Outer Nuclear Layer Damage for Detection of Early Retinal Toxicity of Hydroxychloroquine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Individuals
2.2. Clinical Assessment
2.3. Optical Coherence Tomography Procedure
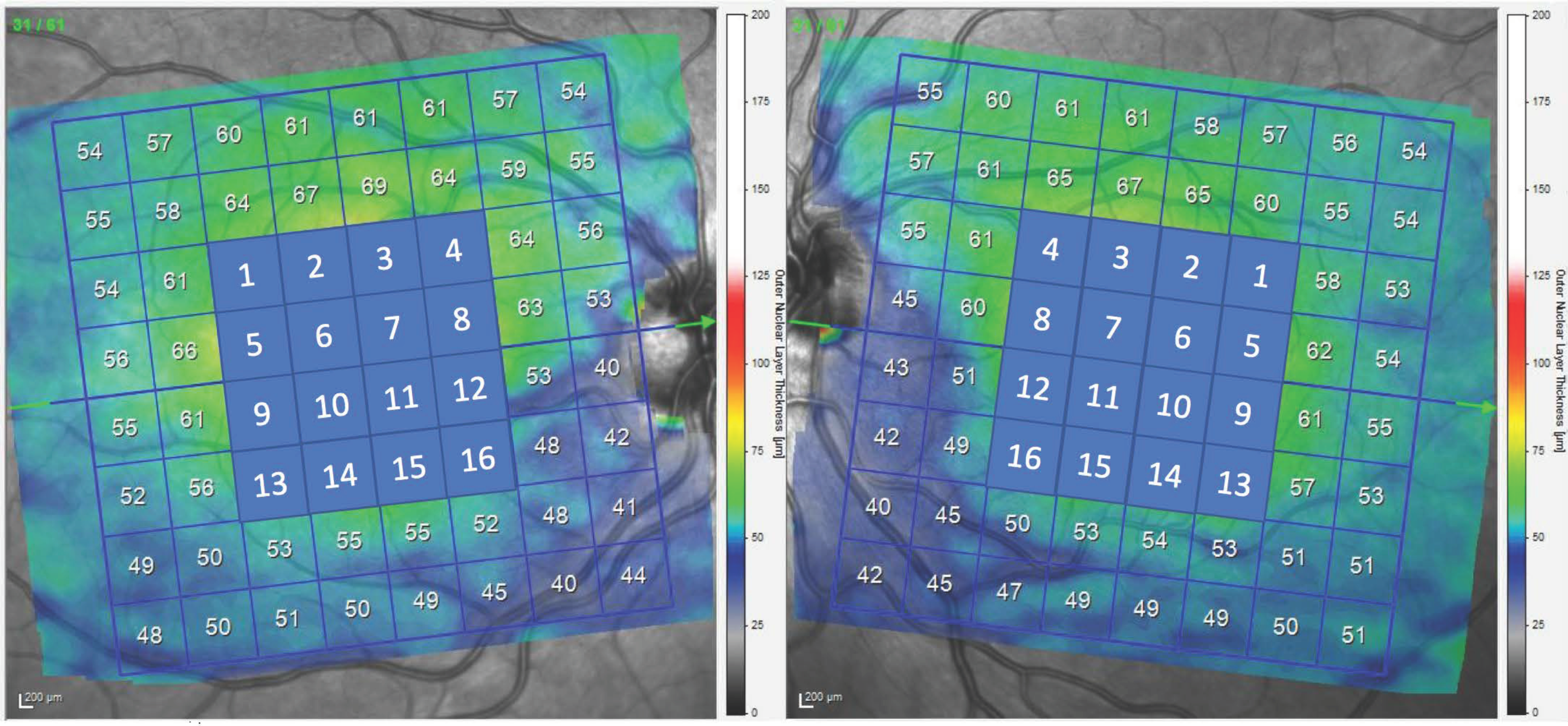
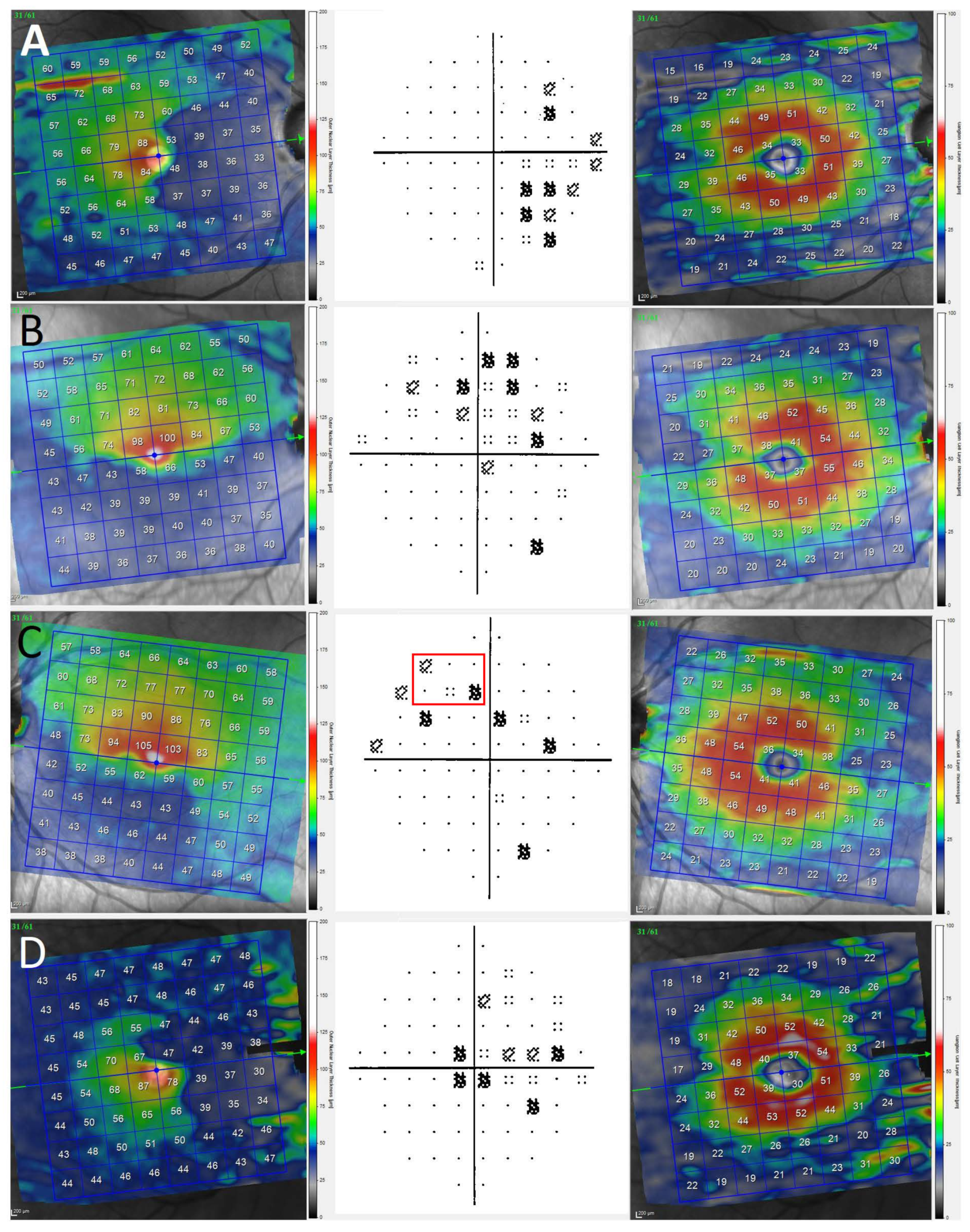
Methodology for Measurement of ONL and GCL
2.4. Visual Field
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Lyons, J.S.; Mieler, W.F.; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011, 118, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Carr, R.E.; Easterbrook, M.; Farjo, A.A.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: A report by the American Academy of Ophthalmology. Ophthalmology 2002, 109, 1377–13821. [Google Scholar] [CrossRef]
- Hobbs, H.E.; Sorsby, A.; Freedman, A. Retinopathy following chloroquine therapy. Lancet 1959, 2, 478–480. [Google Scholar] [CrossRef]
- Bernstein, H.N. Ocular safety of hydroxychloroquine. Ann. Ophthalmol. 1991, 23, 292–296. [Google Scholar] [PubMed]
- Easterbrook, M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can. J. Ophthalmol. 1990, 25, 249–251. [Google Scholar]
- Easterbrook, M. Ocular effects and safety of antimalarial agents. Am. J. Med. 1988, 85 (Suppl. 4A), 23–29. [Google Scholar] [CrossRef]
- Weiner, A.; Sandberg, M.A.; Gaudio, A.R.; Kini, M.M.; Berson, E.L. Hydroxychloroquine retinopathy. Am. J. Ophthalmol. 1991, 112, 528–534. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F.; American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef] [Green Version]
- Korthagen, N.M.; Bastiaans, J.; van Meurs, J.C.; van Bilsen, K.; van Hagen, P.M.; Dik, W.A. Chloroquine and hydroxychloroquine increase retinal pigment epithelial layer permeability. J. Biochem. Mol. Toxicol. 2015, 29, 299–304. [Google Scholar] [CrossRef]
- Lee, M.G.; Kim, S.J.; Ham, D.I.; Kang, S.W.; Kee, C.; Lee, J.; Cha, H.S.; Koh, E.M. Macular retinal ganglion cell inner plexiform layer thickness in patients on hydroxychloroquine therapy. Investig. Ophthalmol. Vis. Sci. 2014, 56, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Kan, E.; Yakar, K.; Demirag, M.D.; Gok, M. Macular ganglion cell-inner plexiform layer thickness for detection of early retinal toxicity of hydroxychloroquine. Int. Ophthalmol. 2018, 38, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- de Sisternes, L.; Hu, J.; Rubin, D.L.; Marmor, M.F. Localization of damage in progressive hydroxychloroquine retinopathy on and off the drug: Inner versus outer retina, parafovea versus peripheral fovea. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3415–3426. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Ryu, S.J.; Lim, H.W.; Lee, B.R. Retina. TOXIC EFFECTS OF HYDROXYCHLOROQUINE ON THE CHOROID: Evidence from Multimodal Imaging. Retina 2019, 39, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Akıdan, M.; Gözkaya, O.; Erol, M.K.; Cengiz, A.; Çay, H.F. Optical coherence tomography angiography for screening of hydroxychloroquine-induced retinal alterations. Graefes. Arch. Clin. Exp. Ophthalmol. 2018, 256, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Ryu, S.J.; Joung, J.Y.; Lee, B.R. Choroidal Thinning Associated with Hydroxychloroquine Retinopathy. Am. J. Ophthalmol. 2017, 183, 56–64. [Google Scholar] [CrossRef]
- Hanaoka, H.; Iida, H.; Kiyokawa, T.; Takakuwa, Y.; Kawahata, K. Hydroxychloroquine Improves the Disease Activity and Allows the Reduction of the Corticosteroid Dose Regardless of Background Treatment in Japanese Patients with Systemic Lupus Erythematosus. Intern. Med. 2019, 58, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.Y.; Lin, Y.S.; Cheng, T.T.; Syu, Y.J.; Lin, M.S.; Lin, H.F.; Su, Y.J.; Chen, Y.C.; Chen, J.F.; Chen, T.H. Adherence to hydroxychloroquine improves long-term survival of patients with systemic lupus erythematosus. Rheumatology 2018, 57, 1743–1751. [Google Scholar] [CrossRef] [Green Version]
- Ponticelli, C.; Moroni, G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin. Drug Saf. 2017, 16, 411–419. [Google Scholar] [CrossRef]
- Rempenault, C.; Combe, B.; Barnetche, T.; Gaujoux-Viala, C.; Lukas, C.; Morel, J.; Hua, C. Clinical and Structural Efficacy of Hydroxychloroquine in Rheumatoid Arthritis: A Systematic Review. Arthritis Care Res. 2020, 72, 36–40. [Google Scholar] [CrossRef]
- Yamashita, T.; Sakamoto, T.; Kakiuchi, N.; Tanaka, M.; Kii, Y.; Nakao, K. Posterior pole asymmetry analyses of retinal thickness of upper and lower sectors and their association with peak retinal nerve fiber layer thickness in healthy young eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5673–5678. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Tanaka, M.; Kii, Y.; Nakao, K.; Sakamoto, T. Association between retinal thickness of 64 sectors in posterior pole determined by optical coherence tomography and axial length and body height. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7478–7482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, N.; Chen, J.; Wei, H.; Jiang, S.M.; Chen, X.M. A new strategy to interpret OCT posterior pole asymmetry analysis for glaucoma diagnosis. Int. J. Ophthalmol. 2017, 10, 1857–1863. [Google Scholar] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Marziani, E.; Pomati, S.; Ramolfo, P.; Cigada, M.; Giani, A.; Mariani, C.; Staurenghi, G. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral- domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5953–5958. [Google Scholar] [CrossRef]
- Eraslan, M.; Cerman, E.; Yildiz Balci, S.; Celiker, H.; Sahin, O.; Temel, A.; Suer, D.; Tuncer Elmaci, N. The choroid and lamina cribrosa is affected in patients with Parkinson’s disease: Enhanced depth imaging optical coherence tomography study. Acta Ophthalmol. 2016, 94, e68–e75. [Google Scholar] [CrossRef]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017, 2, e93621. [Google Scholar] [CrossRef]
- Reiner, A.; Fitzgerald, M.E.C.; Del Mar, N.; Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 2018, 64, 96–130. [Google Scholar] [CrossRef]
| HCQ Retinopathy Eyes (n = 42) | Control Eyes (n = 72) | p | |
|---|---|---|---|
| Age (years) | 63.1 (14.9) | 53.8 (13.5) | 0.118 |
| Female eyes (%) | 38 (90.4) | 66 (91.7) | 0.906 |
| Spherical equivalent (diopters) | 0.55 (0.39) | 0.67 (0.44) | 0.144 |
| IOP | 13.9 (2.4) | 13.4 (2.1) | 0.361 |
| HCQR Eyes (n = 42) | Control Eyes (n = 72) | p | |
|---|---|---|---|
| ONL 1 | 62.5 (6.7) | 62.9 (7.3) | 0.473 |
| ONL 2 | 65.3 (9.6) | 67.0 (8.2) | 0.173 |
| ONL 3 | 63.9 (11.5) | 66.4 (8.2) | 0.177 |
| ONL 4 | 60.4 (9.5) | 62.8 (8.2) | 0.111 |
| ONL 5 | 70.6 (8.5) | 70.7 (8.9) | 0.920 |
| ONL 6 | 78.7 (13.4) | 78.5 (11.7) | 0.947 |
| ONL 7 | 73.9 (15.6) | 75.2 (14.3) | 0.507 |
| ONL 8 | 63.7 (14.1) | 75.2 (14.7) | 0.080 |
| ONL 9 | 67.2 (12.0) | 68.3 (8.8) | 0.664 |
| ONL 10 | 76.1 (13.9) | 79.7 (12.6) | 0.357 |
| ONL 11 | 71.2 (17.5) | 80.2 (13.3) | 0.009 * |
| ONL 12 | 61.5 (14.6) | 68.5 (12.1) | 0.017 * |
| ONL 13 | 56.9 (8.3) | 59.8 (6.7) | 0.279 |
| ONL 14 | 58.5 (11.4) | 63.2 (8.3) | 0.101 |
| ONL 15 | 66.5 (6.8) | 62.4 (9.5) | 0.044 * |
| ONL 16 | 53.6 (10.3) | 58.5 (8.8) | 0.014 * |
| Superior ONL | 539.3 (73.3) | 551.2 (65.3) | 0.161 |
| Inferior ONL | 511.5 (96.4) | 540.5 (68.3) | 0.104 |
| Temporal ONL | 535.9 (58.2) | 550.1 (59.5) | 0.264 |
| Nasal ONL | 514.9 (93.1) | 541.6 (71.7) | 0.032 * |
| Total ONL | 1050.8 (113.8) | 1091.7 (117.4) | 0.072 |
| HCQR Eyes (n = 42) | Control Eyes (n = 72) | p | |
|---|---|---|---|
| GC 1 | 41.9 (4.7) | 43.4 (5.1) | 0.121 |
| GC 2 | 49.3 (5.3) | 50.7 (5.5) | 0.176 |
| GC 3 | 49.9 (5.5) | 50.5 (5.6) | 0.478 |
| GC 4 | 43.4 (5.3) | 43.5 (5.0) | 0.889 |
| GC 5 | 42.2 (7.5) | 45.9 (6.1) | 0.113 |
| GC 6 | 32.9 (5.6) | 33.3 (6.1) | 0.730 |
| GC 7 | 34.7 (6.1) | 35.5 (6.3) | 0.398 |
| GC 8 | 51.3 (6.5) | 52.7 (6.1) | 0.241 |
| GC 9 | 47.3 (7.0) | 49.9 (6.4) | 0.157 |
| GC 10 | 35.1 (6.3) | 35.8 (6.2) | 0.545 |
| GC 11 | 34.9 (6.4) | 35.2 (6.4) | 0.754 |
| GC 12 | 51.5 (6.4) | 53.3 (5.9) | 0.155 |
| GC 13 | 42.3 (4.7) | 43.8 (4.6) | 0.141 |
| GC 14 | 48.9 (4.7) | 50.3 (4.7) | 0.101 |
| GC 15 | 48.5 (5.0) | 50.1 (4.8) | 0.135 |
| GC 16 | 43.3 (5.2) | 44.3 (4.6) | 0.368 |
| Superior GC | 345.6 (39.9) | 355.4 (37.3) | 0.231 |
| Inferior GC | 351.8 (36.3) | 362.6 (36.3) | 0.194 |
| Temporal GC | 339.9 (37.8) | 352.9 (36.3) | 0.188 |
| Nasal GC | 357.5 (38.8) | 365.1 (36.5) | 0.303 |
| Total GC | 697.4 (74.9) | 718.1 (71.5) | 0.180 |
| Superior ONL | Inferior ONL | Temporal ONL | Nasal ONL | p | |
|---|---|---|---|---|---|
| Inferior D | 512.4 (28.9) | 620.7 (75.9) | 532.7 (48.1) | 600.4 (12.4) | 0.347 |
| Superior D | 556.5 (98.2) | 474.6 (92.7) | 541.7 (66.5) | 516.4 (75.3) | 0.029 * |
| Nasal D | 618.0 (46.7) | 553.0 (38.2) | 604.5 (58.7) | 566.5 (26.2) | 0.125 |
| Temporal D | 527.2 (57.3) | 469.2 (51.4) | 550.4 (47.6) | 446.0 (49.8) | 0.008 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado, A.; López-de-Eguileta, A.; Fonseca, S.; Muñoz, P.; Demetrio, R.; Gordo-Vega, M.A.; Cerveró, A. Outer Nuclear Layer Damage for Detection of Early Retinal Toxicity of Hydroxychloroquine. Biomedicines 2020, 8, 54. https://doi.org/10.3390/biomedicines8030054
Casado A, López-de-Eguileta A, Fonseca S, Muñoz P, Demetrio R, Gordo-Vega MA, Cerveró A. Outer Nuclear Layer Damage for Detection of Early Retinal Toxicity of Hydroxychloroquine. Biomedicines. 2020; 8(3):54. https://doi.org/10.3390/biomedicines8030054
Chicago/Turabian StyleCasado, Alfonso, Alicia López-de-Eguileta, Soraya Fonseca, Pedro Muñoz, Rosalía Demetrio, Miguel A. Gordo-Vega, and Andrea Cerveró. 2020. "Outer Nuclear Layer Damage for Detection of Early Retinal Toxicity of Hydroxychloroquine" Biomedicines 8, no. 3: 54. https://doi.org/10.3390/biomedicines8030054
APA StyleCasado, A., López-de-Eguileta, A., Fonseca, S., Muñoz, P., Demetrio, R., Gordo-Vega, M. A., & Cerveró, A. (2020). Outer Nuclear Layer Damage for Detection of Early Retinal Toxicity of Hydroxychloroquine. Biomedicines, 8(3), 54. https://doi.org/10.3390/biomedicines8030054






