Dedifferentiation of Mature Adipocytes and Their Future Potential for Regenerative Medicine Applications
Abstract
1. Introduction
2. Methodology
3. Results
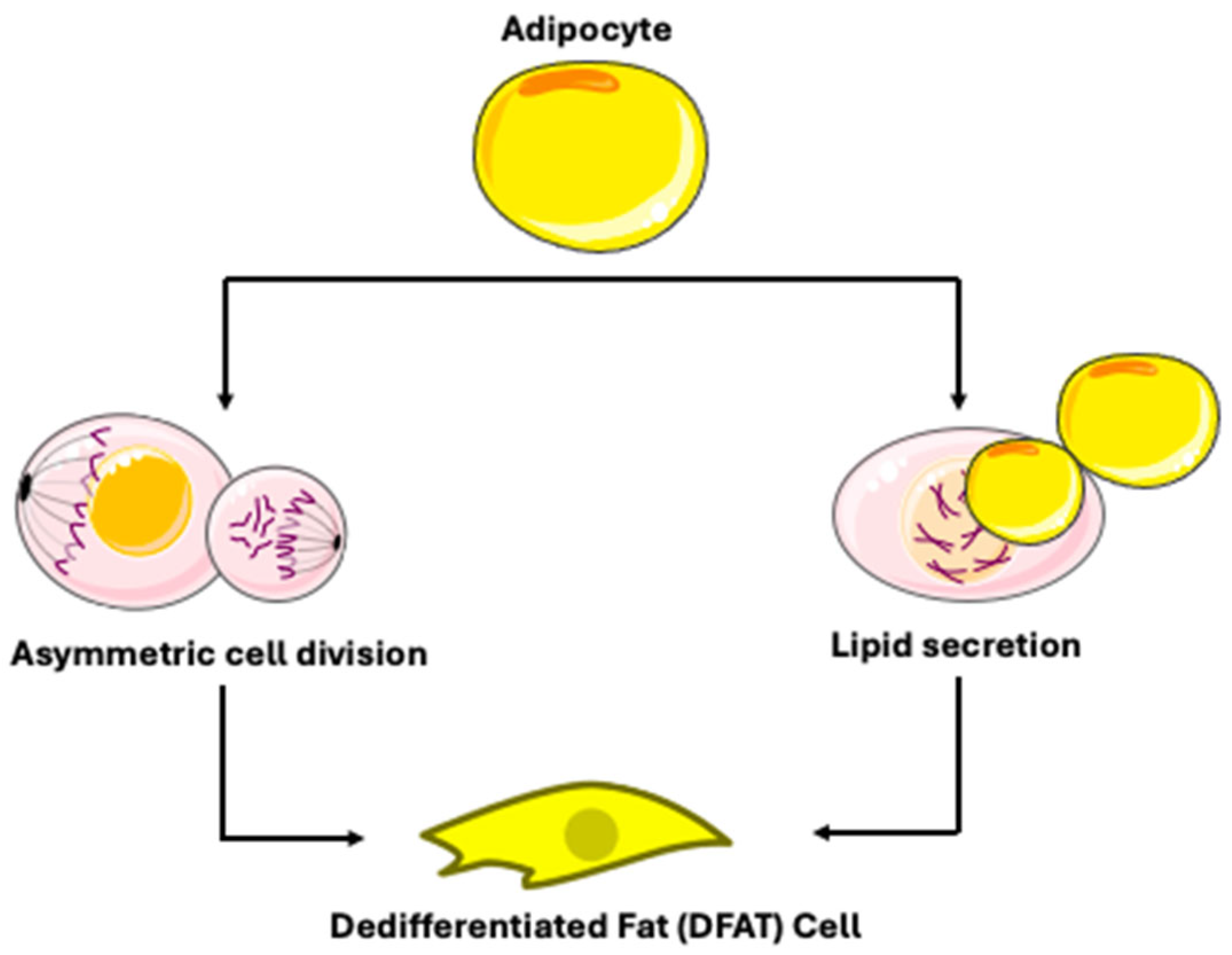
3.1. Mechanisms of Mature Adipocyte Dedifferentiation
3.2. Morphology and Culture Technique of Dedifferentiated Fat (DFAT) Cells
3.3. Physiological and Pathophysiological Roles of Adipocyte Dedifferentiation
In Vivo Evidence for Adipocyte Plasticity
3.4. Implications in Metabolic Dysregulation
3.5. Roles in Cancer, Tissue Repair, and Fibrosis
3.6. Molecular Pathways and Phenotypic Changes in Adipocyte Dedifferentiation
| Dedifferentiation Stage | Molecular Markers | Transcriptional Factors | Selected References and Context |
|---|---|---|---|
| 1. Mature Adipocyte (Initial State) | White Adipocytes: High levels of PLIN1 [73], FABP4 [74], ADIPOQ [75], LEP [37]. Depot-specific markers: TCF21 [76] (visceral), HOXC8/9 [77] (subcutaneous). | Master transcriptional regulators of the mature state: PPARG, C/EBPα [78]. Their downregulation is a hallmark of dedifferentiation initiation [69]. | Markers define the starting point. Their loss is tracked during dedifferentiation [69]. |
| Brown Adipocyte: High UCP1 [79], PRDM16 [80], CIDEA [81], ZIC1 [82]. | |||
| Beige Adipocyte: Inducible UCP1 [79], TMEM26 [83], CD137 [84], TBX1 [57]. | |||
| 2. Early/Initiating Phase | Downregulation: Rapid decrease in mature adipocyte transcripts (PPARG, C/EBPα, ADIPOQ, LPL) [69]. | Inducing Stimuli: Ceiling culture technique; Tumor microenvironment (high osmotic pressure, TNF-α); Wnt3a, TGF-β1 signaling; Cold stimulation [17]. | This phase involves exiting the mature state. In vivo examples include pregnancy/lactation in mammary glands, and dermal adipocytes during hair follicle regression [17]. |
| Upregulation: Early stress/injury response signals and matrix remodeling genes (FAP, DPP4) [69]. | Cellular Process: Activation of “liposecretion”-the secretion of membrane-wrapped lipid droplets, dependent in processes like CDK1 activity [22]. | ||
| 3. Intermediate/Transitional State | Loss: Lipid droplet proteins (e.g., PLIN1) [17]. | Pathway Driving Change: ECM remodeling (sustained FAP, DPP4, MMP1, TGF-β1) [69]; Inflammatory cytokine secretion (IL-6, IL-8) [69]; Modulation of autophagy and cell-cycle re-entry [22]. | Cell adopts a fibroblast-like morphology. This state is observed in pathological conditions, such as cancer-associated adipocytes and skin wound healing [17]. |
| Gain: Re-expression of progenitor/pre-adipocyte markers (e.g., PDGFRA, VIM, COL1A1). Cells may co-express markers of both fates (e.g., perilipin+ and α-SMA+ “transition cells” in fibrosis) [17]. | |||
| 4. Dedifferentiated Fat (DFAT) Cell/Progenitor-like State | Marker Profile: Fibroblast-like morphology; minimal lipid content; Expression of mesenchymal/stem cell markers (e.g., CD90, CD105), absence of hematopoietic marker CD45 [85]. Multilineage potential (adipogenic, osteogenic, chondrogenic) [86]. | Maintained Regulators: Activities that suppress redifferentiation and maintain proliferative capacity [86]. | DFAT cells are considered multipotent and are a focus for regenerative medicine. They can be derived from white, brown, and beige adipocytes [86]. |
3.7. Molecular Crosstalk of Key Signaling Pathways in Adipocyte Dedifferentiation
- PPARG Downregulation is a Central Event: The PPARG is the master transcriptional regulator and essential for establishing and maintaining the mature adipocyte phenotype, including lipid storage and insulin sensitivity. Its downregulation is a hallmark and prerequisite for dedifferentiation to initiate [88].
- Canonical Wnt/β-catenin Pathway as a Primary Repressor: Activation of the canonical Wnt/β-catenin pathway is a potent inhibitor of adipogenesis and a promising driver of dedifferentiation. In the active state, stabilized β-catenin translocates to the nucleus, where it complexes with T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) proteins to transcriptionally repress PPARG gene expression [87,89]. Furthermore, Wnt/β-catenin signaling can inhibit PPARG transactivation via histone-modifying enzymes. In the context of cancer, tumor-derived Wnt ligands (e.g., Wnt3a, Wnt5a) activate this pathway in adjacent adipocytes, directly suppressing PPARG and C/EBPα to induce a dedifferentiated, cancer-associated adipocyte (CAA) phenotype. This pathway’s activity is also upregulated by biophysical stressors such as tissue compression, linking mechanical cues to dedifferentiation [53].
- TGF-β/SMAD Pathway in Fibroblastic Transformation: The TGF-β pathway promotes dedifferentiation towards a fibroblastic or myofibroblastic state [90]. Upon TGF-β1 binding, the receptor SMADs (SMAD2/3) are phosphorylated, complex with SMAD4, and translocate to the nucleus. This signaling directly suppresses adipogenic transcription factors and upregulates the encoding of ECM components (e.g., collagens, fibronectin) and the myofibroblast marker α-SMA. In pathologies such as fibrosis and cancer, TGF-β1 is a major driver of the adipocyte-to-myofibroblast transition [90].
- Notch Signaling Modulates Plasticity: The Notch pathway exerts context-dependent effects on adipocyte plasticity. Activation of Notch signaling, such as through the Notch1 intracellular domain (N1ICD), inhibits adipogenic differentiation by repressing PPARG and C/EBPα expression [91]. Consequently, the gain of Notch function in adipocytes promotes dedifferentiation and is associated with lipodystrophy, while its inhibition can accelerate adipogenesis. Notch interacts with other pathways; for instance, it can inhibit Wnt/β-catenin signaling, creating a complex regulatory network [91].
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ad/N1ICD | Adenovirus expressing the Notch1 intracellular domain |
| Adipoq-Cre | Adiponectin promoter-driver Cre-recombinase |
| ADIPOQ | Adipocyte Q |
| ADSCs | Adipose-derived stem cells |
| AT | Adipose tissue |
| BAT | Brown adipose tissue |
| BrdU | Bromodeoxyuridine |
| CAAs | Cancer-associated adipocytes |
| C/EBPα | C CAAT/Enhancer-Binding Protein Alpha |
| C/EBPβ | C CAAT/Enhancer-Binding Protein Beta |
| DFAT | Dedifferentiated Fat |
| ECs | Endothelial cells |
| FABP4 | Fatty Acid-Binding Protein 4 |
| FASN | Fatty Acid Synthase |
| LDs | Lipid Droplets |
| LIPE | Lipase Hormone-Sensitive |
| LPL | Lipoprotein Lipase |
| LPS | Liposarcoma |
| Mia-PaCa2 | Human pancreatic carcinoma cell line |
| MSCs | Mesenchymal stem cells |
| PDK4 | Pyruvate dehydrogenase kinase 4 |
| PPARG | Peroxisome Proliferator-Activated Receptor Gamma |
| SEM | Scanning Electron Microscopy |
| SREBF1 | Sterol Regulatory Element-Binding Transcription Factor 1 |
| SVF | Stromal vascular fraction |
| TEM | Transmission Electron Microscopy |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| WAT | White Adipose Tissue |
| Wnt/β-catenin | Wingless/Integrated/β-catenin |
| α-SMA | Alpha-smooth muscle actin |
References
- Patel, J.C.; Shukla, M.; Shukla, M. From bench to bedside: Translating mesenchymal stem cell therapies through preclinical and clinical evidence. Front. Bioeng. Biotechnol. 2025, 13, 1639439. [Google Scholar] [CrossRef]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Arner, P. Fat Tissue Growth and Development in Humans. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 37–45. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. Adult cell plasticity in vivo: De-differentiation and trans differentiation are back in style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425. [Google Scholar] [CrossRef]
- Sadowski, D.; Kiel, M.E.; Apicella, M.; Arriola, A.G.; Chen, C.P.; McKinnon, R.D. Teratogenic Potential in Cultures Optimized for Oligodendrocyte Development from Mouse Embryonic Stem Cells. Stem Cells Dev. 2010, 19, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, M.; Perdikari, A.; Rülicke, T.; Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013, 15, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, G.; Marchi, A.; Sbarbati, A. Adipose-derived mesenchymal stem cells: Past, present, and future. Aesthetic Plast. Surg. 2009, 33, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sugawara, A.; Yamashita, J.; Ogura, H.; Sato, S. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. Int. J. Oral. Sci. 2011, 3, 117–124. [Google Scholar] [CrossRef]
- Sugihara, H.; Yonemitsu, N.; Miyabara, S.; Toda, S. Proliferation of unilocular fat cells in the primary culture. J. Lipid Res. 1987, 28, 1038–1045. [Google Scholar] [CrossRef]
- Veronese, S.; Dai Prè, E.; Conti, G.; Busato, A.; Mannucci, S.; Sbarbati, A. Comparative technical analysis of lipoaspirate mechanical processing devices. J. Tissue Eng. Regen. Med. 2020, 14, 1213–1226. [Google Scholar] [CrossRef]
- Kishimoto, N.; Momota, Y.; Hashimoto, Y.; Tatsumi, S.; Ando, K.; Omasa, T.; Kotani, J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin. Oral. Investig. 2014, 18, 1893–1901. [Google Scholar] [CrossRef]
- Jumabay, M.; Abdmaulen, R.; Urs, S.; Heydarkhan-Hagvall, S.; Chazenbalk, G.D.; Jordan, M.C.; Roos, K.P.; Yao, Y.; Boström, K.I. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J. Mol. Cell Cardiol. 2012, 53, 790–800. [Google Scholar] [CrossRef]
- Ohta, Y.; Takenaga, M.; Tokura, Y.; Hamaguchi, A.; Matsumoto, T.; Kano, K.; Mugishima, H.; Okano, H.; Igarashi, R. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008, 17, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Darra, E.; Rizzatti, V.; Budui, S.; Franceschetti, G.; Mazzali, G.; Rossi, A.P.; Fantin, F.; Menegazzi, M.; Cinti, S.; et al. Adipocytes WNT5a mediated dedifferentiation: A possible target in pancreatic cancer microenvironment. Oncotarget 2016, 7, 20223–20235. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liao, Y.; Jiang, W. Insights into the molecular changes of adipocyte dedifferentiation and its future research opportunities. J. Lipid Res. 2024, 65, 100644. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, Z.; Lu, F.; Dong, Z.; Chang, Q.; Gao, J. In Vivo Dedifferentiation of Adult Adipose Cells. PLoS ONE 2015, 10, e0125254. [Google Scholar] [CrossRef]
- Sugihara, H.; Yonemitsu, N.; Miyabara, S.; Yun, K. Primary cultures of unilocular fat cells: Characteristics of growth in vitro and changes in differentiation properties. Differentiation 1986, 31, 42–49. [Google Scholar] [CrossRef]
- Maurizi, G.; Poloni, A.; Mattiucci, D.; Santi, S.; Maurizi, A.; Izzi, V.; Giuliani, A.; Mancini, S.; Zingaretti, M.C.; Perugini, J.; et al. Human White Adipocytes Convert Into “Rainbow” Adipocytes In Vitro. J. Cell. Physiol. 2017, 232, 2887–2899. [Google Scholar] [CrossRef]
- Ostinelli, G.; Gauthier, M.-F.; Vernoux, N.; Bernier, E.; Dubé, T.; Marceau, S.; Lebel, S.; Tremblay, M.-È.; Tchernof, A. Exploring the role of cell cycle regulation in human mature adipocyte dedifferentiation. Front. Cell Dev. Biol. 2025, 13, 1547836. [Google Scholar] [CrossRef]
- Zang, L.; Kothan, S.; Yang, Y.; Zeng, X.; Ye, L.; Pan, J. Insulin negatively regulates dedifferentiation of mouse adipocytes in vitro. Adipocyte 2020, 9, 24–34. [Google Scholar] [CrossRef]
- Li, Y.; Mao, A.S.; Seo, B.R.; Zhao, X.; Gupta, S.K.; Chen, M.; Han, Y.L.; Shih, T.-Y.; Mooney, D.J.; Guo, M. Compression-induced dedifferentiation of adipocytes promotes tumor progression. Sci. Adv. 2020, 6, eaax5611. [Google Scholar] [CrossRef]
- Wei, S.; Duarte, M.S.; Zan, L.; Du, M.; Jiang, Z.; Guan, L.; Chen, J.; Hausman, G.J.; Dodson, M.V. Cellular and molecular implications of mature adipocyte dedifferentiation. J. Genom. 2013, 1, 5–12. [Google Scholar] [CrossRef]
- Côté, J.A.; Ostinelli, G.; Gauthier, M.-F.; Lacasse, A.; Tchernof, A. Focus on dedifferentiated adipocytes: Characteristics, mechanisms, and possible applications. Cell Tissue Res. 2019, 378, 385–398. [Google Scholar] [CrossRef]
- Conti, G.; Benati, D.; Bernardi, P.; Jurga, M.; Rigotti, G.; Sbarbati, A. The post-adipocytic phase of the adipose cell cycle. Tissue Cell 2014, 46, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xia, B.; Dai, Z.; Yang, R.; Chen, R.; Yang, H. Comparative study of dedifferentiated fat cell and adipose-derived stromal cell sheets for periodontal tissue regeneration: In vivo and in vitro evidence. J. Clin. Periodontol. 2022, 49, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.A.; Gauthier, M.-F.; Ostinelli, G.; Brochu, D.; Bellmann, K.; Marette, A.; Julien, F.; Lebel, S.; Tchernof, A. Characterization and visualization of the liposecretion process taking place during ceiling culture of human mature adipocytes. J. Cell Physiol. 2019, 234, 10270–10280. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.-Y.; Choi, S.; Ko, U.H.; Lim, D.-S.; Suh, J.M.; Shin, J.H. Ceiling culture chip reveals dynamic lipid droplet transport during adipocyte dedifferentiation via actin remodeling. Lab. Chip 2022, 22, 3920–3932. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.-F.; Ostinelli, G.; Pelletier, M.; Tchernof, A. Origin of dedifferentiated adipocyte-derived cells (DFAT) during ceiling culture in an Adiponectin Cre-Recombinase mouse model. Biochem. Cell Biol. 2025, 103, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.A.; Song, A.; Chen, W.; Schwalie, P.C.; Zhang, F.; Vishvanath, L.; Jiang, L.; Ye, R.; Shao, M.; Tao, C.; et al. Reversible De-differentiation of Mature White Adipocytes into Preadipocyte-like Precursors during Lactation. Cell Metab. 2018, 28, 282–288.e3. [Google Scholar] [CrossRef]
- Cinti, S. The Lactating Adipose Organ. In Obesity, Type 2 Diabetes and the Adipose Organ: A Pictorial Atlas from Research to Clinical Applications; Cinti, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 337–383. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuédé, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Conti, G.; Calderan, L.; Quintero Sierra, L.A.; Conti, A.; Ossanna, R.; Boschi, F.; Marzola, P.; Ferrarini, F.; Governa, M.; Lievens, P.M.-J.; et al. Tumor and peritumoral adipose tissue crosstalk: De-differentiated adipocytes influence spread of colon carcinoma cells. Tissue Cell 2023, 80, 101990. [Google Scholar] [CrossRef]
- Jumabay, M.; Matsumoto, T.; Yokoyama, S.; Kano, K.; Kusumi, Y.; Masuko, T.; Mitsumata, M.; Saito, S.; Hirayama, A.; Mugishima, H.; et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J. Mol. Cell Cardiol. 2009, 47, 565–575. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, M.; Hepler, C.; Zi, Z.; Zhao, S.; An, Y.A.; Zhu, Y.; Ghaben, A.L.; Wang, M.; Li, N.; et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J. Clin. Investig. 2019, 129, 5327–5342. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.A.; Waterhouse, P.D.; Kasaian, K.; Fang, H.; Gulyaeva, O.; Sul, H.S.; Boutros, P.C.; Khokha, R. PDGFRα+ stromal adipocyte progenitors transition into epithelial cells during lobulo-alveologenesis in the murine mammary gland. Nat. Commun. 2019, 10, 1760. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, G.C.; Shook, B.A.; Andrae, J.; Holtrup, B.; Bollag, K.; Betsholtz, C.; Rodeheffer, M.S.; Horsley, V. Skin adipocyte stem cell self-renewal is regulated by a Pdgfa/Akt signaling axis. Cell Stem Cell 2016, 19, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.; Gonzalez De Los Santos, F.; Wu, Z.; Capelozzi, V.; Phan, S.H.; Liu, T. FIZZ1-Induced Myofibroblast Transdifferentiation from Adipocytes and Its Potential Role in Dermal Fibrosis and Lipoatrophy. Am. J. Pathol. 2015, 185, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Hu, W.; Zhang, H.; Ding, Y.; Zeng, Q.; Liao, X.; Wang, D.; Xie, W.; Hui, H.X.; Deng, T. Single-nucleus RNA sequencing reveals heterogeneity among multiple white adipose tissue depots. Life Metab. 2023, 2, load045. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Sen, B.; Rubin, J.; Pike, J.W. Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow-derived Mesenchymal Stem Cells. J. Biol. Chem. 2016, 291, 17829–17847. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes. Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Ono, H.; Oki, Y.; Bono, H.; Kano, K. Gene expression profiling in multipotent DFAT cells derived from mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 407, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Rybinska, I.; Mangano, N.; Tagliabue, E.; Triulzi, T. Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences. Int. J. Mol. Sci. 2021, 22, 3775. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Hayashi, M.; Uchida, Y.; Cheng, X.W.; Nakayama, T.; Matsushita, T.; Murohara, T.; Takeshita, K. Notch1 haploinsufficiency in mice accelerates adipogenesis. Sci. Rep. 2021, 11, 16761. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. The many secret lives of adipocytes: Implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.D.; Wei, J.; Wood, T.A.; Graham, L.V.; Whitfield, M.L.; Scherer, P.E.; Tourtellotte, W.G.; Varga, J. Myofibroblasts in Murine Cutaneous Fibrosis Originate From Adiponectin-Positive Intradermal Progenitors. Arthritis Rheumatol. 2015, 67, 1062–1073. [Google Scholar] [CrossRef]
- Plikus, M.V.; Guerrero-Juarez, C.F.; Ito, M.; Li, Y.R.; Dedhia, P.H.; Zheng, Y.; Shao, M.; Gay, D.L.; Ramos, R.; Hsi, T.-C.; et al. Regeneration of fat cells from myofibroblasts during wound healing. Science 2017, 355, 748–752. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Palliyaguru, D.L.; Wakabayashi, N.; Khoo, N.K.H.; Schoiswohl, G.; O’Doherty, R.M.; Kensler, T.W. Notch intracellular domain overexpression in adipocytes confers lipodystrophy in mice. Mol. Metab. 2015, 4, 543–550. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, J.; Shen, H.; Xu, W. Cancer-associated adipocytes in the ovarian cancer microenvironment. Am. J. Cancer Res. 2024, 14, 3259–3279. [Google Scholar] [CrossRef]
- Na, H.; Song, Y.; Lee, H.-W. Emphasis on Adipocyte Transformation: Anti-Inflammatory Agents to Prevent the Development of Cancer-Associated Adipocytes. Cancers 2023, 15, 502. [Google Scholar] [CrossRef]
- Gao, T.; Li, J.; Cheng, T.; Wang, X.; Wang, M.; Xu, Z.; Mu, Y.; He, X.; Xing, J.; Liu, S. Ovarian cancer-derived TGF-β1 induces cancer-associated adipocytes formation by activating SMAD3/TRIB3 pathway to establish pre-metastatic niche. Cell Death Dis. 2024, 15, 930. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dong, S.; Huang, R.; Chen, X. Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy. Cancers 2023, 15, 726. [Google Scholar] [CrossRef]
- Markan, K.R.; Boland, L.K.; King-McAlpin, A.Q.; Claflin, K.E.; Leaman, M.P.; Kemerling, M.K.; Stonewall, M.M.; Amendt, B.A.; Ankrum, J.A.; Potthoff, M.J. Adipose TBX1 regulates β-adrenergic sensitivity in subcutaneous adipose tissue and thermogenic capacity in vivo. Mol. Metab. 2020, 36, 100965. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Na, H.; Lee, S.E.; Kim, Y.M.; Moon, J.; Nam, T.W.; Ji, Y.; Jin, Y.; Park, J.H.; Cho, S.C.; et al. Dysfunctional adipocytes promote tumor progression through YAP/TAZ-dependent cancer-associated adipocyte transformation. Nat. Commun. 2024, 15, 4052. [Google Scholar] [CrossRef]
- Nieman, K.M.; Romero, I.L.; Van Houten, B.; Lengyel, E. Adipose tissue and adipocytes supports tumorigenesis and metastasis. Biochim. Biophys. Acta 2013, 1831, 1533–1541. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.; Varga, J. Adipocytic Progenitor Cells Give Rise to Pathogenic Myofibroblasts: Adipocyte-to-Mesenchymal Transition and Its Emerging Role in Fibrosis in Multiple Organs. Curr. Rheumatol. Rep. 2020, 22, 79. [Google Scholar] [CrossRef]
- Shook, B.A.; Wasko, R.R.; Mano, O.; Rutenberg-Schoenberg, M.; Rudolph, M.C.; Zirak, B.; Rivera-Gonzalez, G.C.; López-Giráldez, F.; Zarini, S.; Rezza, A.; et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 2020, 26, 880–895.e6. [Google Scholar] [CrossRef] [PubMed]
- Horsley, V.; Watt, F. Repeal and Replace: Adipocyte Regeneration in Wound Repair. Cell Stem Cell 2017, 20, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.A.; Guénard, F.; Lessard, J.; Lapointe, M.; Biron, S.; Vohl, M.-C.; Tchernof, A. Temporal Changes in Gene Expression Profile during Mature Adipocyte Dedifferentiation. Int. J. Genom. 2017, 2017, 5149362. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef]
- Poloni, A.; Maurizi, G.; Leoni, P.; Serrani, F.; Mancini, S.; Frontini, A.; Zingaretti, M.C.; Siquini, W.; Sarzani, R.; Cinti, S. Human Dedifferentiated Adipocytes Show Similar Properties to Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2012, 30, 965–974. [Google Scholar] [CrossRef]
- Jumabay, M.; Abdmaulen, R.; Ly, A.; Cubberly, M.R.; Shahmirian, L.J.; Heydarkhan-Hagvall, S.; Dumesic, D.A.; Yao, Y.; Boström, K.I. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Transl. Med. 2014, 3, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Tsang, N.-M.; Lee, P.-J.; Sui, Y.-H.; Huang, C.-H.; Liu, T.-T. Epstein-Barr Virus Induces Adipocyte Dedifferentiation to Modulate the Tumor Microenvironment. Cancer Res. 2021, 81, 3283–3294. [Google Scholar] [CrossRef]
- Bielczyk-Maczynska, E. White Adipocyte Plasticity in Physiology and Disease. Cells 2019, 8, 1507. [Google Scholar] [CrossRef]
- Lessard, J.; Pelletier, M.; Biertho, L.; Biron, S.; Marceau, S.; Hould, F.-S.; Lebel, S.; Moustarah, F.; Lescelleur, O.; Marceau, P.; et al. Characterization of Dedifferentiating Human Mature Adipocytes from the Visceral and Subcutaneous Fat Compartments: Fibroblast-Activation Protein Alpha and Dipeptidyl Peptidase 4 as Major Components of Matrix Remodeling. PLoS ONE 2015, 10, e0122065. [Google Scholar] [CrossRef]
- Kou, L.; Lu, X.-W.; Wu, M.-K.; Wang, H.; Zhang, Y.-J.; Sato, S.; Shen, J.-F. The phenotype and tissue-specific nature of multipotent cells derived from human mature adipocytes. Biochem. Biophys. Res. Commun. 2014, 444, 543–548. [Google Scholar] [CrossRef]
- Saler, M.; Caliogna, L.; Botta, L.; Benazzo, F.; Riva, F.; Gastaldi, G. hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In Vitro. Int. J. Mol. Sci. 2017, 18, 2699. [Google Scholar] [CrossRef]
- Bi, P.; Yue, F.; Karki, A.; Castro, B.; Wirbisky, S.E.; Wang, C.; Durkes, A.; Elzey, B.D.; Andrisani, O.M.; Bidwell, C.A.; et al. Notch activation drives adipocyte dedifferentiation and tumorigenic transformation in mice. J. Exp. Med. 2016, 213, 2019–2037. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.-W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, C.; Deng, B.; Gao, P.; Wang, L.; Li, Y.; Shiri, M.; Alkaifi, F.; Zhao, J.; Stephens, J.M.; et al. Tcf21 marks visceral adipose mesenchymal progenitors and functions as a rate-limiting factor during visceral adipose tissue development. Cell Rep. 2023, 42, 112166. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.n.; Zachara, M.; Deplancke, B. A systems perspective on brown adipogenesis and metabolic activation. Obes. Rev. 2017, 18, 65–81. [Google Scholar] [CrossRef] [PubMed]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef]
- Nicholls, D.G.; Locke, R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984, 64, 1–64. [Google Scholar] [CrossRef]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef]
- Zhou, Z.; Yon Toh, S.; Chen, Z.; Guo, K.; Peng Ng, C.; Ponniah, S.; Lin, S.-C.; Hong, W.; Li, P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 2003, 35, 49–56. [Google Scholar] [CrossRef]
- de Jong, J.M.A.; Larsson, O.; Cannon, B.; Nedergaard, J. A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1085–E1105. [Google Scholar] [CrossRef]
- Ussar, S.; Lee, K.Y.; Dankel, S.N.; Boucher, J.; Haering, M.-F.; Kleinridders, A.; Thomou, T.; Xue, R.; Macotela, Y.; Cypess, A.M.; et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 2014, 6, 247ra103. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Moliner, A.; Lee, E.-S.; Nickles, E.; Sim, E.; Liu, C.; Schwarz, H.; Ibáñez, C.F. CD137 negatively affects “browning” of white adipose tissue during cold exposure. J. Biol. Chem. 2020, 295, 2034–2042. [Google Scholar] [CrossRef]
- Liang, Z.; He, Y.; Tang, H.; Li, J.; Cai, J.; Liao, Y. Dedifferentiated fat cells: Current applications and future directions in regenerative medicine. Stem Cell Res. Ther. 2023, 14, 207. [Google Scholar] [CrossRef]
- Yuuki, Y.; Katafuchi, T.; Kazama, T.; Matsumoto, T.; Makishima, M. C3H10T1/2 Mesenchymal Stem Cell Line as a New In Vitro Tool for Studying Adipocyte Dedifferentiation. Biology 2025, 14, 444. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef]
- James, A.W. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica 2013, 2013, 684736. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, T.C.; MacDougald, O.A. Wnt/β-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef]
- Gumede, D.B.; Abrahamse, H.; Houreld, N.N. Targeting Wnt/β-catenin signaling and its interplay with TGF-β and Notch signaling pathways for the treatment of chronic wounds. Cell Commun. Signal 2024, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Kuang, S. Adipocyte dedifferentiation in health and diseases. Clin. Sci. 2019, 133, 2107–2119. [Google Scholar] [CrossRef]
- Nie, F.; Bi, H.; Zhang, C.; Ding, P. Differentiation potential and mRNA profiles of human dedifferentiated adipose cells and adipose-derived stem cells from young donors. Mol. Med. Rep. 2021, 23, 47. [Google Scholar] [CrossRef]
- Li, Y.; Long, J.; Zhang, Z.; Yin, W. Insights into the unique roles of dermal white adipose tissue (dWAT) in wound healing. Front. Physiol. 2024, 15, 1346612. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Jeong, S.; Kwon, H.R.; Olson, L.E. Regulation of adipocyte dedifferentiation at the skin wound edge. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, C.; Lu, F.; Liao, Y.; Cai, J.; Gao, J. Challenges and opportunities in obesity: The role of adipocytes during tissue fibrosis. Front. Endocrinol. 2024, 15, 1365156. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.A.; Lessard, J.; Pelletier, M.; Marceau, S.; Lescelleur, O.; Fradette, J.; Tchernof, A. Role of the TGF-β pathway in dedifferentiation of human mature adipocytes. FEBS Open Bio 2017, 7, 1092–1101. [Google Scholar] [CrossRef]
- Nohawica, M.; Errachid, A.; Wyganowska-Swiatkowska, M. Adipose-PAS interactions in the context of its localised bio-engineering potential (Review). Biomed. Rep. 2021, 15, 70. [Google Scholar] [CrossRef]
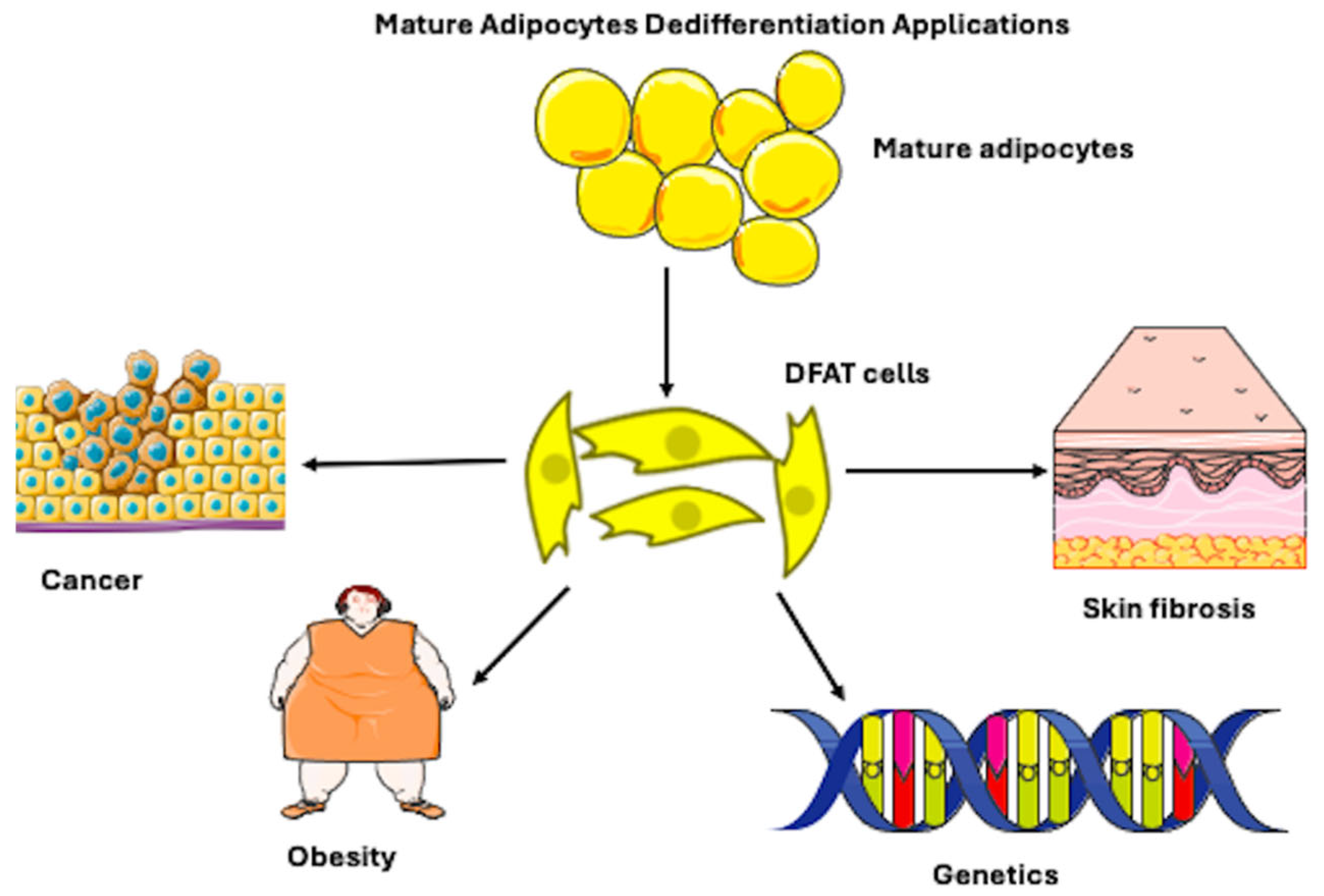
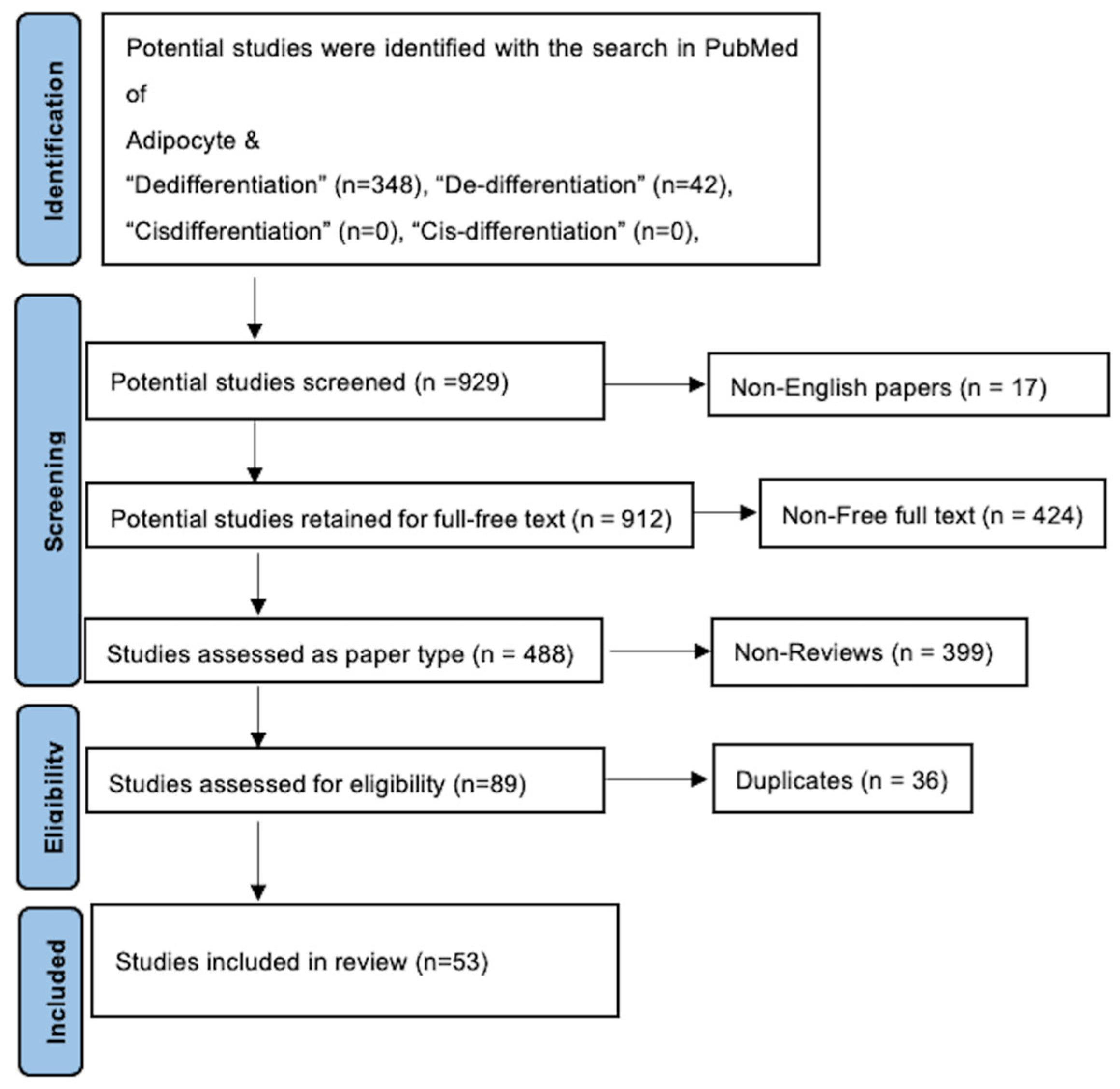
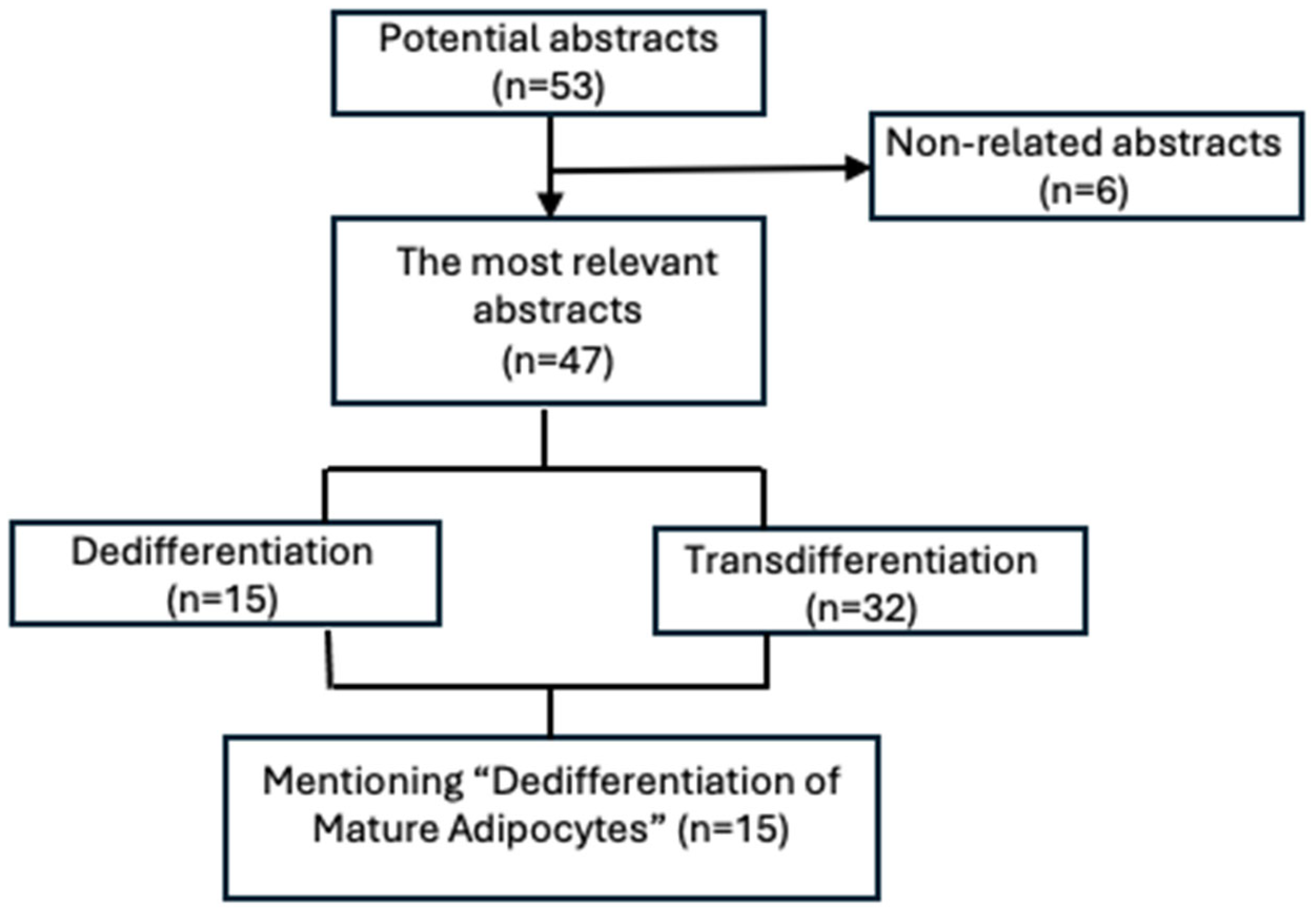
| Subcategory | References |
|---|---|
| Cancer (n = 6) | [19,20,21,22,23,24] |
| Morphology/Cell Culture Techniques (n = 3) | [10,25,26] |
| Obesity/Metabolism (n = 2) | [27,28] |
| Genetic (n = 2) | [17,29] |
| Myofibroblast (Scar Repair/Skin Inflammation) (n = 2) | [30,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bayulgen, D.S.; Veronese, S.; Sbarbati, A. Dedifferentiation of Mature Adipocytes and Their Future Potential for Regenerative Medicine Applications. Biomedicines 2026, 14, 95. https://doi.org/10.3390/biomedicines14010095
Bayulgen DS, Veronese S, Sbarbati A. Dedifferentiation of Mature Adipocytes and Their Future Potential for Regenerative Medicine Applications. Biomedicines. 2026; 14(1):95. https://doi.org/10.3390/biomedicines14010095
Chicago/Turabian StyleBayulgen, Deniz Simal, Sheila Veronese, and Andrea Sbarbati. 2026. "Dedifferentiation of Mature Adipocytes and Their Future Potential for Regenerative Medicine Applications" Biomedicines 14, no. 1: 95. https://doi.org/10.3390/biomedicines14010095
APA StyleBayulgen, D. S., Veronese, S., & Sbarbati, A. (2026). Dedifferentiation of Mature Adipocytes and Their Future Potential for Regenerative Medicine Applications. Biomedicines, 14(1), 95. https://doi.org/10.3390/biomedicines14010095






