Cytotoxicity of Different Nano Composite Resins on Human Gingival and Periodontal Ligament Fibroblast Cell Lines: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Material Samples
2.2. Cell Culture
2.3. Cytotoxicity Test
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ilie, N.; Rencz, A.; Hickel, R. Investigations towards nano-hybrid resin-based composites. Clin. Oral Investig. 2013, 17, 185–193. [Google Scholar] [CrossRef]
- Al-Ahdal, K.; Silikas, N.; Watts, D.C. Development of viscoelastic stability of resin-composites incorporating novel matrices. Dent. Mater. 2015, 31, 1561–1566. [Google Scholar] [CrossRef]
- Putzeys, E.; De Nys, S.; Cokic, S.M.; Duca, R.C.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Long-term elution of monomers from resin-based dental composites. Dent. Mater. 2019, 35, 477–485. [Google Scholar] [CrossRef]
- Camargo, P.; Lagos, R.; Lekovic, V.; Wolinsky, L. Soft tissue root coverage as treatment for cervical abrasion and caries. Gen. Dent. 2001, 49, 299–304. [Google Scholar]
- Martins, T.M.; Bosco, A.F.; Nóbrega, F.J.; Nagata, M.J.; Garcia, V.G.; Fucini, S.E. Periodontal tissue response to coverage of root cavities restored with resin materials: A histomorphometric study in dogs. J. Periodontol. 2007, 78, 1075–1082. [Google Scholar] [CrossRef]
- McGuire, M.K. Soft tissue augmentation on previously restored root surfaces. Int. J. Periodontics Restor. Dent. 1996, 16, 570–581. [Google Scholar]
- Alkan, A.; Keskiner, I.; Yuzbasioglu, E. Connective tissue grafting on resin ionomer in localized gingival recession. J. Periodontol. 2006, 77, 1446–1451. [Google Scholar] [CrossRef]
- Cairo, F.; Pini-Prato, G.P. A technique to identify and reconstruct the cementoenamel junction level using combined periodontal and restorative treatment of gingival recession. A prospective clinical study. Int. J. Periodontics Restor. Dent. 2010, 30, 573–581. [Google Scholar]
- Santamaria, M.P.; da Silva Feitosa, D.; Nociti, F.H., Jr.; Casati, M.Z.; Sallum, A.W.; Sallum, E.A. Cervical restoration and the amount of soft tissue coverage achieved by coronally advanced flap: A 2-year follow-up randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 434–441. [Google Scholar] [CrossRef]
- Santos, V.R.; Lucchesi, J.A.; Cortelli, S.C.; Amaral, C.M.; Feres, M.; Duarte, P.M. Effects of glass ionomer and microfilled composite subgingival restorations on periodontal tissue and subgingival biofilm: A 6-month evaluation. J. Periodontol. 2007, 78, 1522–1528. [Google Scholar] [CrossRef]
- Zucchelli, G.; Testori, T.; De Sanctis, M. Clinical and anatomical factors limiting treatment outcomes of gingival recession: A new method to predetermine the line of root coverage. J. Periodontol. 2006, 77, 714–721. [Google Scholar] [CrossRef]
- Ababneh, K.T.; Al-Omari, M.; Alawneh, T.N.-E. The effect of dental restoration type and material on periodontal health. Oral Health Prev. Dent. 2011, 9, 395–403. [Google Scholar]
- Matthews, D.C.; Tabesh, M. Detection of localized tooth-related factors that predispose to periodontal infections. Periodontology 2000 2004, 34, 136–150. [Google Scholar] [CrossRef]
- Silness, J.; Røynstkand, T. Effects on dental health of spacing of teeth in anterior segments. J. Clin. Periodontol. 1984, 11, 387–398. [Google Scholar] [CrossRef]
- Paolantonio, M.; D’ercole, S.; Perinetti, G.; Tripodi, D.; Catamo, G.; Serra, E.; Bruè, C.; Piccolomini, R. Clinical and microbiological effects of different restorative materials on the periodontal tissues adjacent to subgingival class V restorations: 1-year results. J. Clin. Periodontol. 2004, 31, 200–207. [Google Scholar] [CrossRef]
- Willershausen, B.; Köttgen, C.; Ernst, C. The influence of restorative materials on marginal gingiva. Eur. J. Med. Res. 2001, 6, 433–439. [Google Scholar]
- Geurtsen, W. Biocompatibility of resin-modified filling materials. Crit. Rev. Oral Biol. Med. 2000, 11, 333–355. [Google Scholar] [CrossRef]
- Jandt, K.D.; Sigusch, B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009, 25, 1001–1006. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular toxicology of substances released from resin–based dental restorative materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef]
- Geurtsen, W. Substances released from dental resin composites and glass ionomer cements. Eur. J. Oral Sci. 1998, 106, 687–695. [Google Scholar] [CrossRef]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef]
- Hanks, C.T.; Anderson, M.; Craig, R.G. Cytotoxic effects of dental cements on two cell culture systems. J. Oral Pathol. Med. 1981, 10, 101–112. [Google Scholar] [CrossRef]
- Lee, S.Y.; Huang, H.M.; Lin, C.Y.; Shih, Y.H. Leached components from dental composites in oral simulating fluids and the resultant composite strengths. J. Oral Rehabil. 1998, 25, 575–588. [Google Scholar] [CrossRef]
- Urcan, E.; Haertel, U.; Styllou, M.; Hickel, R.; Scherthan, H.; Reichl, F.X. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent. Mater. 2010, 26, 51–58. [Google Scholar] [CrossRef]
- Reichl, F.X.; Esters, M.; Simon, S.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Folwaczny, M.; Glas, J.; Hickel, R. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch. Toxicol. 2006, 80, 370–377. [Google Scholar] [CrossRef]
- Kleinsasser, N.H.; Wallner, B.C.; Harréus, U.A.; Kleinjung, T.; Folwaczny, M.; Hickel, R.; Kehe, K.; Reichl, F.-X. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J. Dent. 2004, 32, 229–234. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Ahn, S.J.; Yang, H.C. Involvement of oxidative stress in mutagenicity and apoptosis caused by dental resin monomers in cell cultures. Dent. Mater. 2006, 22, 1086–1092. [Google Scholar] [CrossRef]
- Schmalz, G. Use of cell cultures for toxicity testing of dental materials—advantages and limitations. J. Dent. 1994, 22, S6–S11. [Google Scholar] [CrossRef]
- Schubert, A.; Ziegler, C.; Bernhard, A.; Burgers, R.; Miosge, N. Cytotoxic effects to mouse and human gingival fibroblasts of a nanohybrid ormocer versus dimethacrylate-based composites. Clin. Oral Investig. 2019, 23, 133–139. [Google Scholar] [CrossRef]
- Yang, Y.; Reichl, F.-X.; Shi, J.; He, X.; Hickel, R.; Högg, C. Cytotoxicity and DNA double-strand breaks in human gingival fibroblasts exposed to eluates of dental composites. Dent. Mater. 2018, 34, 201–208. [Google Scholar] [CrossRef]
- Cao, T.; Saw, T.Y.; Heng, B.C.; Liu, H.; Yap, A.U.; Ng, M.L. Comparison of different test models for the assessment of cytotoxicity of composite resins. J. Appl. Toxicol. 2005, 25, 101–108. [Google Scholar] [CrossRef]
- Saw, T.Y.; Cao, T.; Yap, A.U.; Lee Ng, M.M. Tooth slice organ culture and established cell line culture models for cytotoxicity assessment of dental materials. Toxicol Vitr. 2005, 19, 145–154. [Google Scholar] [CrossRef]
- Waerhaug, J. Effect of rough surfaces upon gingival tissue. J. Dent. Res. 1956, 35, 323–325. [Google Scholar] [CrossRef]
- Broadbent, J.M.; Williams, K.B.; Thomson, W.M.; Williams, S.M. Dental restorations: A risk factor for periodontal attachment loss? J. Clin. Periodontol. 2006, 33, 803–810. [Google Scholar] [CrossRef]
- Kostoryz, E.L.; Zhu, Q.; Zhao, H.; Glaros, A.G.; Eick, J.D. Assessment of cytotoxicity and DNA damage exhibited by siloranes and oxiranes in cultured mammalian cells. Mutat. Res. 2007, 634, 156–162. [Google Scholar] [CrossRef]
- van Dijken, J.W.; Sjöström, S.; Wing, K. Development of gingivitis around different types of composite resin. J. Clin. Periodontol. 1987, 14, 257–260. [Google Scholar] [CrossRef]
- Franz, A.; Konig, F.; Skolka, A.; Sperr, W.; Bauer, P.; Lucas, T.; Watts, D.C.; Schedle, A. Cytotoxicity of resin composites as a function of interface area. Dent. Mater. 2007, 23, 1438–1446. [Google Scholar] [CrossRef]
- De Melo, W.M.; Maximiano, W.M.A.; Antunes, A.A.; Beloti, M.M.; Rosa, A.L.; de Oliveira, P.T. Cytotoxicity testing of methyl and ethyl 2-cyanoacrylate using direct contact assay on osteoblast cell cultures. J. Oral Maxillofac. Surg. 2013, 71, 35–41. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef]
- Basak, S.C.; Grunwald, G.D.; Gute, B.D.; Balasubramanian, K.; Opitz, D. Use of Statistical and Neural Net Approaches in Predicting Toxicity of Chemicals. J. Chem. Inf. Comput. Sci. 2000, 40, 885–890. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Eric Hesselberth, P.; Scurria, M.A.; Daily, W.J.; Riss, T.L. A homogeneous assay to measure live and dead cells in the same sample by detecting different protease markers. Anal. Biochem. 2007, 366, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.S.; Balasubramanian, V. Effect of resin chemistry on depth of cure and cytotoxicity of dental resin composites. Mater. Sci. Eng. B 2014, 181, 33–38. [Google Scholar] [CrossRef]
- Darmani, H.; Al-Hiyasat, A.S.; Milhem, M.M. Cytotoxicity of dental composites and their leached components. Quintessence Int. 2007, 38, 789–795. [Google Scholar]
- Issa, Y.; Watts, D.C.; Brunton, P.A.; Waters, C.M.; Duxbury, A.J. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent. Mater. 2004, 20, 12–20. [Google Scholar] [CrossRef]
- Van Landuyt, K.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- ISO. Biological Evaluation of Medical Devices; ISO 10993-5-1999; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- Thonemann, B.; Schmalz, G.; Hiller, K.A.; Schweikl, H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater. 2002, 18, 318–323. [Google Scholar] [CrossRef]
- Koohpeima, F.; Mokhtari, M.J.; Doozandeh, M.; Jowkar, Z.; Yazdanshenas, F. Comparison of Cytotoxicity of New Nanohybrid Composite, Giomer, Glass Ionomer and Silver Reinforced Glass Ionomer using Human Gingival Fibroblast Cell Line. J. Clin. Pediatric Dent. 2017, 41, 368–373. [Google Scholar] [CrossRef]
- Schulz, S.D.; Ruppell, C.; Tomakidi, P.; Steinberg, T.; Reichl, F.X.; Hellwig, E.; Polydorou, O. Gene expression analysis of conventional and interactive human gingival cell systems exposed to dental composites. Dent. Mater. 2015, 31, 1321–1334. [Google Scholar] [CrossRef]
- Manojlovic, D.; Radisic, M.; Vasiljevic, T.; Zivkovic, S.; Lausevic, M.; Miletic, V. Monomer elution from nanohybrid and ormocer-based composites cured with different light sources. Dent. Mater. 2011, 27, 371–378. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Qualitative and quantitative characterization of monomers of uncured bulk-fill and conventional resin-composites using liquid chromatography/mass spectrometry. Dent. Mater. 2015, 31, 711–720. [Google Scholar] [CrossRef]
- Frauscher, K.E.; Ilie, N. Degree of conversion of nano-hybrid resin-based composites with novel and conventional matrix formulation. Clin. Oral Investig. 2013, 17, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Mielert, J.; Kleinheinz, J.; Dammaschke, T. Human oral cells’ response to different endodontic restorative materials: An in vitro study. Head Face Med. 2014, 10, 55. [Google Scholar] [CrossRef] [PubMed]
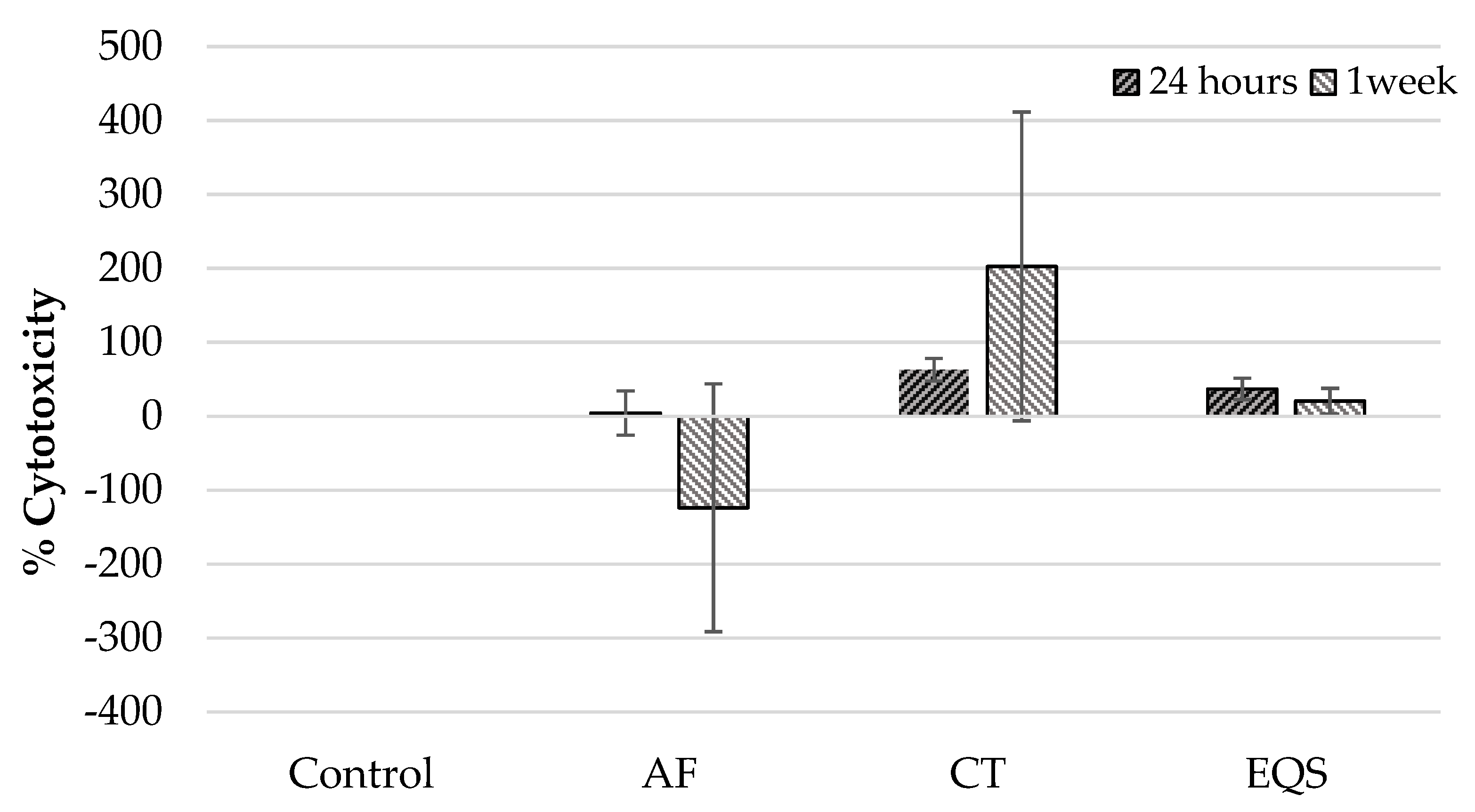

| Cell/Time | % Cytotoxicity Groups (n= 6) Median Min–Max (Mean ± SD) | Paired Comparisons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con a | AF b | CT c | EQS d | p* | p#(a,b) | p#(a–c) | p#(a–d) | p#(b,c) | p#(b–d) | p#(c,d) | |
| hGF/24 h | 0.00 0.00–0.00 (0.00 ± 0.00) | 20.95 −38.09–30.20 (4.53 ± 32.77) | 67.29 39.92–78.82 (62.91 ± 6.58) | 46.14 15.01–48.78 (37.12 ± 15.75) | 0.001 | 1.00 | 0.002 | 0.08 | 0.031 | 0.59 | 1.00 |
| hGF/one week | 0.00 0.00–0.00 (0.00 ± 0.00) | −69.44 −488.09–1.09 (123.77 ± 183.63) | 101.78 48.61–655.55 (202.67 ± 228.76) | 17.36 4.16–55.55 (20.69 ± 19.02) | 0.001 | 1.00 | 0.009 | 0.46 | 0.00 | 0.03 | 0.97 |
| hPDLF/24 h | 0.00 0.00–0.00 (0.00 ± 0.00) | −28.73 −50.00–−18.96 (-21.73 ± 10.66) | −22.91 −38.18–−1.56 (-21.23 ± 12.25) | −44.44 −123.63–−20.31 (-60.70 ± 40.76) | 0.000 | 0.01 | 0.17 | 0.001 | 1.00 | 1.00 | 0.63 |
| hPDLF/one week | 0.00 0.00–0.00 (0.00 ± 0.00) | 6.17 −1.76–12.29 (7.02 ± 4.06) | 31.02 13.27–37.90 (27.10 ± 9.88) | 2.86 −6.36–6.89 (1.97 ± 5.29) | 0.001 | 0.2 | 0.01 | 1.00 | 0.3 | 1.00 | 0.008 |
| Cell/Time | % Viability Groups (n= 6) Median Min–Max (Mean ±SD) | Paired Comparisons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con a | AF b | CT c | EQS d | p* | p#(a,b) | p#(a–c) | p#(a–d) | p#(b,c) | p#(b–d) | p#(c,d) | |
| hGF/ 24 h | 100 100–100 (100 ± 0.00) | 79.04 69.79–138.09 (95.46 ± 32.77) | 32.70 21.17–60.07 (37.06 ± 16.58) | 53.85 51.21–84.98 (62.87 ± 15.75) | 0.001 | 1.00 | 0.002 | 0.08 | 0.031 | 0.59 | 1.00 |
| hGF/ one week | 100 100–100 (100 ± 0.00) | 169.44 98.90–588.88 (223.77 ± 183.63) | −1.78 −555.55–51.38 (−102.67 ± 228.76) | 82.63 44.44–95.83 (79.30 ± 19.02) | 0.000 | 1.00 | 0.009 | 0.46 | 0.00 | 0.03 | 0.97 |
| hPDLF/ 24 h | 100 100–100 (100 ± 0.00) | 128.73 118.96–150.00 (130.62 ± 10.66) | 122.91 101.56–138.18 (121.23 ± 12.25) | 144.44 120.31–223.63 (160.70 ± 40.76) | 0.01 | 0.01 | 0.17 | 0.001 | 1.00 | 1.00 | 0.63 |
| hPDLF/ one week | 100 100–100 (100 ± 0.00) | 93.82 87.70–98.23 (92.97 ± 4.06) | 68.97 62.09–86.72 (72.89 ± 9.88) | 97.13 93.10–106.36 (98.02 ± 5.29) | 0.001 | 0.2 | 0.001 | 1.00 | 0.36 | 1.00 | 0.008 |
| Cells | CRs | Time Median Min–Max (Mean ± SD) | p * | |
|---|---|---|---|---|
| 24 h | One Week | |||
| Cytotoxicity of hGF% | Control | 0.00 0.00–0.00 (0.00 ± 0.00) | 0.00 0.00–0.00 (0.00 ± 0.00) | 1.00 |
| AF | 20.95 −38.09–30.20 (4.53 ± 32.77) | −69.44 −488.88–1.09 (−123.77 ± 183.63) | 0.11 | |
| CT | 67.29 39.92–78.82 (62.93 ± 16.58) | 101.78 48.61–655.55 (202.67 ± 228.76) | 0.04 | |
| EQS | 46.14 15.01–48.78 (37.12 ± 15.75) | 17.36 416–55.55 (20.69 ± 19.02) | 0.34 | |
| Cytotoxicity of hPDLF% | Control | 0.00 0.00–0.00 (0.00 ± 0.00) | 0.00 0.00–0.00 (0.00 ± 0.00) | 1.00 |
| AF | −28.73 −50.00–−18.96 (−21.13 ± 10.66) | 6.17 1.76–12.29 (7.02 ± 4.06) | 0.02 | |
| CT | −22.91 −38.18–−1.56 (−21.23 ± 12.25) | 31.02 13.27–37.90 (27.10 ± 9.88) | 0.02 | |
| EQS | −44.44 −123.63–−20.31 (−60.70 ± 40.76) | 2.86 −6.36–6.89 (1.97 ± 5.29) | 0.04 | |
| Cells | CRs | Time Median Min–Max (Mean ± SD) | p * | |
|---|---|---|---|---|
| 24 h | One Week | |||
| Viability of hGF% | Control | 100 100–100 (100 ± 0.00) | 100 100–100 (100 ± 0.00) | 1.00 |
| AF | 79.04 69.79–138.09 (95.46 ± 32.77) | 169.44 98.90–588.88 (223.77 ± 83.63) | 0.11 | |
| CT | 67.29 39.92–78.82 (62.93 ± 16.58) | 101.78 48.61–655.55 (202.67 ± 228.76) | 0.04 | |
| EQS | 46.14 15.01–48.78 (37.12 ± 15.75) | 17.36 4.16–55.55 (20.69 ± 19.02) | 0.34 | |
| Viability of hPDLF% | Control | 100 100–100 (100 ± 0.00) | 100 100–100 (100 ± 0.00) | 1.00 |
| AF | 128.73 118.96–150.00 (130.62 ± 10.66) | 93.82 87.70–98.73 (92.97 ± 4.06) | 0.02 | |
| CT | 122.91 101.56–138.18 (121.23 ± 12.25) | 68.97 62.09–86.72 (72.89 ± 9.88) | 0.02 | |
| EQS | 144.44 120.31–223.63 (160.70 ± 40.76) | 97.13 93.10–106.36 (98.02 ± 5.29) | 0.04 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavuncu, G.; Yilmaz, A.M.; Karademir Yilmaz, B.; Yilmaz Atali, P.; Altunok, E.C.; Kuru, L.; Agrali, O.B. Cytotoxicity of Different Nano Composite Resins on Human Gingival and Periodontal Ligament Fibroblast Cell Lines: An In Vitro Study. Biomedicines 2020, 8, 48. https://doi.org/10.3390/biomedicines8030048
Kavuncu G, Yilmaz AM, Karademir Yilmaz B, Yilmaz Atali P, Altunok EC, Kuru L, Agrali OB. Cytotoxicity of Different Nano Composite Resins on Human Gingival and Periodontal Ligament Fibroblast Cell Lines: An In Vitro Study. Biomedicines. 2020; 8(3):48. https://doi.org/10.3390/biomedicines8030048
Chicago/Turabian StyleKavuncu, Gamze, Ayse Mine Yilmaz, Betul Karademir Yilmaz, Pinar Yilmaz Atali, Elif Cigdem Altunok, Leyla Kuru, and Omer Birkan Agrali. 2020. "Cytotoxicity of Different Nano Composite Resins on Human Gingival and Periodontal Ligament Fibroblast Cell Lines: An In Vitro Study" Biomedicines 8, no. 3: 48. https://doi.org/10.3390/biomedicines8030048
APA StyleKavuncu, G., Yilmaz, A. M., Karademir Yilmaz, B., Yilmaz Atali, P., Altunok, E. C., Kuru, L., & Agrali, O. B. (2020). Cytotoxicity of Different Nano Composite Resins on Human Gingival and Periodontal Ligament Fibroblast Cell Lines: An In Vitro Study. Biomedicines, 8(3), 48. https://doi.org/10.3390/biomedicines8030048






