Single-Cell Transcriptomics on PRPF31-Mutated Retinal Organoids Reveal Early Müller Glial Activation and Progressive Photoreceptor Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
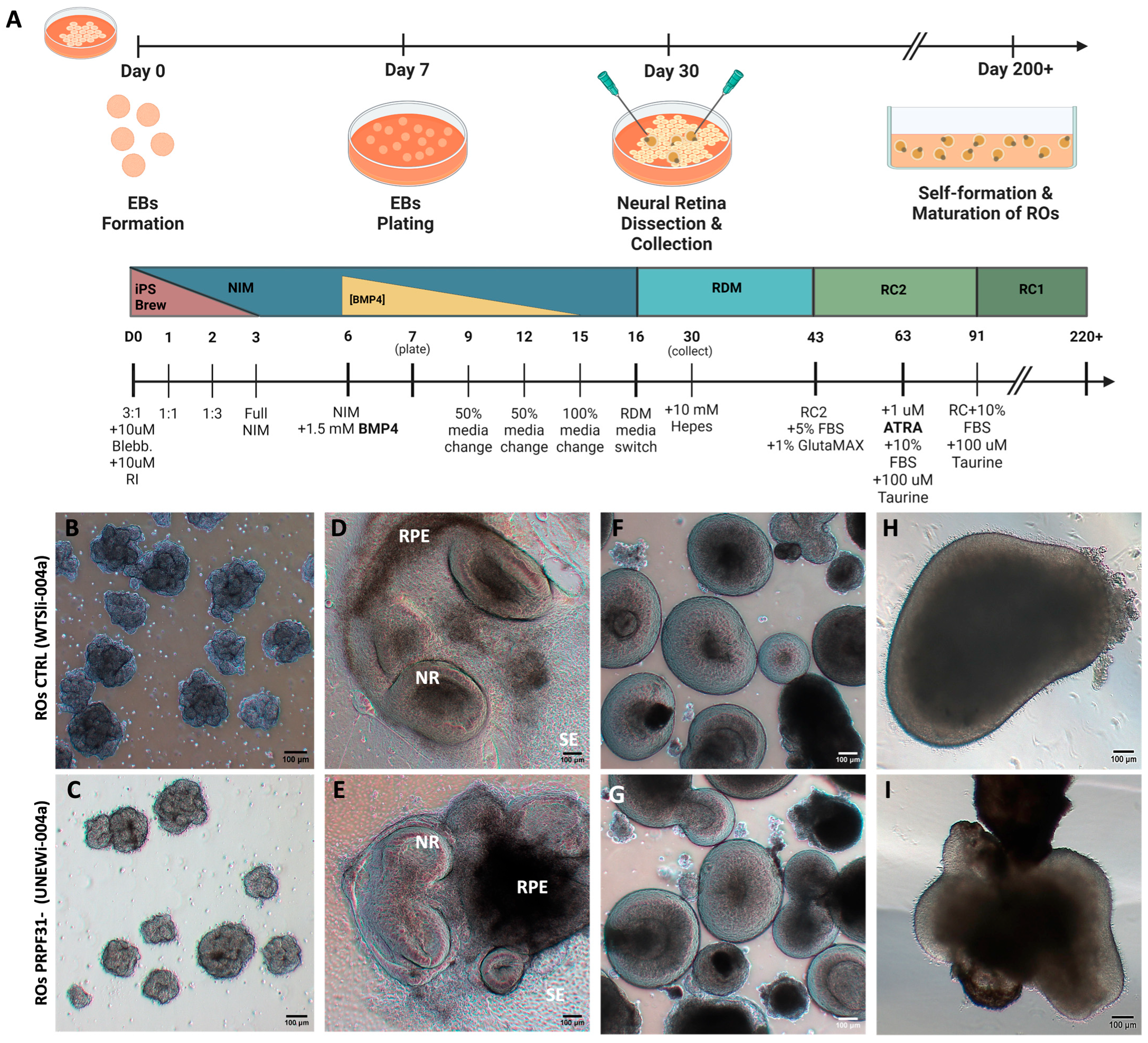
2.2. Retinal Organoid Differentiation
2.3. Cells or Organoid Fixation and Cryoprotection
2.4. Immunostaining, Imaging, and Cell Counting
2.5. Flow Cytometry
2.6. RNA Extraction and cDNA Synthesis
2.7. RTqPCR
2.8. PCR Amplification of PRPF31 Gene
2.9. Multi-Electrode Array (MEA) Recording
2.10. Single-Cell RNA Sequencing (scRNAseq)
2.11. scRNAseq Data Analyses
2.12. Statistical Analysis
2.13. Data Availability
3. Results
3.1. Generation and Characterization of ROs
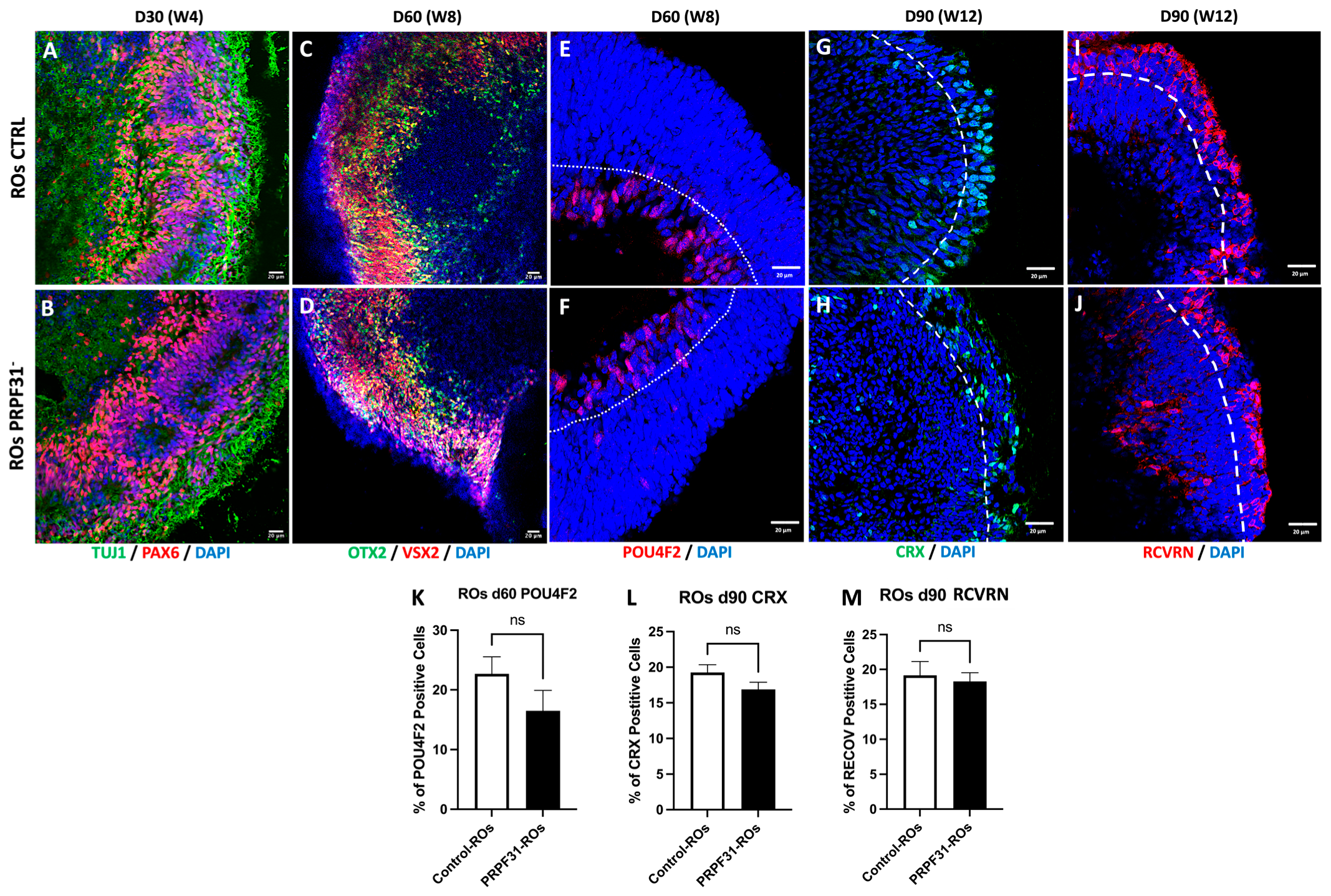
3.2. Early Effects of the PRPF31 Mutation on ROs Development
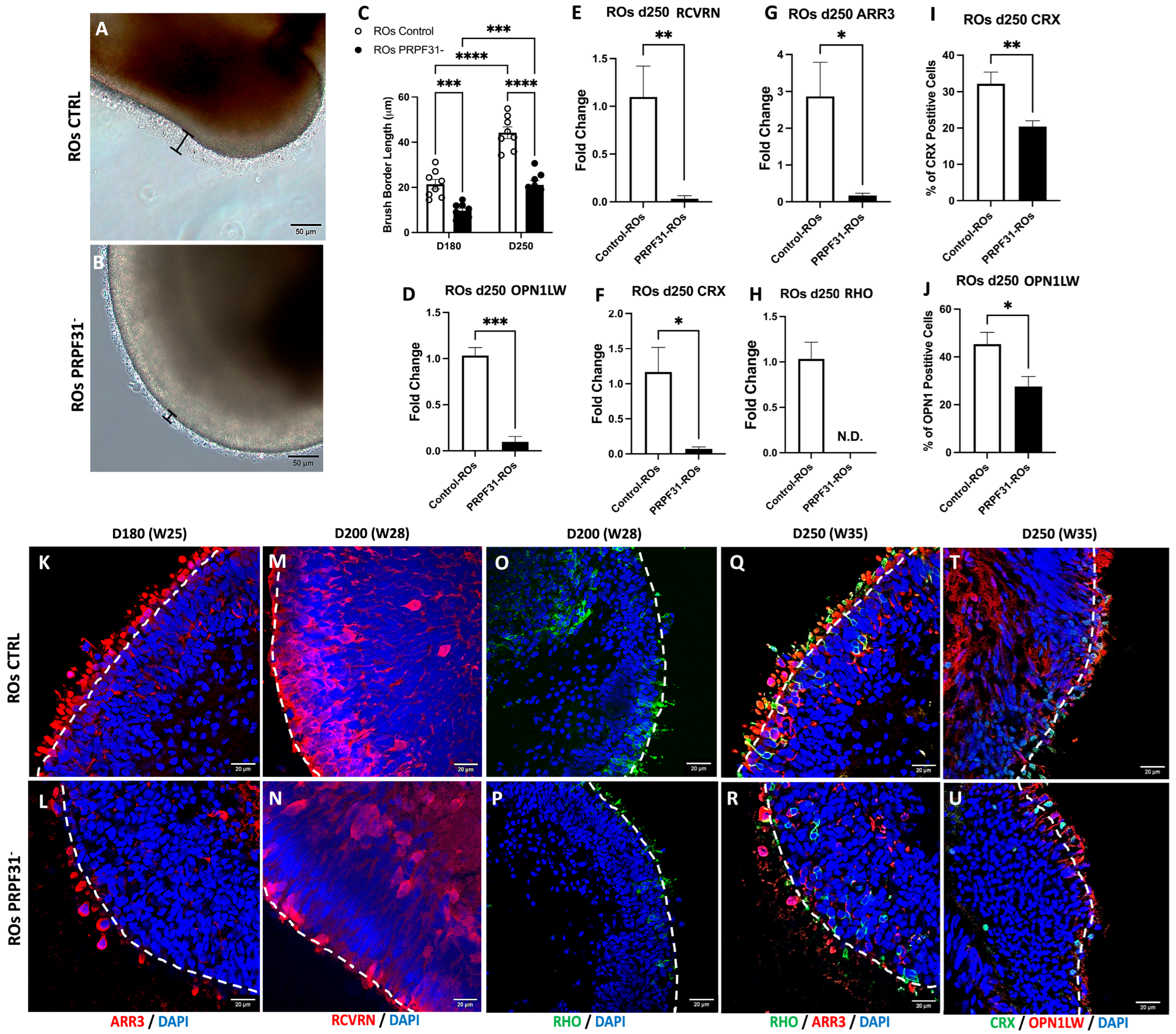
3.3. The PRPF31 Mutation Leads to Photoreceptor Disorganization and Impaired Marker Expression in ROs
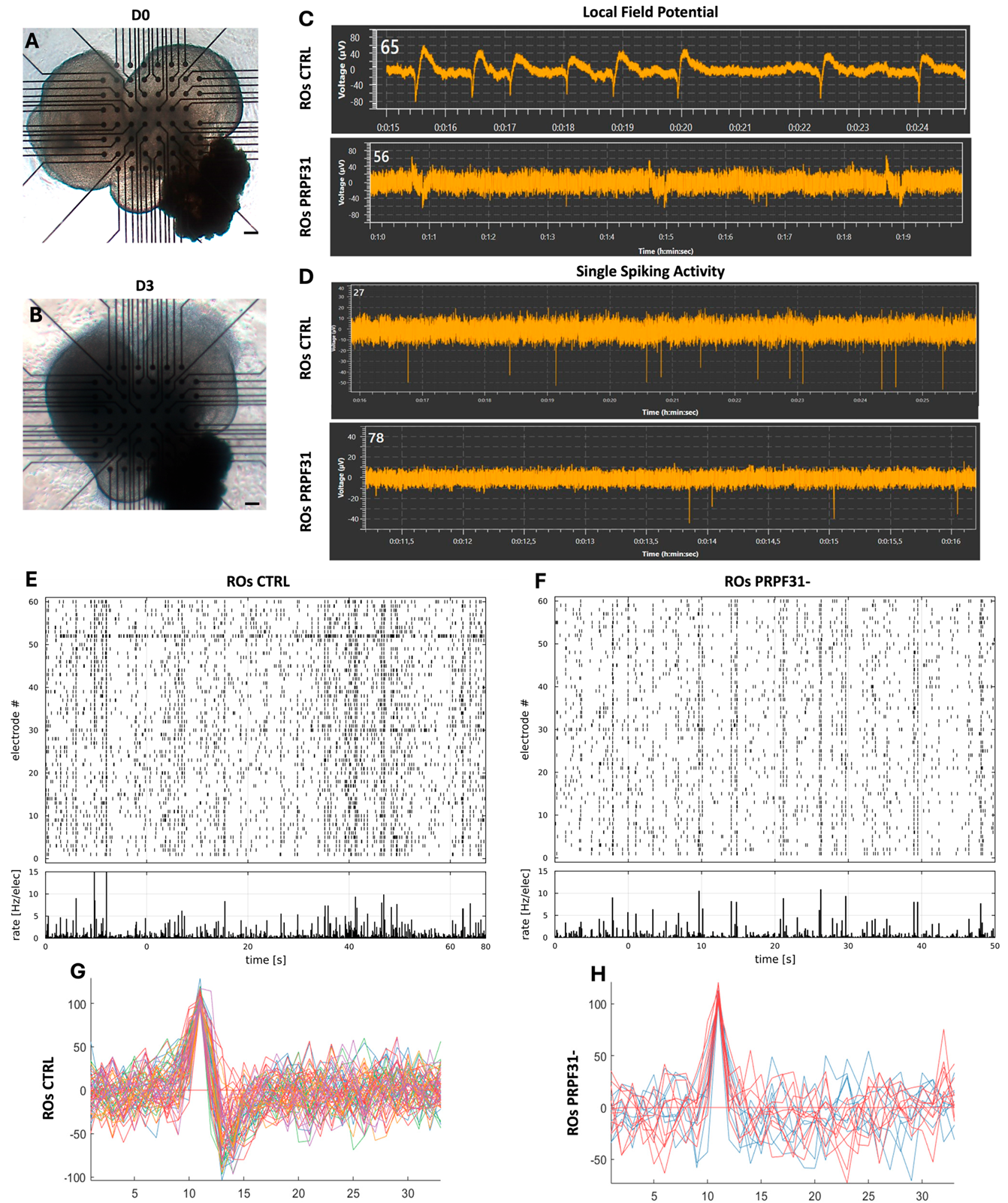
3.4. Impaired Electrophysiological Responses in PRPF31-ROs
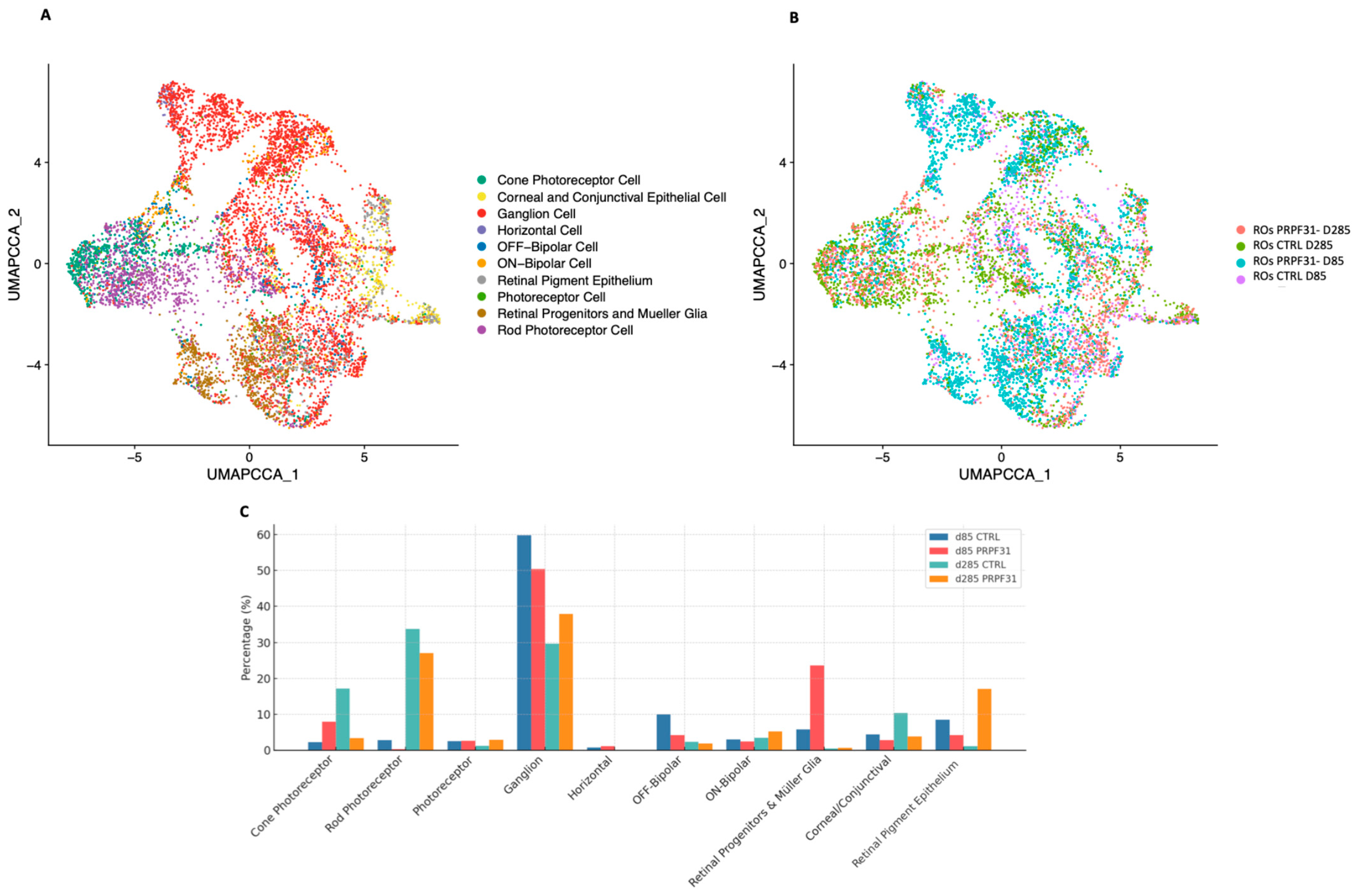
3.5. scRNAseq Revealed Early Müller Glia Marker Expression and Late Photoreceptor Transcriptomic Collapse in PRPF31-ROs
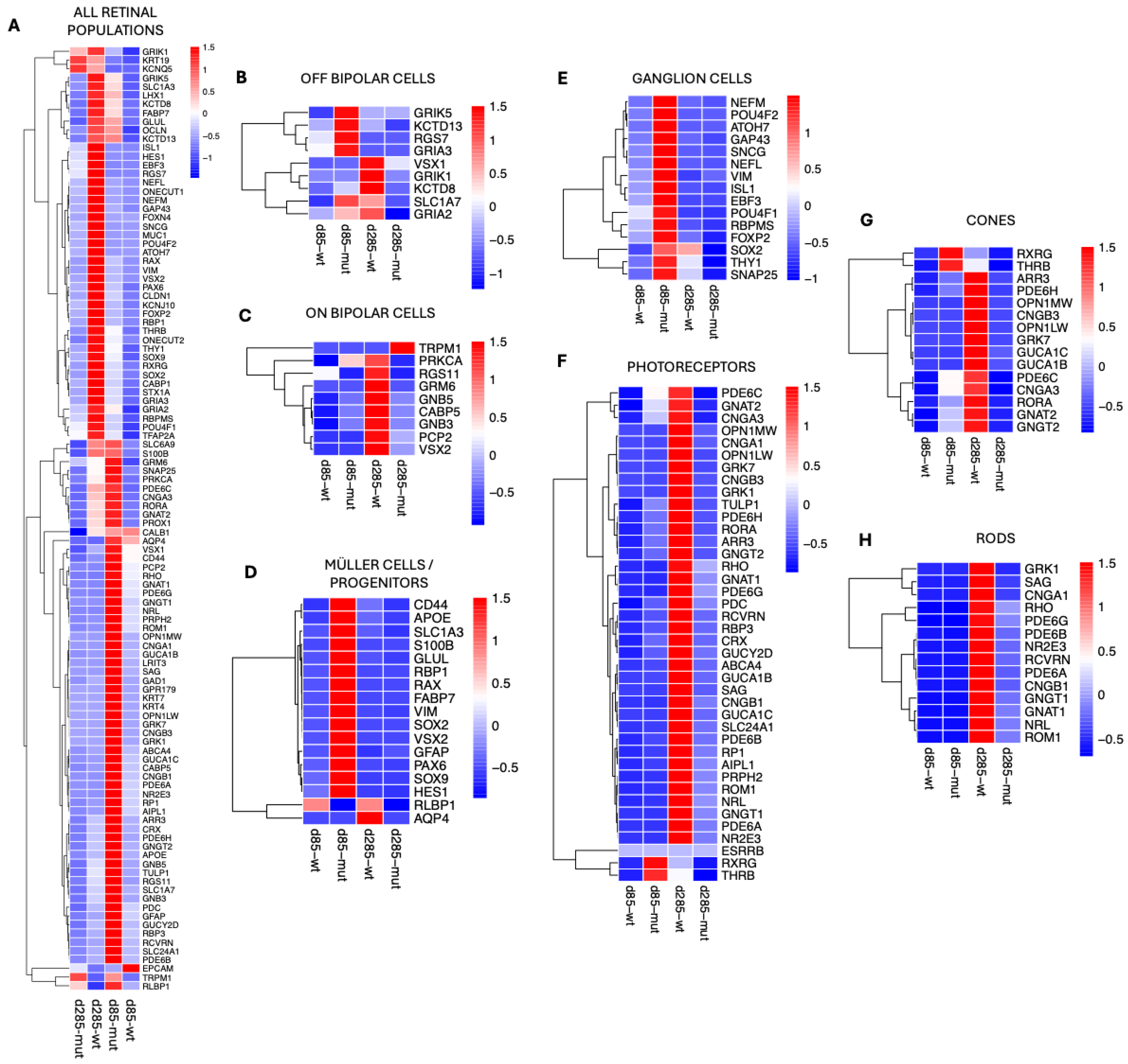
3.6. Cell-Type-Specific Molecular Responses to the PRPF31 Mutation in ROs Drive Early Müller Glial Activation and Progressive Retinal Degeneration
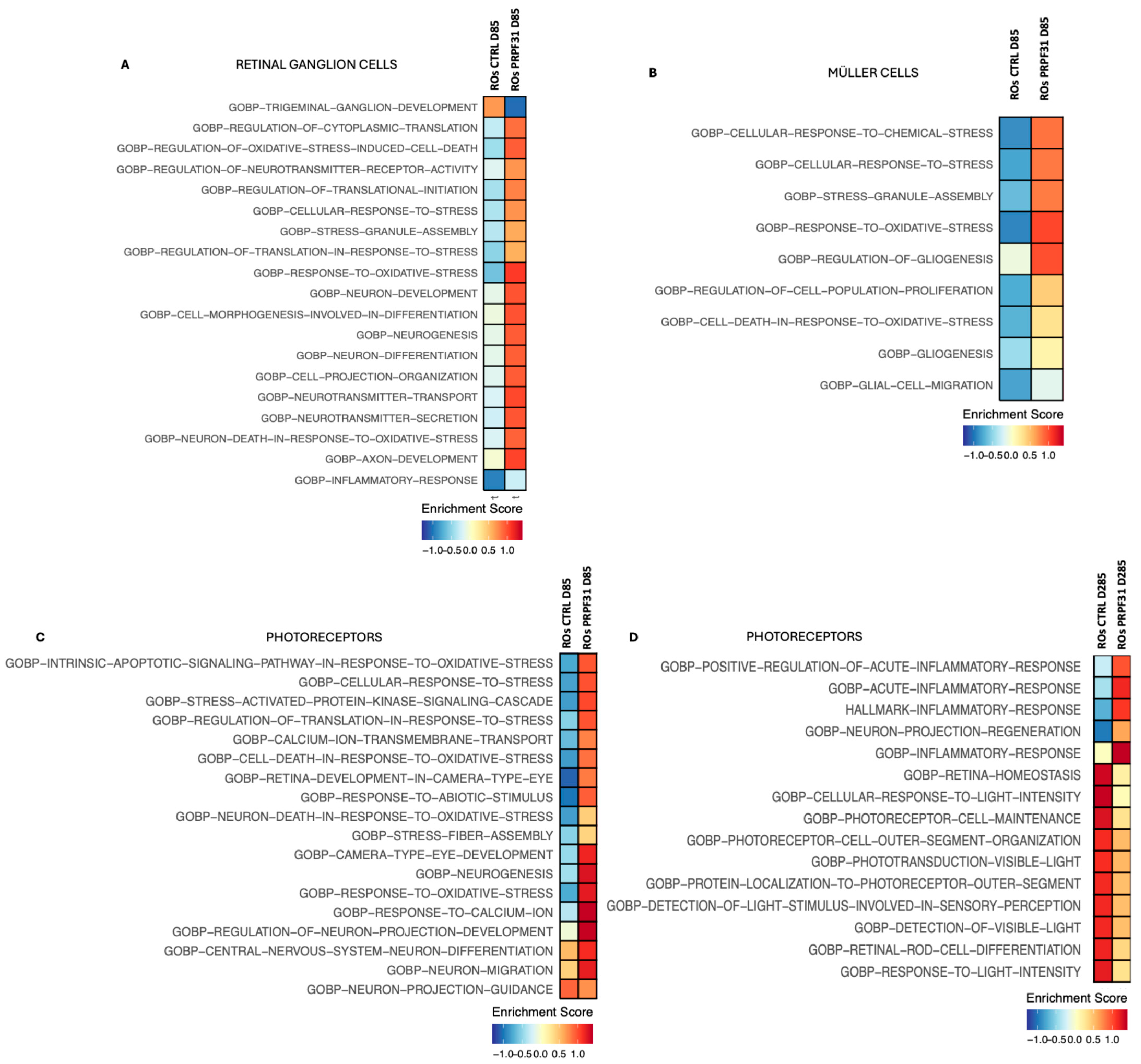
3.7. Pathway Enrichement on scRNAseq Data Revealed Alterated Pathways Involved in Phototransduction, Oxidative Stress, and Inflammation
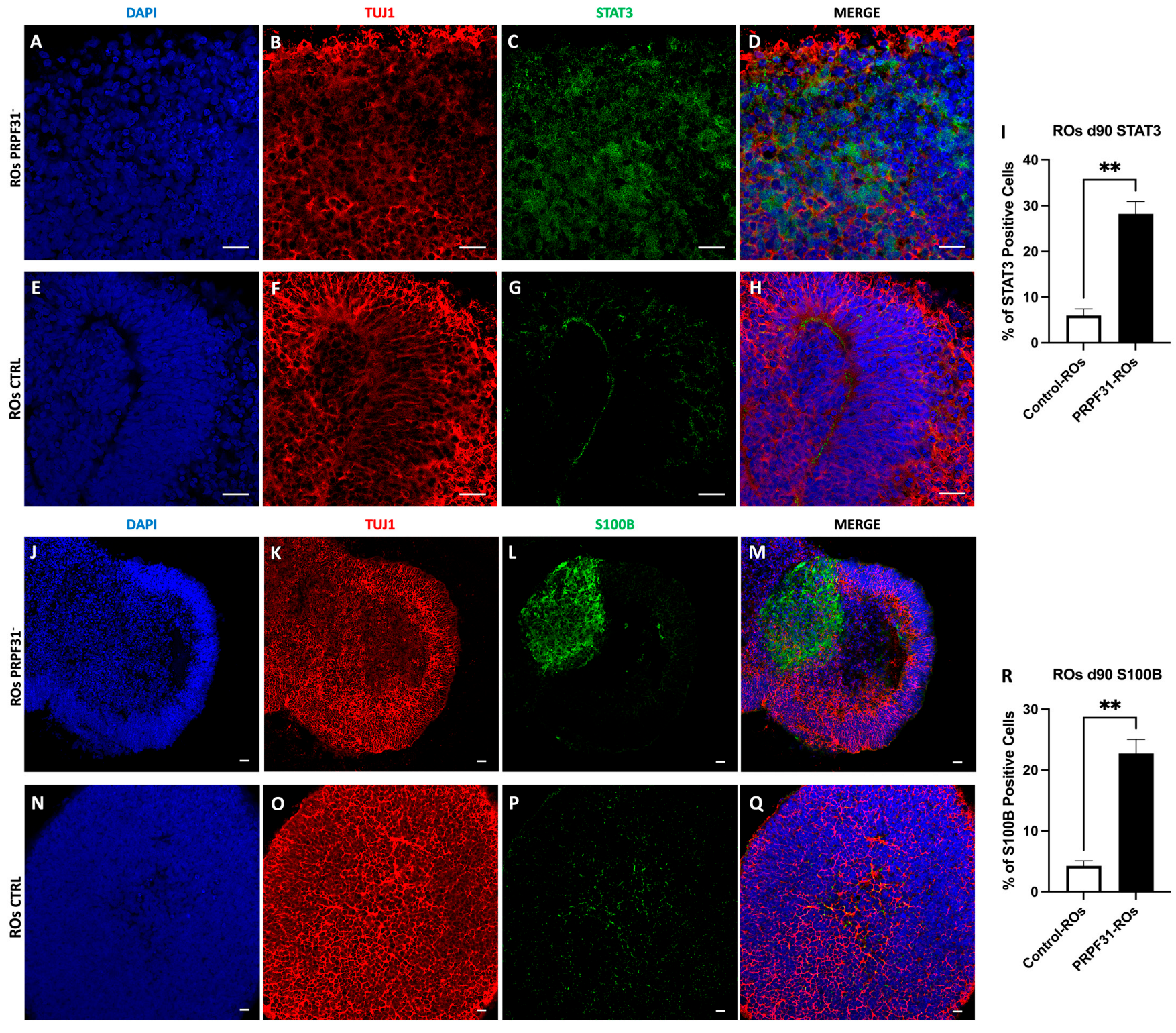
3.8. Upregulation of STAT3 and S100B in PRPF31-ROs
4. Discussion
- Impaired photoreceptors at late-stage PRPF31-ROs
- Reduced electrical activity in PRPF31-ROs
- Müller glia activation in PRPF31-ROs
- Inner retinal Dysfunction
- Photoreceptor Degenerative Expression Dynamics in PRPF31-ROs
- Limitations of the study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| adRP | Autosomal Dominant Retinitis Pigmentosa |
| arRP | Autosomal Recessive Retinitis Pigmentosa |
| BC | Bipolar Cell |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EB | Embryoid Bodies |
| GFR | Growth Factor Reduced |
| GCL | Ganglion Cell Layer |
| GO | Gene Ontology |
| hiPSC | Human Induced Pluripotent Stem Cell |
| IF | Immunofluorescence |
| KOSR | Knock-Out Serum Replacement |
| MEA | Micro Electrode Array |
| NGS | Next Generation Sequencing |
| NIM | Neural Induction Media |
| NR | Neural Retina |
| OLM | Outer Limiting Membrane |
| ONL | Outer Nuclear Layer |
| OPV | Organic Photo-Voltaic |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| PR | Photoreceptor |
| RC1 | Retinal Constituent 1 |
| RC2 | Retinal Constituent 2 |
| RDM | Retinal Differentiation Media |
| RGC | Retinal Ganglion Cell |
| RO | Retinal Organoid |
| RP | Retinitis Pigmentosa |
| RPE | Retinal Pigment Epithelium |
| scRNAseq | Single-cell RNA Sequencing |
| SEM | Standard Error of the Mean |
| ssGSEA | single-sample Gene Set Enrichment Analysis |
| TBS | TRIS-Buffered Saline |
| UMAP | Uniform Manifold Approximation and Projection |
| UPR | Unfolded Protein Response |
| WT | Wild Type |
| xlRP | X-linked Retinitis Pigmentosa |
References
- Galan, A.; Chizzolini, M.; Milan, E.; Sebastiani, A.; Costagliola, C.; Parmeggiani, F. Good Epidemiologic Practice in Retinitis Pigmentosa: From Phenotyping to Biobanking. Curr. Genom. 2011, 12, 260–266. [Google Scholar] [CrossRef]
- Ali-Nasser, T.; Zayit-Soudry, S.; Banin, E.; Sharon, D.; Ben-Yosef, T. Autosomal dominant retinitis pigmentosa with incomplete penetrance due to an intronic mutation of the PRPF31 gene. Mol. Vis. 2022, 28, 359–368. [Google Scholar] [PubMed]
- Lukovic, D.; Artero-Castro, A.; García-Delgado, A.B.; Bernal, M.d.l.A.M.; Luna-Peláez, N.; Lloret, A.D.; Espejo, R.P.; Kamenarova, K.; Fernández-Sánchez, L.; Cuenca, N.; et al. Human iPSC derived disease model of MERTK-associated retinitis pigmentosa. Sci. Rep. 2015, 5, 12910. [Google Scholar] [CrossRef]
- Nazlamova, L.; Vasquez, S.S.V.; Lord, J.; Karthik, V.; Cheung, M.-K.; Lakowski, J.; Wheway, G. Microtubule modification defects underlie cilium degeneration in cell models of retinitis pigmentosa associated with pre-mRNA splicing factor mutations. Front. Genet. 2022, 13, 1009430. [Google Scholar] [CrossRef] [PubMed]
- Shanks, M.; Downes, S.M.; Copley, R.R.; Lise, S.; Broxholme, J.; Hudspith, K.A.; Kwasniewska, A.; Davies, W.I.L.; Hankins, M.W.; Packham, E.; et al. Next-generation sequencing (NGS) as a diagnostic tool for retinal degeneration reveals a much higher detection rate in early-onset disease. Eur. J. Hum. Genet. 2012, 21, 274–280. [Google Scholar] [CrossRef]
- Bravo-Gil, N.; Pozo, M.G.; Martín-Sánchez, M.; Méndez-Vidal, C.; Rodríguez-de-la-Rúa-Franch, E.; Borrego, S.; Antiñolo, G. Unravelling the genetic basis of simplex Retinitis Pigmentosa cases. Sci. Rep. 2017, 7, 41937. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, S.; Li, P.; Yao, K. Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies. Int. J. Mol. Sci. 2022, 23, 4883. [Google Scholar] [CrossRef]
- González-Del Pozo, M.; Fernández-Suárez, E.; Martín-Sánchez, M.; Bravo-Gil, N.; Méndez-Vidal, C.; Rodríguez-de la Rúa, E.; Borrego, S.; Antiñolo, G. Unmasking Retinitis Pigmentosa complex cases by a whole genome sequencing algorithm based on open-access tools: Hidden recessive inheritance and potential oligogenic variants. J. Transl. Med. 2020, 18, 73. [Google Scholar] [CrossRef]
- Aweidah, H.; Xi, Z.; Sahel, J.-A.; Byrne, L.C. PRPF31-retinitis pigmentosa: Challenges and opportunities for clinical translation. Vis. Res. 2023, 213, 108315. [Google Scholar] [CrossRef]
- Roshandel, D.; Thompson, J.A.; Jeffery, R.C.H.; Zhang, D.; Lamey, T.M.; McLaren, T.L.; Roach, J.N.D.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Clinical Evidence for the Importance of the Wild-Type PRPF31 Allele in the Phenotypic Expression of RP11. Genes 2021, 12, 915. [Google Scholar] [CrossRef]
- Yang, C.; Georgiou, M.; Atkinson, R.; Collin, J.; Al-Aama, J.Y.; Nagaraja-Grellscheid, S.; Johnson, C.A.; Ali, R.R.; Armstrong, L.; Mozaffari-Jovin, S.; et al. Pre-mRNA Processing Factors and Retinitis Pigmentosa: RNA Splicing and Beyond. Front. Cell Dev. Biol. 2021, 9, 700276. [Google Scholar] [CrossRef]
- Buskin, A.; Zhu, L.; Chichagova, V.; Basu, B.; Mozaffari-Jovin, S.; Dolan, D.; Droop, A.; Collin, J.; Bronstein, R.; Mehrotra, S.; et al. Disrupted alternative splicing for genes implicated in splicing and ciliogenesis causes PRPF31 retinitis pigmentosa. Nat. Commun. 2018, 9, 4234. [Google Scholar] [CrossRef]
- Farkas, M.H.; Lew, D.; Sousa, M.E.; Bujakowska, K.M.; Chatagnon, J.; Bhattacharya, S.S.; Pierce, E.A.; Nandrot, E.F. Mutations in Pre-mRNA Processing Factors 3, 8, and 31 Cause Dysfunction of the Retinal Pigment Epithelium. Am. J. Pathol. 2014, 184, 2641–2652. [Google Scholar] [CrossRef]
- Georgiou, M.; Grewal, P.S.; Narayan, A.; Alser, M.; Ali, N.; Fujinami, K.; Webster, A.R.; Michaelides, M. Sector Retinitis Pigmentosa: Extending the Molecular Genetics Basis and Elucidating the Natural History. Am. J. Pathol. 2020, 221, 299–310. [Google Scholar] [CrossRef]
- Watson, A.; Lako, M. Retinal organoids provide unique insights into molecular signatures of inherited retinal disease throughout retinogenesis. J. Anat. 2022, 243, 186–203. [Google Scholar] [CrossRef]
- Bellapianta, A.; Cetkovic, A.; Bolz, M.; Salti, A. Retinal Organoids and Retinal Prostheses: An Overview. Int. J. Mol. Sci. 2022, 23, 2922. [Google Scholar] [CrossRef]
- Cetkovic, A.; Bellapianta, A.; Irimia-Vladu, M.; Hofinger, J.; Yumusak, C.; Corna, A.; Scharber, M.C.; Zeck, G.; Sariciftci, N.S.; Bolz, M.; et al. In Vitro Cytotoxicity of D18 and Y6 as Potential Organic Photovoltaic Materials for Retinal Prostheses. Int. J. Mol. Sci. 2022, 23, 8666. [Google Scholar] [CrossRef] [PubMed]
- Ouaidat, S.; Bellapianta, A.; Ammer-Pickhardt, F.; Taghipour, T.; Bolz, M.; Salti, A. Exploring organoid and assembloid technologies: A focus on retina and brain. Expert Rev. Mol. Med. 2025, 27, e14. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Atkinson, R.; Mozaffari-Jovin, S.; Lako, M. Progressive accumulation of cytoplasmic aggregates in PRPF31 retinal pigment epithelium cells interferes with cell survival. Clin. Transl. Discov. 2022, 2, e89. [Google Scholar] [CrossRef]
- Rodrigues, A.; Slembrouck-Brec, A.; Nanteau, C.; Terray, A.; Tymoshenko, Y.; Zagar, Y.; Reichman, S.; Xi, Z.; Sahel, J.-A.; Fouquet, S.; et al. Modeling PRPF31 retinitis pigmentosa using retinal pigment epithelium and organoids combined with gene augmentation rescue. NPJ Regen. Med. 2022, 7, 39. [Google Scholar] [CrossRef]
- Fligor, C.M.; Huang, K.-C.; Lavekar, S.S.; VanderWall, K.B.; Meyer, J.S. Differentiation of retinal organoids from human pluripotent stem cells. Methods Cell Biol. 2020, 159, 279–302. [Google Scholar] [CrossRef]
- Hallam, D.; Hilgen, G.; Dorgau, B.; Zhu, L.; Yu, M.; Bojic, S.; Hewitt, P.; Schmitt, M.; Uteng, M.; Kustermann, S.; et al. Human-Induced Pluripotent Stem Cells Generate Light Responsive Retinal Organoids with Variable and Nutrient-Dependent Efficiency. Stem Cells 2018, 36, 1535–1551. [Google Scholar] [CrossRef]
- Cowan, C.S.; Renner, M.; Gennaro, M.D.; Gross-Scherf, B.; Goldblum, D.; Hou, Y.; Munz, M.; Rodrigues, T.M.; Król, J.; Szikra, T.; et al. Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell 2020, 182, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Buskin, A.; Zhu, L.; Chichagova, V.; Basu, B.; Mozaffari-Jovin, S.; Dolan, D.; Droop, A.; Collin, J.; Bronstein, R.; Mehrotra, S.; et al. Human iPSC-derived RPE and retinal organoids reveal impaired alternative splicing of genes involved in pre-mRNA splicing in PRPF31 autosomal dominant retinitis pigmentosa. bioRxiv 2017. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, S.; Basu, B.; Suresh, S.; Meera, V.; Riya, P.A.; Parvathy, S.; Das, A.V.; Sivakumar, K.C.; Nelson-Sathi, S.; James, J. Pax6 modulates intra-retinal axon guidance and fasciculation of retinal ganglion cells during retinogenesis. Sci. Rep. 2020, 10, 16075. [Google Scholar] [CrossRef] [PubMed]
- Diacou, R.; Nandigrami, P.; Fiser, A.; Liu, W.; Ashery-Padan, R.; Cvekl, A. Cell fate decisions, transcription factors and signaling during early retinal development. Prog. Retin. Eye Res. 2022, 91, 101093. [Google Scholar] [CrossRef]
- Wright, L.S.; Pinilla, I.; Saha, J.; Clermont, J.; Lien, J.; Borys, K.D.; Capowski, E.E.; Phillips, M.J.; Gamm, D.M. VSX2 and ASCL1 Are Indicators of Neurogenic Competence in Human Retinal Progenitor Cultures. PLoS ONE 2015, 10, e0135830. [Google Scholar] [CrossRef]
- Brzezinski, J.A.; Reh, T.A. Photoreceptor cell fate specification in vertebrates. Development 2015, 142, 3263–3273. [Google Scholar] [CrossRef]
- Fuhrmann, S. Eye Morphogenesis and Patterning of the Optic Vesicle. Curr. Top. Dev. Biol. 2010, 93, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Capowski, E.E.; Samimi, K.; Mayerl, S.J.; Phillips, M.J.; Pinilla, I.; Howden, S.E.; Saha, J.; Jansen, A.D.; Edwards, K.L.; Jager, L.D.; et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 2019, 146, dev171686. [Google Scholar] [CrossRef]
- Gu, L.; Cong, P.; Ning, Q.; Jiang, B.; Wang, J.; Cui, H. The causal mutation in ARR3 gene for high myopia and progressive color vision defect. Sci. Rep. 2023, 13, 8986. [Google Scholar] [CrossRef]
- Sakurai, K.; Chen, J.; Khani, S.C.; Kefalov, V.J. Regulation of Mammalian Cone Phototransduction by Recoverin and Rhodopsin Kinase. J. Biol. Chem. 2015, 290, 9239–9250. [Google Scholar] [CrossRef]
- Klenchin, V.A.; Calvert, P.D.; Bownds, M.D. Inhibition of Rhodopsin Kinase by Recoverin. J. Biol. Chem. 1995, 270, 16147–16152. [Google Scholar] [CrossRef]
- Furukawa, T.; Morrow, E.M.; Cepko, C.L. Crx, a Novel otx-like Homeobox Gene, Shows Photoreceptor-Specific Expression and Regulates Photoreceptor Differentiation. Cell 1997, 91, 531–541. [Google Scholar] [CrossRef]
- Ruzycki, P.A.; Tran, N.M.; Kolesnikov, A.V.; Kefalov, V.J.; Chen, S. Graded gene expression changes determine phenotype severity in mouse models of CRX-associated retinopathies. Genome Biol. 2015, 16, 171. [Google Scholar] [CrossRef]
- Hussey, K.A.; Hadyniak, S.E.; Johnston, R.J. Patterning and Development of Photoreceptors in the Human Retina. Front. Cell Dev. Biol. 2022, 10, 878350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Najafov, A.; Py, B.F. Roles of Caspases in Necrotic Cell Death. Cell 2016, 167, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Erskine, L.; Herrera, E. Connecting the Retina to the Brain. ASN Neuro 2014, 6, 1759091414562107. [Google Scholar] [CrossRef] [PubMed]
- Sluch, V.M.; Chamling, X.; Liu, M.M.; Berlinicke, C.; Cheng, J.; Mitchell, K.L.; Welsbie, D.S.; Zack, D.J. Enhanced Stem Cell Differentiation and Immunopurification of Genome Engineered Human Retinal Ganglion Cells. Stem Cells Transl. Med. 2017, 6, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; O’Day, D.R.; Pliner, H.A.; Kingsley, P.D.; Deng, M.; Daza, R.M.; Zager, M.A.; Aldinger, K.A.; Blecher-Gonen, R.; Zhang, F.; et al. A human cell atlas of fetal gene expression. Science 2020, 370, eaba7721. [Google Scholar] [CrossRef] [PubMed]
- Collin, J.; Queen, R.; Zerti, D.; Dorgau, B.; Hussain, R.; Coxhead, J.; Cockell, S.; Lako, M. Deconstructing Retinal Organoids: Single Cell RNA-Seq Reveals the Cellular Components of Human Pluripotent Stem Cell-Derived Retina. Stem Cells 2019, 37, 593–598. [Google Scholar] [CrossRef]
- Haumann, I.; Junghans, D.; Anstötz, M.; Frotscher, M. Presynaptic localization of GluK5 in rod photoreceptors suggests a novel function of high affinity glutamate receptors in the mammalian retina. PLoS ONE 2017, 12, e0172967. [Google Scholar] [CrossRef]
- Lindstrom, S.H.; Ryan, D.G.; Shi, J.; DeVries, S.H. Kainate receptor subunit diversity underlying response diversity in retinal off bipolar cells. J. Physiol. 2014, 592, 1457–1477. [Google Scholar] [CrossRef]
- Shim, H.; Wang, C.-T.; Chen, Y.-L.; Chau, V.Q.; Fu, K.G.; Yang, J.; McQuiston, A.R.; Fisher, R.A.; Chen, C.-K. Defective Retinal Depolarizing Bipolar Cells in Regulators of G Protein Signaling (RGS) 7 and 11 Double Null Mice. J. Biol. Chem. 2012, 287, 14873–14879. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Aouacheria, A.; Lionnard, L.; Metz, K.A.; Soane, L.; Kamiya, A.; Hardwick, J.M. KCTD: A new gene family involved in neurodevelopmental and neuropsychiatric disorders. CNS Neurosci. Ther. 2019, 25, 887–902. [Google Scholar] [CrossRef]
- Gécz, J.; Barnett, S.; Liu, J.; Hollway, G.; Donnelly, A.; Eyre, H.; Eshkevari, H.S.; Baltazar, R.; Grunn, A.; Nagaraja, R.; et al. Characterization of the Human Glutamate Receptor Subunit 3 Gene (GRIA3), a Candidate for Bipolar Disorder and Nonspecific X-Linked Mental Retardation. Genomics 1999, 62, 356–368. [Google Scholar] [CrossRef]
- Gilhooley, M.J.; Hickey, D.G.; Lindner, M.; Palumaa, T.; Hughes, S.; Peirson, S.N.; MacLaren, R.E.; Hankins, M.W. ON-bipolar cell gene expression during retinal degeneration: Implications for optogenetic visual restoration. Exp. Eye Res. 2021, 207, 108553. [Google Scholar] [CrossRef]
- Xu, Y.; Sulaiman, P.; Feddersen, R.M.; Liu, J.; Smith, R.G.; Vardi, N. Retinal ON bipolar cells express a new PCP2 splice variant that accelerates the light response. J. Neurosci. 2008, 28, 8873–8884. [Google Scholar] [CrossRef]
- Saxena, A.; Scaini, G.; Bavaresco, D.V.; Leite, C.; Valvassori, S.S.; Carvalho, A.F.; Quevedo, J. Role of Protein Kinase C in Bipolar Disorder: A Review of the Current Literature. Mol. Neuropsychiatry 2017, 3, 108–124. [Google Scholar] [CrossRef]
- Liu, J.-B.; Yuan, H.-L.; Zhang, G.; Ke, J.-B. Comprehensive Characterization of a Subfamily of Ca2+ -Binding Proteins in Mouse and Human Retinal Neurons at Single-Cell Resolution. Eneuro 2024, 11, ENEURO.0145-24.2024. [Google Scholar] [CrossRef]
- Cao, Y.; Pahlberg, J.; Sarria, I.; Kamasawa, N.; Sampath, A.P.; Martemyanov, K.A. Regulators of G protein signaling RGS7 and RGS11 determine the onset of the light response in ON bipolar neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 7905–7910. [Google Scholar] [CrossRef]
- Shao, Z.; Tumber, A.; Maynes, J.; Tavares, E.; Kannu, P.; Heon, E.; Vincent, A. Unique retinal signaling defect in GNB5-related disease. Doc. Ophthalmol. 2020, 140, 273–277. [Google Scholar] [CrossRef]
- Gayet-Primo, J.; Puthussery, T. Alterations in Kainate Receptor and TRPM1 Localization in Bipolar Cells after Retinal Photoreceptor Degeneration. Front. Cell. Neurosci. 2015, 9, 486. [Google Scholar] [CrossRef]
- Koike, C.; Obara, T.; Uriu, Y.; Numata, T.; Sanuki, R.; Miyata, K.; Koyasu, T.; Ueno, S.; Funabiki, K.; Tani, A.; et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc. Natl. Acad. Sci. USA 2010, 107, 332–337. [Google Scholar] [CrossRef]
- Erickson, P.A.; Fisher, S.K.; Guérin, C.J.; Anderson, D.H.; Kaska, D.D. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp. Eye Res. 1987, 44, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Verardo, M.R.; Lewis, G.P.; Takeda, M.; Linberg, K.A.; Byun, J.; Luna, G.; Wilhelmsson, U.; Pekny, M.; Chen, D.; Fisher, S.K. Abnormal Reactivity of Müller Cells after Retinal Detachment in Mice Deficient in GFAP and Vimentin. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3659–3665. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.L.; Liem, R.K.H. The neuronal intermediate filament, α-internexin is transiently expressed in amacrine cells in the developing mouse retina. Exp. Eye Res. 1995, 61, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Harada, C.; Watanabe, M.; Inoue, Y.; Sakagawa, T.; Nakayama, N.; Sasaki, S.; Okuyama, S.; Watase, K.; Wada, K.; et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc. Natl. Acad. Sci. USA 1998, 95, 4663–4666. [Google Scholar] [CrossRef]
- Bringmann, A.; Grosche, A.; Pannicke, T.; Reichenbach, A. GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells. Front. Endocrinol. 2013, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Amaratunga, A.; Abraham, C.R.; Edwards, R.B.; Sandell, J.H.; Schreiber, B.M.; Fine, R.E. Apolipoprotein E Is Synthesized in the Retina by Müller Glial Cells, Secreted into the Vitreous, and Rapidly Transported into the Optic Nerve by Retinal Ganglion Cells. J. Biol. Chem. 1996, 271, 5628–5632. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Madden, A.F.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef]
- Campbell, W.A.; Tangeman, A.; El-Hodiri, H.M.; Hawthorn, E.C.; Hathoot, M.; Blum, S.A.; Hoang, T.; Blackshaw, S.; Fischer, A.J. Fatty acid-binding proteins and fatty acid synthase influence glial reactivity and promote the formation of Müller glia-derived progenitor cells in the chick retina. Development 2022, 149, dev200127. [Google Scholar] [CrossRef]
- Catsicas, S.; Catsicas, M.; Keyser, K.T.; Karten, H.J.; Wilson, M.C.; Milner, R.J. Differential expression of the presynaptic protein SNAP-25 in mammalian retina. J. Neurosci. Res. 1992, 33, 1–9. [Google Scholar] [CrossRef]
- Hsiao, Y.-T.; Shu, W.-C.; Chen, P.-C.; Yang, H.-J.; Chen, H.-Y.; Hsu, S.-P.; Huang, Y.-T.; Yang, C.-C.; Chen, Y.-J.; Yu, N.-Y.; et al. Presynaptic SNAP-25 regulates retinal waves and retinogeniculate projection via phosphorylation. Proc. Natl. Acad. Sci. USA 2019, 116, 3262–3267. [Google Scholar] [CrossRef]
- Dijk, F.; Bergen, A.A.; Kamphuis, W. GAP-43 expression is upregulated in retinal ganglion cells after ischemia/reperfusion-induced damage. Exp. Eye Res. 2007, 84, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, C.; Severin, C.; Sretavan, D.W. GAP-43 mediates retinal axon interaction with lateral diencephalon cells during optic tract formation. Development 2000, 127, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Rheaume, B.A.; Jereen, A.; Bolisetty, M.; Sajid, M.S.; Yang, Y.; Renna, K.; Sun, L.; Robson, P.; Trakhtenberg, E.F. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat. Commun. 2018, 9, 2759. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; De Sevilla Müller, L.P.; Brecha, N.C. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Dash, N.; Mazo, K.W.; Chopra, M.; Avila, M.P.; Patel, A.; Wong, R.M.; Jia, C.; Do, H.; Cheng, J.; et al. Human retinal ganglion cell neurons generated by synchronous BMP inhibition and transcription factor mediated reprogramming. NPJ Regen. Med. 2023, 8, 55. [Google Scholar] [CrossRef]
- Huang, L.; Hu, F.; Xie, X.; Harder, J.; Fernandes, K.; Zeng, X.; Libby, R.; Gan, L. Pou4f1 and pou4f2 are dispensable for the long-term survival of adult retinal ganglion cells in mice. PLoS ONE 2014, 9, e94173. [Google Scholar] [CrossRef]
- Huang, W.; Fileta, J.; Guo, Y.; Grosskreutz, C.L. Downregulation of Thy1 in Retinal Ganglion Cells in Experimental Glaucoma. Curr. Eye Res. 2006, 31, 265–271. [Google Scholar] [CrossRef]
- Laboissonniere, L.A.; Goetz, J.J.; Martin, G.M.; Bi, R.; Lund, T.J.S.; Ellson, L.; Lynch, M.R.; Mooney, B.; Wickham, H.; Liu, P.; et al. Molecular signatures of retinal ganglion cells revealed through single cell profiling. Sci. Rep. 2019, 9, 15778. [Google Scholar] [CrossRef] [PubMed]
- Taranova, O.; Magness, S.T.; Fagan, B.M.; Wu, Y.; Surzenko, N.; Hutton, S.R.; Pevny, L. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006, 20, 1187–1202. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Baumann, B.; Rosenberg, T.; Kellner, U.; Lorenz, B.; Vadalà, M.; Jacobson, S.G.; Wissinger, B. Mutations in the Cone Photoreceptor G-Protein α-Subunit Gene GNAT2 in Patients with Achromatopsia. Am. J. Hum. Genet. 2002, 71, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Robson, A.G.; Singh, N.; Pontikos, N.; Kane, T.; Hirji, N.; Ripamonti, C.; Rotsos, T.; Dubra, A.; Kalitzeos, A.; et al. Deep Phenotyping of PDE6C-Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5112–5123. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Cote, R. Phosphodiesterase 6H, cone-specific inhibitor. AfCS-Nat. Mol. Pages 2011, 2011, A001758. [Google Scholar] [CrossRef]
- Gerhardt, M.J.; Petersen-Jones, S.M.; Michalakis, S. CNG channel-related retinitis pigmentosa. Vis. Res. 2023, 208, 108232. [Google Scholar] [CrossRef]
- Nassisi, M.; Smirnov, V.M.; Solis Hernandez, C.; Mohand-Saïd, S.; Condroyer, C.; Antonio, A.; Kühlewein, L.; Kempf, M.; Kohl, S.; Wissinger, B.; et al. CNGB1-related rod-cone dystrophy: A mutation review and update. Hum. Mutat. 2021, 42, 641–666. [Google Scholar] [CrossRef]
- Kandaswamy, S.; Zobel, L.; John, B.; Santhiya, S.T.; Bogedein, J.; Przemeck, G.K.H.; Gailus-Durner, V.; Fuchs, H.; Biel, M.; De Angelis, M.H.; et al. Mutations within the cGMP-binding domain of CNGA1 causing autosomal recessive retinitis pigmentosa in human and animal model. Cell Death Discov. 2022, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- López-Begines, S.; Plana-Bonamaisó, A.; Méndez, A. Molecular determinants of Guanylate Cyclase Activating Protein subcellular distribution in photoreceptor cells of the retina. Sci. Rep. 2018, 8, 2903. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Holder, D.L.; Alshehri, A.H.; Tréguier, J.; Lakowski, J.; Sowden, J.C. NRL-/- gene edited human embryonic stem cells generate rod-deficient retinal organoids enriched in S-cone-like photoreceptors. Stem Cells 2021, 39, 414–428. [Google Scholar] [CrossRef]
- Fujieda, H.; Bremner, R.; Mears, A.J.; Sasaki, H. Retinoic acid receptor-related orphan receptor α regulates a subset of cone genes during mouse retinal development. J. Neurochem. 2009, 108, 91–101. [Google Scholar] [CrossRef]
- Roberts, M.R.; Hendrickson, A.; McGuire, C.R.; Reh, T.A. Retinoid X Receptor γ Is Necessary to Establish the S-opsin Gradient in Cone Photoreceptors of the Developing Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2897–2904. [Google Scholar] [CrossRef]
- Aramaki, M.; Wu, X.; Liu, H.; Liu, Y.; Cho, Y.-W.; Song, M.; Fu, Y.; Ng, L.; Forrest, D. Transcriptional control of cone photoreceptor diversity by a thyroid hormone receptor. Proc. Natl. Acad. Sci. USA 2022, 119, e2209884119. [Google Scholar] [CrossRef]
- Ng, L.; Ma, M.; Curran, T.; Forrest, D. Developmental expression of thyroid hormone receptor beta2 protein in cone photoreceptors in the mouse. Neuroreport 2009, 20, 627–631. [Google Scholar] [CrossRef]
- McNerney, C.; Johnston, R.J. Thyroid hormone signaling specifies cone photoreceptor subtypes during eye development: Insights from model organisms and human stem cell-derived retinal organoids. Vitam. Horm. 2021, 116, 51–90. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Z.-L.; Zhang, X.; Wang, W.; Huang, Z.-Q.; Sun, S.-N.; Jin, Z.-B. Modeling autosomal dominant retinitis pigmentosa by using patient-specific retinal organoids with a class-3 RHO mutation. Exp. Eye Res. 2024, 241, 109856. [Google Scholar] [CrossRef]
- Kallman, A.; Capowski, E.E.; Wang, J.; Kaushik, A.M.; Jansen, A.D.; Edwards, K.L.; Chen, L.; Berlinicke, C.A.; Joseph Phillips, M.; Pierce, E.A.; et al. Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Commun. Biol. 2020, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Coppieters, F.; Meire, F.; Schaich, S.; Roosing, S.; Brennenstuhl, C.; Bolz, S.; van Genderen, M.M.; Riemslag, F.C.C.; Lukowski, R.; et al. A Nonsense Mutation in PDE6H Causes Autosomal-Recessive Incomplete Achromatopsia. Am. J. Hum. Genet. 2012, 91, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Wheway, G.; Douglas, A.G.L.; Baralle, D.; Guillot, E.G. Mutation spectrum of PRPF31, genotype-phenotype correlation in retinitis pigmentosa, and opportunities for therapy. Exp. Eye Res. 2020, 192, 107950. [Google Scholar] [CrossRef] [PubMed]
- Pormehr, L.A.; Ahmadian, S.; Daftarian, N.; Mousavi, S.A.; Shafiezadeh, M. PRPF31 reduction causes mis-splicing of the phototransduction genes in human organotypic retinal culture. Eur. J. Hum. Genet. 2019, 28, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Urrestizala-Arenaza, N.; Cerchio, S.; Cavaliere, F.; Magliaro, C. Limitations of human brain organoids to study neurodegenerative diseases: A manual to survive. Front. Cell. Neurosci. 2024, 18, 1419526. [Google Scholar] [CrossRef]
- Wang, W.; Tan, S.; Zuo, X.; Gao, X.; Ma, L.; Sun, R.; Liang, G.; Yin, L.; Pu, Y.; Zhang, J. Brain Organoids in Neurodegenerative Disease Modeling: Advances, Applications and Future Perspectives. Mol. Neurobiol. 2026, 63, 142. [Google Scholar] [CrossRef]
- Biswas, S.; Haselier, C.; Mataruga, A.; Thumann, G.; Walter, P.; Müller, F. Pharmacological Analysis of Intrinsic Neuronal Oscillations in rd10 Retina. PLoS ONE 2014, 9, e99075. [Google Scholar] [CrossRef]
- Duan, C.; Ding, C.; Sun, X.; Mao, S.; Liang, Y.; Liu, X.; Ding, X.; Chen, J.; Tang, S. Retinal organoids with X-linked retinoschisis RS1 (E72K) mutation exhibit a photoreceptor developmental delay and are rescued by gene augmentation therapy. Stem Cell Res. Ther. 2024, 15, 152. [Google Scholar] [CrossRef]
- Taylor, O.B.; El-Hodiri, H.M.; Palazzo, I.; Todd, L.; Fischer, A.J. Regulating the formation of Müller glia-derived progenitor cells in the retina. Glia 2025, 73, 4–24. [Google Scholar] [CrossRef]
- Chucair-Elliott, A.J.; Ocañas, S.R.; Pham, K.; Van Der Veldt, M.; Cheyney, A.; Stanford, D.; Gurley, J.; Elliott, M.H.; Freeman, W.M. Translatomic response of retinal Müller glia to acute and chronic stress. Neurobiol. Dis. 2022, 175, 105931. [Google Scholar] [CrossRef]
- Rossi, S.; Bucolo, C.; Sanderson, J. Editorial: Chronic Inflammation and Neurodegeneration in Retinal Disease, Volume II. Front. Pharmacol. 2022, 13, 219–232. [Google Scholar] [CrossRef]
- Tomita, Y.; Qiu, C.; Bull, E.; Allen, W.E.; Kotoda, Y.; Talukdar, S.; Smith, L.E.H.; Fu, Z. Müller glial responses compensate for degenerating photoreceptors in retinitis pigmentosa. Exp. Mol. Med. 2021, 53, 1748–1758. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Müller Cell Alterations During Retinal Degeneration in a Transgenic Rat Model of Retinitis Pigmentosa. Front. Cell. Neurosci. 2015, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Eastlake, K.; Luis, J.; Limb, G.A. Potential of Müller Glia for Retina Neuroprotection. Curr. Eye Res. 2019, 45, 339–348. [Google Scholar] [CrossRef]
- Peterson, W.M.; Wang, Q.; Tzekova, R.; Wiegand, S.J. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J. Neurosci. 2000, 20, 4081–4090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Liu, M.-G.; Kawasaki, A.; Fu, X.-Y.; Barnstable, C.J.; Zhang, S.S. STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp. Eye Res. 2008, 86, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Beach, K.M.; Wang, J.; Otteson, D.C. Regulation of Stem Cell Properties of Müller Glia by JAK/STAT and MAPK Signaling in the Mammalian Retina. Stem Cells Int. 2017, 2017, 1610691. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, Q.; Zeng, Y.; Zhang, Y.; Zhang, M. Regulations of Retinal Inflammation: Focusing on Müller Glia. Front. Cell Dev. Biol. 2022, 10, 898652. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Benning, L.; Reinehr, S.; Grotegut, P.; Kuehn, S.; Stute, G.; Dick, H.B.; Joachim, S.C. Synapse and Receptor Alterations in Two Different S100B-Induced Glaucoma-Like Models. Int. J. Mol. Sci. 2020, 21, 6998. [Google Scholar] [CrossRef]
- Grotegut, P.; Perumal, N.; Kuehn, S.; Smit, A.; Dick, H.B.; Grus, F.H.; Joachim, S.C. Minocycline reduces inflammatory response and cell death in a S100B retina degeneration model. J. Neuroinflamm. 2020, 17, 375. [Google Scholar] [CrossRef]
- Kirsch, M.; Trautmann, N.; Ernst, M.; Hofmann, H. Involvement of gp130-associated cytokine signaling in Müller cell activation following optic nerve lesion. Glia 2010, 58, 768–779. [Google Scholar] [CrossRef]
- Livne-Bar, I.; Lam, S.; Chan, D.; Guo, X.; Askar, I.; Nahirnyj, A.; Flanagan, J.G.; Sivak, J.M. Pharmacologic inhibition of reactive gliosis blocks TNF-α-mediated neuronal apoptosis. Cell Death Dis. 2016, 7, e2386. [Google Scholar] [CrossRef]
- Reichenbach, N.; Delekate, A.; Plescher, M.; Schmitt, F.; Krauss, S.; Blank, N.; Halle, A.; Petzold, G.C. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol. Med. 2019, 11, e9665. [Google Scholar] [CrossRef]
- Balzamino, B.O.; Cacciamani, A.; Dinice, L.; Cecere, M.; Pesci, F.R.; Ripandelli, G.; Micera, A. Retinal Inflammation and Reactive Müller Cells: Neurotrophins’ Release and Neuroprotective Strategies. Biology 2024, 13, 1030. [Google Scholar] [CrossRef]
- Peña, J.S.; Vázquez, M. Harnessing the Neuroprotective Behaviors of Müller Glia for Retinal Repair. Front. Biosci. 2022, 27, 169. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.M.K.; Caprioli, J.; Piri, N. RNA Binding Protein with Multiple Splicing: A New Marker for Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1052–1058. [Google Scholar] [CrossRef]
- O’Brien, E.E.; Greferath, U.; Fletcher, E.L. The effect of photoreceptor degeneration on ganglion cell morphology. J. Comp. Neurol. 2014, 522, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Vidal-Sanz, M.; Villegas-Pérez, M.P. Retinal Ganglion Cell Death as a Late Remodeling Effect of Photoreceptor Degeneration. Int. J. Mol. Sci. 2019, 20, 4649. [Google Scholar] [CrossRef]
- Tapia, M.L.; Nascimento-Dos-Santos, G.; Park, K.K. Subtype-specific survival and regeneration of retinal ganglion cells in response to injury. Front. Cell Dev. Biol. 2022, 10, 956279. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, C.K. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005, 1057, 17–28. [Google Scholar] [CrossRef]
- Morgans, C.W.; Zhang, J.; Jeffrey, B.G.; Nelson, S.M.; Burke, N.S.; Duvoisin, R.M.; Brown, R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19174–19178. [Google Scholar] [CrossRef]
- Dhingra, A.; Jiang, M.; Wang, T.-L.; Lyubarsky, A.; Savchenko, A.; Bar-Yehuda, T.; Sterling, P.; Birnbaumer, L.; Vardi, N. Light Response of Retinal ON Bipolar Cells Requires a Specific Splice Variant of Gαo. J. Neurosci. 2002, 22, 4878–4884. [Google Scholar] [CrossRef]
- Dhingra, A.; Ramakrishnan, H.; Neinstein, A.; Fina, M.E.; Xu, Y.; Li, J.; Chung, D.C.; Lyubarsky, A.; Vardi, N. Gβ3 is required for normal light ON responses and synaptic maintenance. J. Neurosci. 2012, 32, 11343–11355. [Google Scholar] [CrossRef] [PubMed]
- Strettoi, E.; Porciatti, V.; Falsini, B.; Pignatelli, V.; Rossi, C. Morphological and Functional Abnormalities in the Inner Retina of the rd/rd Mouse. J. Neurosci. 2002, 22, 5492–5504. [Google Scholar] [CrossRef]
- Puthussery, T.; Gayet-Primo, J.; Pandey, S.; Duvoisin, R.M.; Taylor, W.R. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. Eur. J. Neurosci. 2009, 29, 1533–1542. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Hanna, J.; David, L.A.; Touahri, Y.; Fleming, T.; Screaton, R.A.; Schuurmans, C. Beyond Genetics: The Role of Metabolism in Photoreceptor Survival, Development and Repair. Front. Cell Dev. Biol. 2022, 10, 887764. [Google Scholar] [CrossRef] [PubMed]
- López-del Hoyo, N.; Fazioli, L.; López-Begines, S.; Fernández-Sánchez, L.; Cuenca, N.; Llorens, J.; De La Villa, P.; Méndez, A. Overexpression of Guanylate Cyclase Activating Protein 2 in Rod Photoreceptors In Vivo Leads to Morphological Changes at the Synaptic Ribbon. PLoS ONE 2012, 7, e42994. [Google Scholar] [CrossRef]
- Jiang, K.; Mondal, A.K.; Adlakha, Y.K.; Gumerson, J.; Aponte, A.; Gieser, L.; Kim, J.-W.; Boleda, A.; Brooks, M.J.; Nellissery, J.; et al. Early mitochondrial stress and metabolic imbalance lead to photoreceptor cell death in retinal degeneration. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bighinati, A.; Adani, E.; Stanzani, A.; D’Alessandro, S.; Marigo, V. Molecular mechanisms underlying inherited photoreceptor degeneration as targets for therapeutic intervention. Front. Cell. Neurosci. 2024, 18, 1343544. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Mustafi, D.; Pepple, K.L. Immunology of Retinitis Pigmentosa and Gene Therapy–Associated Uveitis. Cold Spring Harb. Perspect. Med. 2023, 14, a041305. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, T.J.; Hubbard, M.G.; Levi, H.J.; White, W.A.; Wang, X.; Simpson, R.N.; Jablonski, M.M.; Gross, A.K. Proinflammatory Pathways Are Activated in the Human Q344X Rhodopsin Knock-In Mouse Model of Retinitis Pigmentosa. Biomolecules 2021, 11, 1163. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Roh, H.; Kim, S.-H.; Lee, K.; Im, M.; Oh, S.J. Effective protection of photoreceptors using an inflammation-responsive hydrogel to attenuate outer retinal degeneration. NPJ Regen. Med. 2023, 8, 68. [Google Scholar] [CrossRef]
- Eriksen, K.O.; Eidet, J.R.; Kjellström, U.; Baldesi, J.; Bragadóttir, R.; Colombo, L.; Holtan, J.P. Characterising PRPF31-associated retinal dystrophy: Clinical insights from baseline data in a natural history study. Acta Ophthalmol. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bellapianta, A.; Qi, J.; Giugliano, M.; Ouaidat, S.; El Rawas, R.; Bolz, M.; Salti, A. Single-Cell Transcriptomics on PRPF31-Mutated Retinal Organoids Reveal Early Müller Glial Activation and Progressive Photoreceptor Degeneration. Biomedicines 2026, 14, 45. https://doi.org/10.3390/biomedicines14010045
Bellapianta A, Qi J, Giugliano M, Ouaidat S, El Rawas R, Bolz M, Salti A. Single-Cell Transcriptomics on PRPF31-Mutated Retinal Organoids Reveal Early Müller Glial Activation and Progressive Photoreceptor Degeneration. Biomedicines. 2026; 14(1):45. https://doi.org/10.3390/biomedicines14010045
Chicago/Turabian StyleBellapianta, Alessandro, Jingjing Qi, Michele Giugliano, Sara Ouaidat, Rana El Rawas, Matthias Bolz, and Ahmad Salti. 2026. "Single-Cell Transcriptomics on PRPF31-Mutated Retinal Organoids Reveal Early Müller Glial Activation and Progressive Photoreceptor Degeneration" Biomedicines 14, no. 1: 45. https://doi.org/10.3390/biomedicines14010045
APA StyleBellapianta, A., Qi, J., Giugliano, M., Ouaidat, S., El Rawas, R., Bolz, M., & Salti, A. (2026). Single-Cell Transcriptomics on PRPF31-Mutated Retinal Organoids Reveal Early Müller Glial Activation and Progressive Photoreceptor Degeneration. Biomedicines, 14(1), 45. https://doi.org/10.3390/biomedicines14010045







