Evaluation of Posaconazole Pharmacokinetics in Adult Patients with Invasive Fungal Infection
Abstract
:1. Introduction
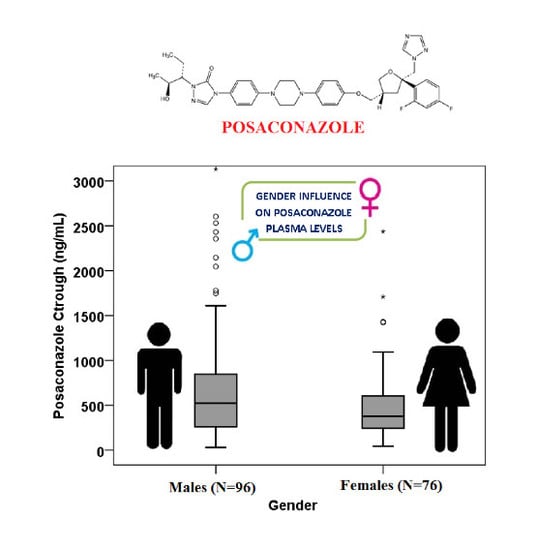
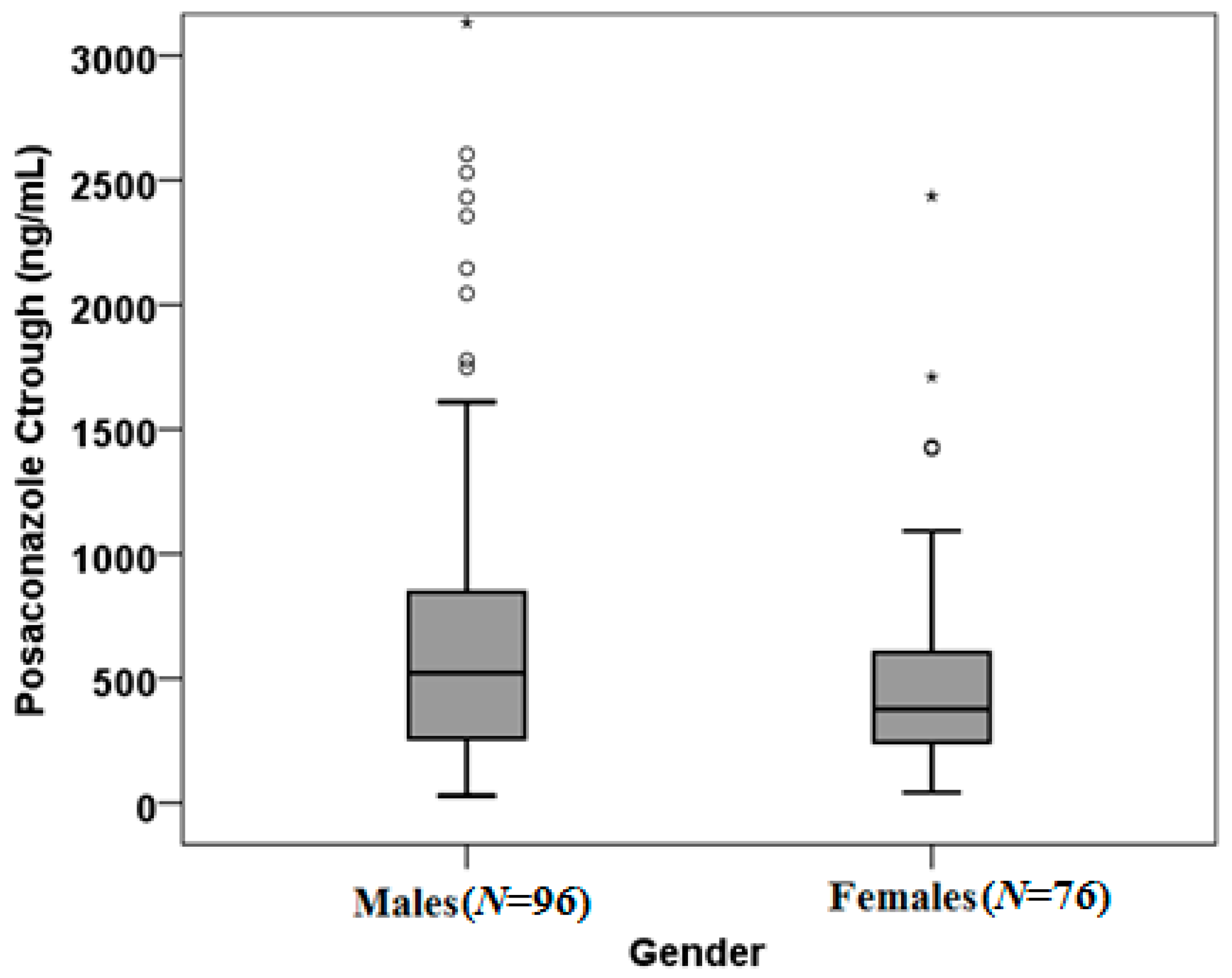
2. Results
3. Discussion
4. Material and Methods
4.1. Patients and Inclusion Criteria
4.2. Determinations of Posaconazole Plasma Concentration
4.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drew, R.H.; Townsend, M.L.; Pound, M.W.; Johnson, S.W.; Perfect, J.R. Recent advances in the treatment of life-threatening, invasive fungal infections. Expert Opin. Pharmacother. 2013, 14, 2361–2374. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Mantadakis, E.; Samonis, G. Systemic mycoses in the immunocompromised host: An update in antifungal therapy. J. Hosp. Infect. 2003, 53, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Bernal-Martinez, L.; Buitrago, M.J.; Castelli, M.V.; Gomez-Lopez, A.; Zaragoza, O.; Rodriguez-Tudela, J.L. Update on the epidemiology and diagnosis of invasive fungal infection. Int. J. Antimicrob. Agents 2008, 32 (Suppl. 2), S143–S147. [Google Scholar] [CrossRef]
- Sheng, C.; Zhang, W. New lead structures in antifungal drug discovery. Curr. Med. Chem. 2011, 18, 733–766. [Google Scholar] [CrossRef] [PubMed]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R., 3rd; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Fleming, S.; Yannakou, C.K.; Haeusler, G.M.; Clark, J.; Grigg, A.; Heath, C.H.; Bajel, A.; van Hal, S.J.; Chen, S.C.; Milliken, S.T.; et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern. Med. J. 2014, 44, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, F.; Patel, R.; Mann, P.A.; Mendrick, C.A.; Norris, C.C.; Hare, R.; Loebenberg, D.; Black, T.A.; McNicholas, P.M. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin b against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 2006, 50, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R.; Pai, S.; Laughlin, M.; Lim, J.; Batra, V. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 2003, 47, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Eiden, C.; Meniane, J.C.; Peyriere, H.; Eymard-Duvernay, S.; Le Falher, G.; Ceballos, P.; Fegueux, N.; Cociglio, M.; Reynes, J.; Hillaire-Buys, D. Therapeutic drug monitoring of posaconazole in hematology adults under posaconazole prophylaxis: Influence of food intake. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (tdm) of antifungal agents: Guidelines from the british society for medical mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Imagawa, Y.; Yamazaki, H. Drug interactions between nine antifungal agents and drugs metabolized by human cytochromes p450. Curr. Drug. Metab. 2014, 15, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Kraft, W.K.; Chang, P.S.; van Iersel, M.L.; Waskin, H.; Krishna, G.; Kersemaekers, W.M. Posaconazole tablet pharmacokinetics: Lack of effect of concomitant medications altering gastric ph and gastric motility in healthy subjects. Antimicrob. Agents Chemother. 2014, 58, 4020–4025. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). EMA Warns that Noxafil Tablets and Oral Suspension Have Different Doses and Are Not Interchangeable; EMA: London, UK, 2016. [Google Scholar]
- Cattaneo, C.; Panzali, A.; Passi, A.; Borlenghi, E.; Lamorgese, C.; Petulla, M.; Re, A.; Caimi, L.; Rossi, G. Serum posaconazole levels during acute myeloid leukaemia induction therapy: Correlations with breakthrough invasive fungal infections. Mycoses 2015, 58, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.J.; Ray, J.E.; Chen, S.C.; Ng, K.; Pont, L.; McLachlan, A.J. Multicenter study of posaconazole therapeutic drug monitoring: Exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 2012, 56, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Wannemuehler, K.A.; Marr, K.A.; Hadley, S.; Kontoyiannis, D.P.; Walsh, T.J.; Fridkin, S.K.; Pappas, P.G.; Warnock, D.W. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: Interim results of a prospective multicenter surveillance program. Med. Mycol. 2005, 43 (Suppl. 1), S49–S58. [Google Scholar] [CrossRef] [PubMed]
- Hummert, S.E.; Green, M.R. Therapeutic drug monitoring and dose adjustment of posaconazole oral suspension in adults with acute myeloid leukemia. Ther. Drug. Monit. 2015, 37, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Vaes, M.; Hites, M.; Cotton, F.; Bourguignon, A.M.; Csergo, M.; Rasson, C.; Ameye, L.; Bron, D.; Jacobs, F.; Aoun, M. Therapeutic drug monitoring of posaconazole in patients with acute myeloid leukemia or myelodysplastic syndrome. Antimicrob. Agents Chemother. 2012, 56, 6298–6303. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Cho, J.Y.; Park, S.I.; Kim, E.J.; Yoon, S.; Yoon, S.H.; Lee, J.O.; Koh, Y.; Song, K.H.; Choe, P.G.; et al. Effectiveness of increasing the frequency of posaconazole syrup administration to achieve optimal plasma concentrations in patients with haematological malignancy. Int. J. Antimicrob. Agents 2016, 48, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.J.; Ray, J.E.; Marriott, D.; McLachlan, A.J. Posaconazole exposure-response relationship: Evaluating the utility of therapeutic drug monitoring. Antimicrob. Agents Chemother. 2012, 56, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Mouton, J.W.; Verweij, P.E.; Bruggemann, R.J. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev. Anti. Infect. Ther. 2013, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- EMA. European Medicines Agency, Committee for Medicinal Products for Human Use. Assessment Report Noxafil. Available online: http//www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000610/WC500168187.pdf (accessed on 20 February 2014).
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of america. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [PubMed]
- Girmenia, C.; Iori, A.P. An update on the safety and interactions of antifungal drugs in stem cell transplant recipients. Expert Opin. Drug. Saf. 2016, 16, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. A new, broad-spectrum azole antifungal: Posaconazol—Mechanisms of action and resistance, spectrum of activity. Mycoses 2006, 49 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Kohl, V.; Muller, C.; Cornely, O.A.; Abduljalil, K.; Fuhr, U.; Vehreschild, J.J.; Scheid, C.; Hallek, M.; Ruping, M.J. Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob. Agents Chemother. 2010, 54, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Vehreschild, J.J.; Muller, C.; Farowski, F.; Vehreschild, M.J.; Cornely, O.A.; Fuhr, U.; Kreuzer, K.A.; Hallek, M.; Kohl, V. Factors influencing the pharmacokinetics of prophylactic posaconazole oral suspension in patients with acute myeloid leukemia or myelodysplastic syndrome. Eur. J. Clin. Pharmacol. 2012, 68, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Beierle, I.; Meibohm, B.; Derendorf, H. Gender differences in pharmacokinetics and pharmacodynamics. Int. J. Clin. Pharmacol. Ther. 1999, 37, 529–547. [Google Scholar] [PubMed]
- Sansone-Parsons, A.; Krishna, G.; Simon, J.; Soni, P.; Kantesaria, B.; Herron, J.; Stoltz, R. Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 2007, 51, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Jeu, L.; Piacenti, F.J.; Lyakhovetskiy, A.G.; Fung, H.B. Voriconazole. Clin. Ther. 2003, 25, 1321–1381. [Google Scholar] [CrossRef]
- Baietto, L.; Corcione, S.; Pacini, G.; Perri, G.D.; D’Avolio, A.; De Rosa, F.G. A 30-years review on pharmacokinetics of antibiotics: Is the right time for pharmacogenetics? Curr. Drug Metab. 2014, 15, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Baietto, L.; D’Avolio, A.; Ventimiglia, G.; De Rosa, F.G.; Siccardi, M.; Simiele, M.; Sciandra, M.; Di Perri, G. Development, validation, and routine application of a high-performance liquid chromatography method coupled with a single mass detector for quantification of itraconazole, voriconazole, and posaconazole in human plasma. Antimicrob. Agents Chemother. 2010, 54, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Baietto, L.; D’Avolio, A.; Marra, C.; Simiele, M.; Cusato, J.; Pace, S.; Ariaudo, A.; De Rosa, F.G.; Di Perri, G. Development and validation of a new method to simultaneously quantify triazoles in plasma spotted on dry sample spot devices and analysed by hplc-ms. J. Antimicrob. Chemother. 2012, 67, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
| Variable | N = 172 | |||
|---|---|---|---|---|
| Mean | Standard Deviation | Median | IQR | |
| Age (years) | 47.14 | 18.952 | 49.50 | 27.00–64.00 |
| BMI Kg/m2 | 24.49 | 4.342 | 24.16 | 21.83–27.01 |
| PSC Ctrough ng/mL | 726.71 | 914.443 | 419.50 | 252.50–778.75 |
| N = 172 | |||
|---|---|---|---|
| PSC Dose | Dose Score | N | % |
| 100 t.d. | 1 | 1 | 0.6 |
| 100 th.d. | 2 | 1 | 0.6 |
| 200 o.d. | 3 | 27 | 15.7 |
| 200 t.d. | 4 | 84 | 48.8 |
| 200 th.d | 5 | 13 | 7.6 |
| 300 t.d. | 6 | 1 | 0.6 |
| 300 th.d. | 7 | 2 | 1.2 |
| 400 o.d. | 8 | 1 | 0.6 |
| 400 t.d. | 9 | 30 | 17.4 |
| 400 th.d. | 10 | 7 | 4.1 |
| 500 th.d. | 11 | 3 | 1.7 |
| 800 o.d. | 12 | 2 | 1.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, S.; Fatiguso, G.; De Francia, S.; Favata, F.; Pirro, E.; Carcieri, C.; De Nicolò, A.; Cusato, J.; Di Perri, G.; D’Avolio, A. Evaluation of Posaconazole Pharmacokinetics in Adult Patients with Invasive Fungal Infection. Biomedicines 2017, 5, 66. https://doi.org/10.3390/biomedicines5040066
Allegra S, Fatiguso G, De Francia S, Favata F, Pirro E, Carcieri C, De Nicolò A, Cusato J, Di Perri G, D’Avolio A. Evaluation of Posaconazole Pharmacokinetics in Adult Patients with Invasive Fungal Infection. Biomedicines. 2017; 5(4):66. https://doi.org/10.3390/biomedicines5040066
Chicago/Turabian StyleAllegra, Sarah, Giovanna Fatiguso, Silvia De Francia, Fabio Favata, Elisa Pirro, Chiara Carcieri, Amedeo De Nicolò, Jessica Cusato, Giovanni Di Perri, and Antonio D’Avolio. 2017. "Evaluation of Posaconazole Pharmacokinetics in Adult Patients with Invasive Fungal Infection" Biomedicines 5, no. 4: 66. https://doi.org/10.3390/biomedicines5040066
APA StyleAllegra, S., Fatiguso, G., De Francia, S., Favata, F., Pirro, E., Carcieri, C., De Nicolò, A., Cusato, J., Di Perri, G., & D’Avolio, A. (2017). Evaluation of Posaconazole Pharmacokinetics in Adult Patients with Invasive Fungal Infection. Biomedicines, 5(4), 66. https://doi.org/10.3390/biomedicines5040066