, N.; Konishi, T.; Terada, Y.; Shimada, Y. Cellular localization of uranium in the renal proximal tubules during acute renal uranium toxicity. J. Appl. Toxicol. 2015, 35, 1594–1600. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Numako, C.; Kitahara, K.; Yoshida, T.; Oikawa, M.; Terada, Y.; Kokubo, T.; Shimada, Y. Phosphorus Localization and Its Involvement in the Formation of Concentrated Uranium in the Renal Proximal Tubules of Rats Exposed to Uranyl Acetate. Int. J. Mol. Sci. 2019, 20, 4677. [Google Scholar] [CrossRef]
- Travis, E.L.; Harley, R.A.; Fenn, J.O.; Klobukowski, C.J.; Hargrove, H.B. Pathologic changes in the lung following single and multi-fraction irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 475–490. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Koukourakis, M.I. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Williams, J.P.; Hernady, E.; Reed, C.; Fenton, B.; Love, T.; Finkelstein, J.N.; Johnston, C.J. A Potential Biomarker for Predicting the Risk of Radiation-Induced Fibrosis in the Lung. Radiat. Res. 2018, 190, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.L.; Barkauskas, C.E.; Chapman, H.A.; Epstein, J.A.; Jain, R.; Hsia, C.C.; Niklason, L.; Calle, E.; Le, A.; Randell, S.H.; et al. Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 2014, 15, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.S.; Johnston, C.J.; Groves, A.M.; DeDiego, M.L.; St Martin, J.; Reed, C.; Hernady, E.; Miller, J.N.; Love, T.; Finkelstein, J.N.; et al. Examining the Effects of External or Internal Radiation Exposure of Juvenile Mice on Late Morbidity after Infection with Influenza A. Radiat. Res. 2015, 184, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, P.K.; Reid, L. New observations of rat airway epithelium: A quantitative and electron microscopic study. J. Anat. 1975, 120, 295–320. [Google Scholar]
- Dormans, J.A. The ultrastructure of various cell types in the lung of the rat: A survey. Exp. Pathol. 1983, 24, 15–33. [Google Scholar] [CrossRef]
- Mercer, R.R.; Russell, M.L.; Roggli, V.L.; Crapo, J.D. Cell number and distribution in human and rat airways. Am. J. Respir. Cell Mol. Biol. 1994, 10, 613–624. [Google Scholar] [CrossRef]
- Houghton, A.M.; Quintero, P.A.; Perkins, D.L.; Kobayashi, D.K.; Kelley, D.G.; Marconcini, L.A.; Mecham, R.P.; Senior, R.M.; Shapiro, S.D. Elastin fragments drive disease progression in a murine model of emphysema. J. Clin. Investig. 2006, 116, 753–759. [Google Scholar] [CrossRef]
- Hahn, F.F.; Scott, B.; Lundgren, D.L. Comparative stochastic effects of alpha, beta or x-irradiation of the lung of rats. Health Phys. 2010, 99, 363–366. [Google Scholar] [CrossRef]
- Liebow, A.A.; Warren, S.; DeCoursey, E. Pathology of atomic bomb casualties. Am. J. Pathol. 1949, 25, 853–1027. [Google Scholar]
- Lundgren, D.L.; Damon, E.G.; Diel, J.H.; Hahn, F.F. The deposition, distribution and retention of inhaled 239PuO2 in the lungs of rats with pulmonary emphysema. Health Phys. 1981, 40, 231–235. [Google Scholar] [PubMed]
- Pasquali-Ronchetti, I.; Baccarani-Contri, M. Elastic fiber during development and aging. Microsc. Res. Tech. 1997, 38, 428–435. [Google Scholar] [CrossRef]
- Bailey, A.J. Molecular mechanisms of ageing in connective tissues. Mech. Ageing. Dev. 2001, 122, 735–755. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Abrams, W.R.; Mecham, R. Extracellular matrix 4: The elastic fiber. FASEB J. 1993, 7, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Coggle, J.E.; Lambert, B.E.; Moores, S.R. Radiation effects in the lung. Environ. Health Perspect. 1986, 70, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.F.; Kim, Y.T.; Molteni, A.; Ts’ao, C.; Hinz, J.M. Pentoxifylline does not spare acute radiation reactions in rat lung and skin. Radiat. Res. 1992, 129, 107–111. [Google Scholar] [CrossRef]
- ICRP. The 2007 Recommendation of the International Commission in Radiological Protection; ICRP Pubrication 103; Annals of the ICRP 37 (2–4), Paragrah 78 and Table A.3.4.; ICRP: Ottawa, ON, Canada, 2007; p. 167. [Google Scholar]
- Cook, J.C.; West, H.J.; Kraft, J.W. The treatment of lung cancer by split-dose irradiation. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1968, 103, 772–777. [Google Scholar] [CrossRef][Green Version]
- Van den Brenk, H.A.S. Radiation effects on the pulmonary system. In Pathology of Irradiation; Berdjis, C.C., Ed.; Williams and Wilkin: Baltimore, MD, USA, 1971; pp. 569–591. [Google Scholar]
- Tomonaga, M. The Atomic Bombings of Hiroshima and Nagasaki: A Summary of the Human Consequences, 1945–2018, and Lessons for Homo sapiens to End the Nuclear Weapon Age. J. Peace Nucl. Disarm. 2019. [Google Scholar] [CrossRef]
- Shmatov, M.L. Potential to raise the efficiency of neutron and neutrons-photon therapy using metal nonradioactive nanoparticles. Phys. Part. Nucl. Lett. 2016, 13, 514–520. [Google Scholar] [CrossRef]
- Kim, K.; Seol, S.; Kim, T.; Kim, H.; Chung, H.; Kim, R.; Ye, J. Enhanced proton treatment in mouse tumors trough proton irradiated nanoradiator effects on metallic nanoparticles. Phys. Med. Biol. 2012, 57, 8309–8323. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.; Bhattaray, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishman, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Schuemann, J.; Berbeco, R.; Chithrani, B.D.; Cho, S.; Kumar, R.; McMahon, S.; Sridhar, S.; Krishnan, S. Roadmap to clinical use of gold nanoparticles for radiosensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Taggart1, L.; McMahon, S.J.; Currell, F.J.; Prise, K.M.; Butterworth, K.T. The role of mitochondrial function in gold nanoparticle mediated radiosensitisation. Cancer Nanotechnol. 2014, 5, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Shmatov, M.L. Importance of electric fields from ionized nanoparticles for radiation therapy. Phys. Part. Nucl. Lett. 2017, 14, 533–536. [Google Scholar] [CrossRef]
- Fuss, M.C.; Boscolo, D.; Durante, M.; Scifoni, E.; Krämer, M. Systematic quantification of nanoscopic dose enhancement of gold nanoparticles in ion beams. Phys. Med. Biol. 2020. [Google Scholar] [CrossRef]
| Group of Experiment | 56Mn(1×) | 56Mn(2×) | 56Mn(4×) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | A0 (kBq/g) | D (Gy) | A0 (kBq/g) | D (Gy) | A0 (kBq/g) | D (Gy) | ||||||||||||
| Liver | 4.14 | ± | 0.35 | 0.015 | ± | 0.001 | 1.40 | ± | 0.33 | 0.005 | ± | 0.001 | 3.00 | ± | 0.70 | 0.011 | ± | 0.003 |
| Heart | 5.46 | ± | 0.60 | 0.016 | ± | 0.021 | 2.00 | ± | 0.30 | 0.006 | ± | 0.001 | 4.50 | ± | 0.94 | 0.014 | ± | 0.004 |
| Kidney | 3.97 | ± | 0.48 | 0.013 | ± | 0.002 | 2.30 | ± | 0.51 | 0.008 | ± | 0.002 | 3.60 | ± | 0.60 | 0.012 | ± | 0.003 |
| Tongue | 45.0 | ± | 5.4 | 0.069 | ± | 0.011 | 12.0 | ± | 2.8 | 0.018 | ± | 0.005 | 28.0 | ± | 5.0 | 0.043 | ± | 0.012 |
| Lungs | 71.8 | ± | 9.3 | 0.100 | ± | 0.014 | 37 | ± | 7 | 0.051 | ± | 0.011 | 81 | ± | 13 | 0.110 | ± | 0.023 |
| Esophagus | 25.5 | ± | 3.6 | 0.050 | ± | 0.009 | 6.4 | ± | 1.6 | 0.012 | ± | 0.003 | 16 | ± | 3.3 | 0.030 | ± | 0.007 |
| Stomach | 148 | ± | 16 | 0.24 | ± | 0.03 | 140 | ± | 28 | 0.22 | ± | 0.06 | 210 | ± | 47 | 0.33 | ± | 0.08 |
| small intestine | 811 | ± | 93 | 1.33 | ± | 0.17 | 350 | ± | 79 | 0.58 | ± | 0.14 | 890 | ± | 210 | 1.48 | ± | 0.37 |
| Large intestine | 1011 | ± | 100 | 1.65 | ± | 0.18 | 410 | ± | 87 | 0.69 | ± | 0.17 | 1160 | ± | 270 | 1.90 | ± | 0.47 |
| Trachea | 5.79 | ± | 0.75 | 0.014 | ± | 0.002 | 3.90 | ± | 0.77 | 0.010 | ± | 0.002 | 6.50 | ± | 1.40 | 0.016 | ± | 0.004 |
| Eyes | 13.2 | ± | 1.7 | 0.021 | ± | 0.003 | 19.0 | ± | 4.4 | 0.031 | ± | 0.008 | 24.0 | ± | 5.3 | 0.004 | ± | 0.010 |
| Skin | 40.6 | ± | 4.9 | 0.076 | ± | 0.010 | 45.0 | ± | 8.2 | 0.086 | ± | 0.020 | 49.0 | ± | 9.0 | 0.095 | ± | 0.020 |
| Whole body | 83.4 | ± | 11.0 | 0.150 | ± | 0.025 | 51.0 | ± | 11.0 | 0.091 | ± | 0.026 | 77.0 | ± | 15.0 | 0.140 | ± | 0.030 |
| 56Mn(1×); Initial total activity of 100 mg 56MnO2 powder sprayed was 2.74 × 108 Bq [5]. | ||||||||||||||||||
| 56Mn(2×); Initial total activity of 100 mg 56MnO2 powder sprayed was 5.48 × 108 Bq. | ||||||||||||||||||
| 56Mn(4×); Initial total activity of 100 mg 56MnO2 powder sprayed was 1.10 × 109 Bq. | ||||||||||||||||||
| Group | Hour6 | Day3 | Day14 | Month2 | Month6 | Group | Month8 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemorrhage | 56Mn(4×) | 3a) | (+~++)b) | 3 | (++~+++) | 2 | (+) | 3 | (+~++) | 4 | (+~+++) | 56Mn(1×) | 6 | (++~+++) |
| Emphysema | 2 | (++~+++) | 2 | (++) | 2 | (+~++) | 3 | (+~++) | 4 | (+~++) | 6 | (++~+++) | ||
| Inflammation | 3 | (+~+++) | 3 | (++) | 2 | (+~++) | 3 | (+~+++) | 4 | (++~+++) | 6 | (++~+++) | ||
| Atelectasis | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 2 | (++~+++) | 3 | (++~+++) | ||
| Pneumonia | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (+) | 0 | (-) | ||
| Granuloma | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (+) | 0 | (-) | ||
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 4) | (n = 6) | |||||||||
| Hemorrhage | 56Mn(2×) | 3 | (+~++) | 3 | (++~+++) | 2 | (+~++) | 3 | (++) | 2 | (+~++) | Mn | 1 | (+) |
| Emphysema | 2 | (+) | 3 | (+~++) | 3 | (+~+++) | 3 | (+~++) | 2 | (+~++) | stable | 1 | (+++) | |
| Inflammation | 3 | (+~++) | 3 | (++~+++) | 3 | (+~+++) | 3 | (++) | 2 | (++~+++) | 1 | (+) | ||
| Atelectasis | 0 | (-) | 1 | (++) | 0 | (-) | 0 | (-) | 1 | (+) | 1 | (-) | ||
| Pneumonia | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (+) | 0 | (-) | ||
| Granuloma | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | ||
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 4) | |||||||||
| Hemorrhage | γ 2.0 Gy | 3 | (+~++) | 0 | (-) | 1 | (+) | 1 | (+) | 1 | (+) | γ 2.0Gy | 1 | (++) |
| Emphysema | 2 | (+~++) | 2 | (+) | 0 | (-) | 1 | (+) | 0 | (-) | 2 | (+) | ||
| Inflammation | 3 | (+) | 1 | (+) | 1 | (+) | 1 | (+) | 1 | (+) | 2 | (+~+++) | ||
| Atelectasis | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (+) | ||
| Pneumonia | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | ||
| Granuloma | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | ||
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 4) | (n = 4) | |||||||||
| Hemorrhage | Control | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | Control | 0 | (-) |
| Emphysema | 0 | (-) | 1 | (+) | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (+) | ||
| Inflammation | 1 | (+) | 1 | (+) | 0 | (-) | 1 | (+) | 0 | (-) | 0 | (-) | ||
| Atelectasis | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 1 | (+) | ||
| Pneumonia | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | ||
| Granuloma | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | 0 | (-) | ||
| (n = 3) | (n = 3) | (n = 3) | (n = 3) | (n = 4) | (n = 5) | |||||||||
| Distance from Center (μm) | 56Mn Initial Dose Rate (Gy/h) | Accumulated Dose of 6 h (Gy) | Accumulated Dose of ∞ (Gy) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diameter (μm) | Diameter (μm) | Diameter (μm) | |||||||
| 3 | 5 | 10 | 3 | 5 | 10 | 3 | 5 | 10 | |
| 1.5 | 1.67 | 4.98 | 6.22 | ||||||
| 2 | 7.21 × 10−1 | 2.15 | 2.68 | ||||||
| 2.5 | 4.38 × 10−1 | 2.70 | 1.30 | 8.05 | 1.63 | 10.05 | |||
| 3 | 2.91 × 10−1 | 1.52 | 8.67 × 10−1 | 4.52 | 1.08 | 5.64 | |||
| 5 | 9.99 × 10−2 | 4.72 × 10−1 | 5.19 | 2.98 × 10−1 | 1.41 | 15.5 | 3.72 × 10−1 | 1.76 | 19.3 |
| 10 | 2.41 × 10−2 | 1.11 × 10−1 | 9.07 × 10−1 | 7.19 × 10−2 | 3.31 × 10−1 | 2.70 | 8.97 × 10−2 | 4.14 × 10−1 | 3.37 |
| 50 | 8.94 × 10−4 | 4.12 × 10−3 | 3.27 × 10−2 | 2.67 × 10−3 | 1.23 × 10−2 | 9.74 × 10−2 | 3.33 × 10−3 | 1.53 × 10−2 | 1.22 × 10−1 |
| 100 | 2.15 × 10−4 | 9.92 × 10−4 | 7.88 × 10−3 | 6.40 × 10−4 | 2.96 × 10−3 | 2.35 × 10−2 | 8.00 × 10−4 | 3.69 × 10−3 | 2.93 × 10−2 |
| 500 | 7.43 × 10−6 | 3.43 × 10−5 | 2.73 × 10−4 | 2.21 × 10−5 | 1.02 × 10−4 | 8.14 × 10−4 | 2.77 × 10−5 | 1.27 × 10−4 | 1.02 × 10−3 |
| 1000 | 1.60 × 10−6 | 7.38 × 10−6 | 5.87 × 10−5 | 4.76 × 10−6 | 2.20 × 10−5 | 1.75 × 10−4 | 5.95 × 10−6 | 2.74 × 10−5 | 2.18 × 10−4 |
| 2000 | 2.97 × 10−7 | 1.38 × 10−6 | 1.10 × 10−5 | 8.85 × 10−7 | 4.10 × 10−6 | 3.27 × 10−5 | 1.11 × 10−6 | 5.12 × 10−6 | 4.08 × 10−5 |
| 3000 | 1.03 × 10−7 | 4.77 × 10−7 | 3.80 × 10−6 | 3.07 × 10−7 | 1.42 × 10−6 | 1.13 × 10−5 | 3.84 × 10−7 | 1.78 × 10−6 | 1.42 × 10−5 |
| 5000 | 2.61 × 10−8 | 1.21 × 10−7 | 9.67 × 10−7 | 7.78 × 10−8 | 3.61 × 10−7 | 2.88 × 10−6 | 9.72 × 10−8 | 4.51 × 10−7 | 3.60 × 10−6 |
| 8000 | 5.17 × 10−9 | 2.39 × 10−8 | 1.90 × 10−7 | 1.54 × 10−8 | 7.12 × 10−8 | 5.67 × 10−7 | 1.92 × 10−8 | 8.90 × 10−8 | 7.08 × 10−7 |
| 10,000 | 1.47 × 10−9 | 6.73 × 10−9 | 5.38 × 10−8 | 4.39 × 10−9 | 2.01 × 10−8 | 1.60 × 10−7 | 5.48 × 10−9 | 2.51 × 10−8 | 2.00 × 10−7 |
| 12,000 | 2.28 × 10−10 | 1.03 × 10−9 | 8.21 × 10−9 | 6.79 × 10−10 | 3.08 × 10−9 | 2.45 × 10−8 | 8.48 × 10−10 | 3.84 × 10−9 | 3.05 × 10−8 |
| 13,000 | 4.57 × 10−11 | 2.11 × 10−10 | 1.65 × 10−9 | 1.36 × 10−10 | 6.29 × 10−10 | 4.91 × 10−9 | 1.70 × 10−10 | 7.85 × 10−10 | 6.13 × 10−9 |
| 14,000 | 2.17 × 10−12 | 1.00 × 10−11 | 7.39 × 10−11 | 6.47 × 10−12 | 2.98 × 10−11 | 2.20 × 10−10 | 8.07 × 10−12 | 3.72 × 10−11 | 2.75 × 10−10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shichijo, K.; Takatsuji, T.; Abishev, Z.; Uzbekov, D.; Chaizhunusova, N.; Shabdarbaeva, D.; Niino, D.; Kurisu, M.; Takahashi, Y.; Stepanenko, V.; et al. Impact of Local High Doses of Radiation by Neutron Activated Mn Dioxide Powder in Rat Lungs: Protracted Pathologic Damage Initiated by Internal Exposure. Biomedicines 2020, 8, 171. https://doi.org/10.3390/biomedicines8060171
Shichijo K, Takatsuji T, Abishev Z, Uzbekov D, Chaizhunusova N, Shabdarbaeva D, Niino D, Kurisu M, Takahashi Y, Stepanenko V, et al. Impact of Local High Doses of Radiation by Neutron Activated Mn Dioxide Powder in Rat Lungs: Protracted Pathologic Damage Initiated by Internal Exposure. Biomedicines. 2020; 8(6):171. https://doi.org/10.3390/biomedicines8060171
Chicago/Turabian StyleShichijo, Kazuko, Toshihiro Takatsuji, Zhaslan Abishev, Darkhan Uzbekov, Nailya Chaizhunusova, Dariya Shabdarbaeva, Daisuke Niino, Minako Kurisu, Yoshio Takahashi, Valeriy Stepanenko, and et al. 2020. "Impact of Local High Doses of Radiation by Neutron Activated Mn Dioxide Powder in Rat Lungs: Protracted Pathologic Damage Initiated by Internal Exposure" Biomedicines 8, no. 6: 171. https://doi.org/10.3390/biomedicines8060171
APA StyleShichijo, K., Takatsuji, T., Abishev, Z., Uzbekov, D., Chaizhunusova, N., Shabdarbaeva, D., Niino, D., Kurisu, M., Takahashi, Y., Stepanenko, V., Azhimkhanov, A., & Hoshi, M. (2020). Impact of Local High Doses of Radiation by Neutron Activated Mn Dioxide Powder in Rat Lungs: Protracted Pathologic Damage Initiated by Internal Exposure. Biomedicines, 8(6), 171. https://doi.org/10.3390/biomedicines8060171





