Impact of Post-Traumatic Stress Disorder Management Through Reconsolidation Therapy on Fibromyalgia Syndrome: A Pilot Study
Abstract
1. Introduction
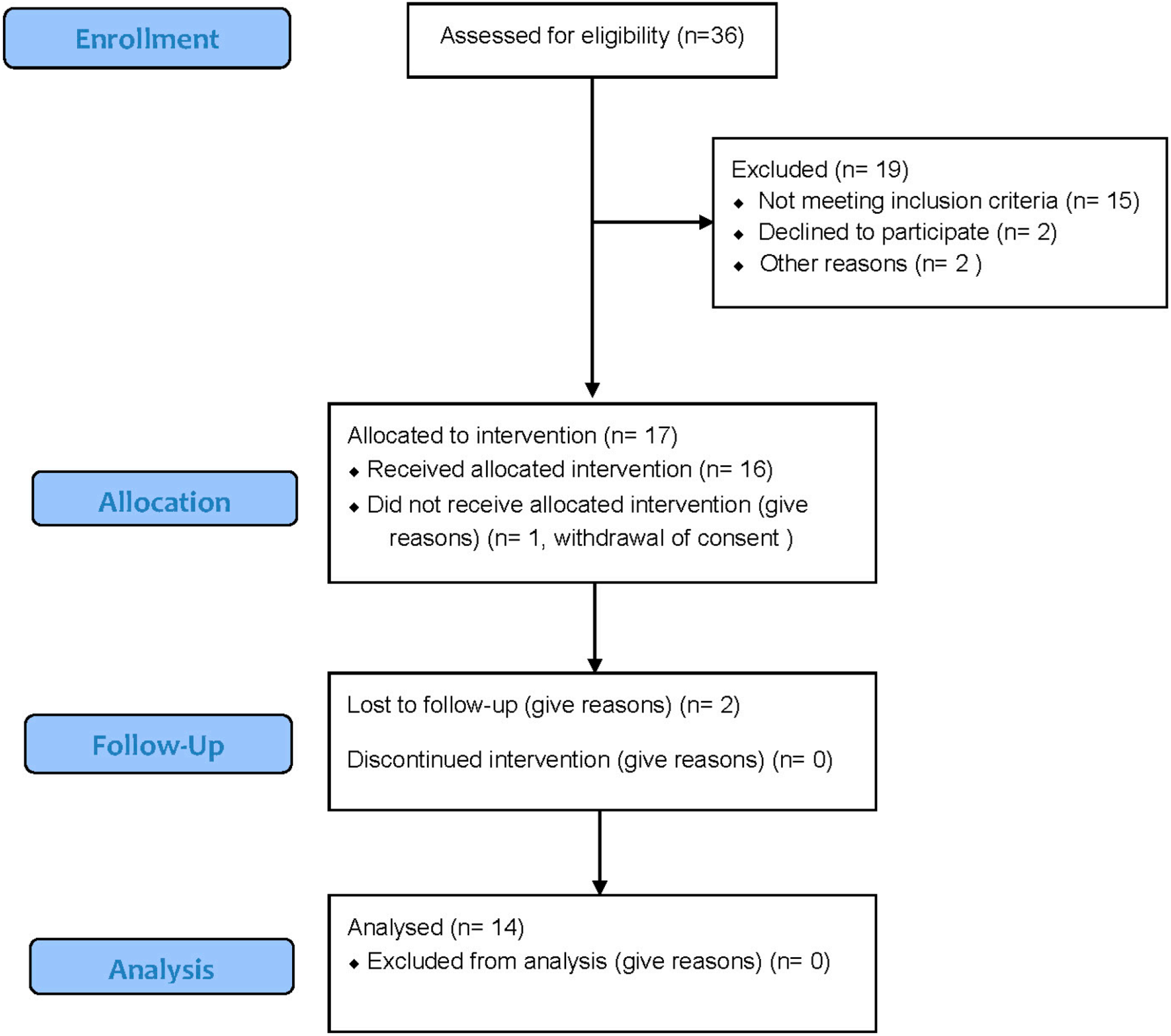
2. Materials and Methods
2.1. Study Design
2.2. Ethics Approval
2.3. Participants
2.4. Procedure
2.5. Study Treatment
2.6. Outcomes Assessment
2.7. Statistical Analyses
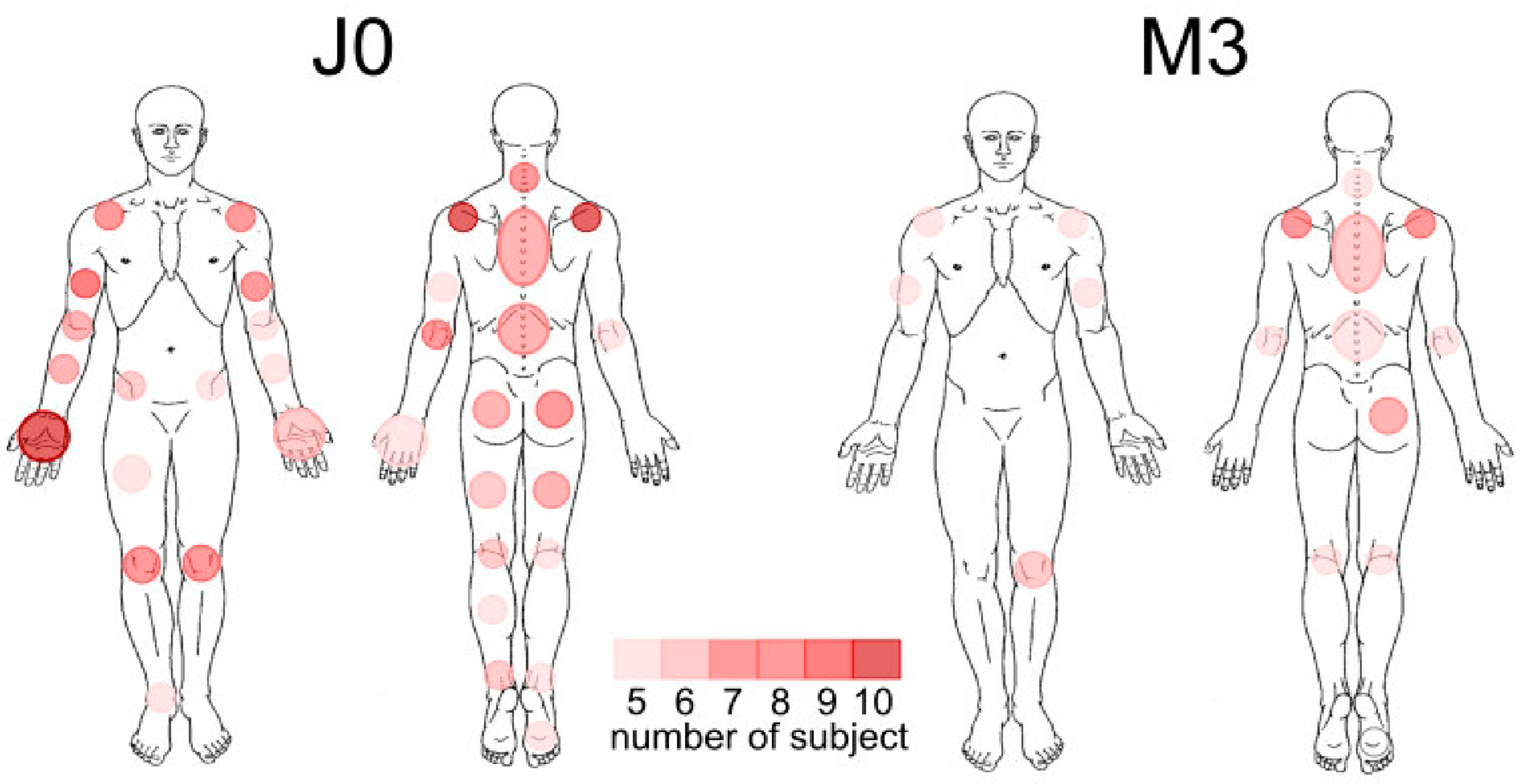
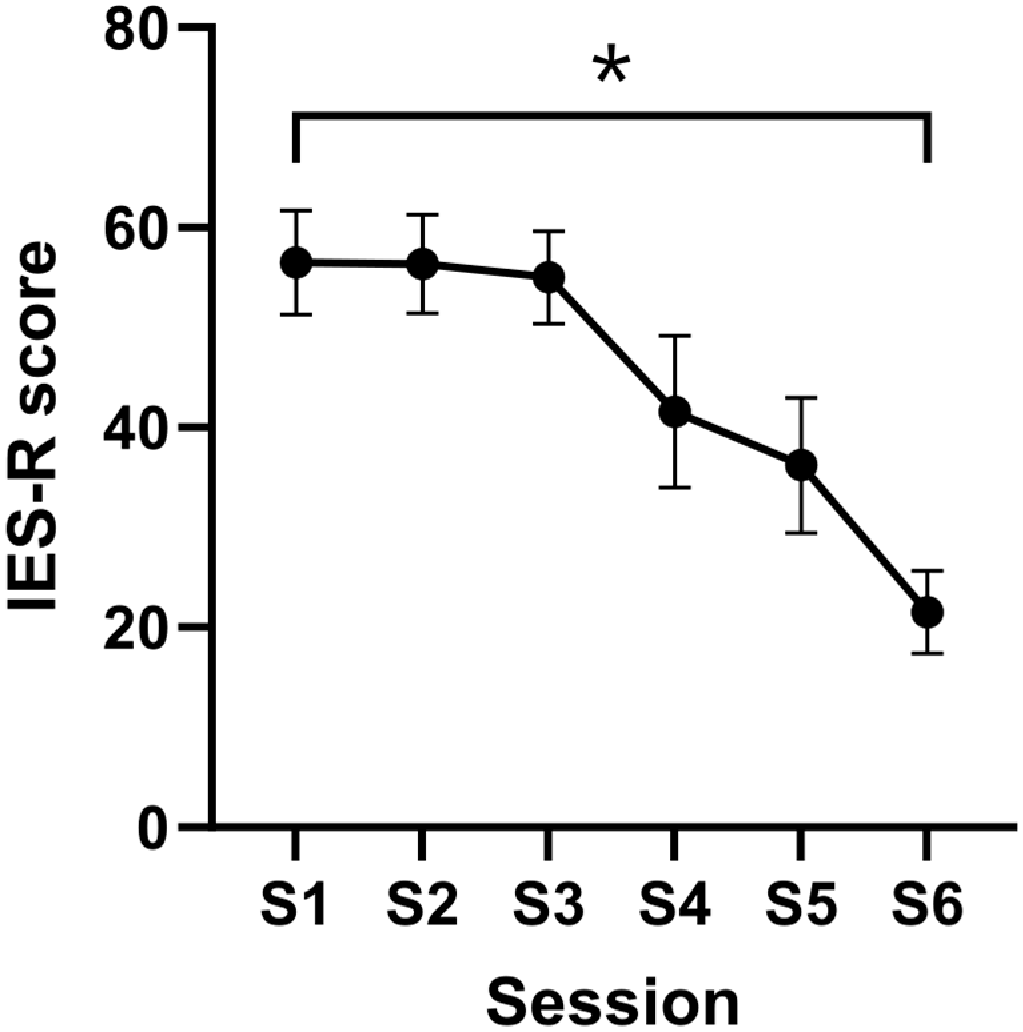
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, G.T.; Atzeni, F.; Beasley, M.; Flüß, E.; Sarzi-Puttini, P.; Macfarlane, G.J. The Prevalence of Fibromyalgia in the General Population: A Comparison of the American College of Rheumatology 1990, 2010, and Modified 2010 Classification Criteria. Arthritis Rheumatol. 2015, 67, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, B.; Blotman, F.; Roué-Le Lay, K.; Caubère, J.P.; André, E.; Taïeb, C. Étude de la prévalence de la fibromyalgie dans la population française. Rev. Rhum. 2009, 76, 274–278. [Google Scholar] [CrossRef]
- Carneiro, B.D.; Torres, S.; Costa-Pereira, J.T.; Pozza, D.H.; Tavares, I. Descending Pain Modulation in Fibromyalgia: A Short Review of Mechanisms and Biomarkers. Diagnostics 2025, 15, 2702. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Silva, A. Fibromyalgia: A new set of diagnostic criteria based on the biopsychosocial model. Rheumatology 2024, 63, 2037–2039. [Google Scholar] [CrossRef]
- Sommer, C.; Häuser, W.; Burgmer, M.; Engelhardt, R.; Gerhold, K.; Petzke, F.; Schmidt-Wilcke, T.; Späth, M.; Toelle, T.; Üçeyler, N.; et al. Etiology and pathophysiology of fibromyalgia syndrome. Der Schmerz 2012, 26, 259–267. [Google Scholar] [CrossRef]
- Gardoki-Souto, I.; Redolar-Ripoll, D.; Fontana, M.; Hogg, B.; Castro, M.J.; Blanch, J.M.; Ojeda, F.; Solanes, A.; Radua, J.; Valiente-Gómez, A.; et al. Prevalence and Characterization of Psychological Trauma in Patients with Fibromyalgia: A Cross-Sectional Study. Pain Res. Manag. 2022, 2022, 2114451. [Google Scholar] [CrossRef]
- Keers, R.; Uher, R.; Huezo-Diaz, P.; Smith, R.; Jaffee, S.; Rietschel, M.; Henigsberg, N.; Kozel, D.; Mors, O.; Maier, W.; et al. Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenom. J. 2011, 11, 138–145. [Google Scholar] [CrossRef]
- Häuser, W.; Ablin, J.; Walitt, B. PTSD and Fibromyalgia Syndrome: Focus on Prevalence, Mechanisms, and Impact. In Comprehensive Guide to Post-Traumatic Stress Disorder; Martin, C.R., Preedy, V.R., Patel, V.B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–13. Available online: https://link.springer.com/10.1007/978-3-319-08613-2_52-1 (accessed on 3 April 2024).
- Luís, M.; Pinto, A.M.; Häuser, W.; Jacobs, J.W.; Saraiva, A.; Giorgi, V.; Sarzi-Puttini, P.; Castelo-Branco, M.; Geenen, R.; Pereira da Silva, J.A. Fibromyalgia and Post-Traumatic Stress Disorder: Different Parts of an Elephant? Clinical and Experimental Rheumatology [Internet]. 27 June 2025. Available online: https://www.clinexprheumatol.org/abstract.asp?a=22504 (accessed on 26 November 2025).
- Maire, C.; Miró, E.; Sánchez, A.I.; Cáliz, R.; Martínez, M.P. Trauma and psychological impact in fibromyalgia and other central sensitization syndromes: The role of anxiety and pain acceptance. BMC Psychol. 2025, 13, 1251. [Google Scholar] [CrossRef]
- Nater, U.M.; Fischer, S.; Ehlert, U. Stress as a Pathophysiological Factor in Functional Somatic Syndromes. Curr. Psychiatry Rev. 2011, 7, 152–169. [Google Scholar] [CrossRef]
- Cohen, H.; Neumann, L.; Haiman, Y.; Matar, M.A.; Press, J.; Buskila, D. Prevalence of post-traumatic stress disorder in fibromyalgia patients: Overlapping syndromes or post-traumatic fibromyalgia syndrome? Semin. Arthritis Rheum. 2002, 32, 38–50. [Google Scholar] [CrossRef]
- Häuser, W.; Galek, A.; Erbslöh-Möller, B.; Köllner, V.; Kühn-Becker, H.; Langhorst, J.; Petermann, F.; Prothmann, U.; Winkelmann, A.; Schmutzer, G.; et al. Posttraumatic stress disorder in fibromyalgia syndrome: Prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013, 154, 1216–1223. [Google Scholar] [CrossRef]
- Sherman, J.J.; Turk, D.C.; Okifuji, A. Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. Clin. J. Pain 2000, 16, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Turk, D.C.; Dworkin, R.H. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION systematic review. Semin Arthritis Rheum. 2021, 51, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Consoli, G.; Conversano, C.; Ramacciotti, C.E.; Musetti, L.; Massimetti, E.; Pergentini, I.; Corsi, M.; Ciapparelli, A.; et al. Lifetime post-traumatic stress symptoms are related to the health-related quality of life and severity of pain/fatigue in patients with fibromyalgia. Clin. Exp. Rheumatol. 2011, 29, S73–S78. [Google Scholar] [PubMed]
- Amir, M.; Kaplan, Z.; Neumann, L.; Sharabani, R.; Shani, N.; Buskila, D. Posttraumatic stress disorder, tenderness and fibromyalgia. J. Psychosom. Res. 1997, 42, 607–613. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Lumley, M.A.; Schubiner, H.; Lockhart, N.A.; Kidwell, K.M.; Harte, S.E.; Clauw, D.J.; Williams, D.A. Emotional awareness and expression therapy, cognitive behavioral therapy, and education for fibromyalgia: A cluster-randomized controlled trial. Pain 2017, 158, 2354–2363. [Google Scholar] [CrossRef]
- Brunet, A.; Orr, S.P.; Tremblay, J.; Robertson, K.; Nader, K.; Pitman, R.K. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J. Psychiatr. Res. 2008, 42, 503–506. [Google Scholar] [CrossRef]
- Brunet, A.; Saumier, D.; Liu, A.; Streiner, D.L.; Tremblay, J.; Pitman, R.K. Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am. J. Psychiatry 2018, 175, 427–433. [Google Scholar] [CrossRef]
- Pigeon, S.; Lonergan, M.; Rotondo, O.; Pitman, R.K.; Brunet, A. Impairing memory reconsolidation with propranolol in healthy and clinical samples: A meta-analysis. J. Psychiatry Neurosci. 2022, 47, E109–E122. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Balker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33; quiz 34–57. [Google Scholar]
- Wortmann, J.H.; Jordan, A.H.; Weathers, F.W.; Resick, P.A.; Dondanville, K.A.; Hall-Clark, B.; Foa, E.B.; Young-McCaughan, S.; Yarvis, J.S.; Hembree, E.A.; et al. Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychol. Assess. 2016, 28, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. The fibromyalgia impact questionnaire: Development and validation. J. Rheumatol. 1991, 18, 728–733. [Google Scholar]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar] [PubMed]
- Beck, A. A new instrument for measuring insight: The Beck Cognitive Insight Scale. Schizophr. Res. 2004, 68, 319–329. [Google Scholar] [CrossRef]
- Rosenberg, M. Rosenberg Self-Esteem Scale [Internet]. 2011. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft01038-000 (accessed on 27 November 2025).
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Beiner, E.; Lucas, V.; Reichert, J.; Buhai, D.V.; Jesinghaus, M.; Vock, S.; Drusko, A.; Baumeister, D.; Eich, W.; Friederich, H.-C.; et al. Stress biomarkers in individuals with fibromyalgia syndrome: A systematic review with meta-analysis. Pain 2023, 164, 1416–1427. [Google Scholar] [CrossRef]
- Bennett, R.M.; Friend, R.; Jones, K.D.; Ward, R.; Han, B.K.; Ross, R.L. The Revised Fibromyalgia Impact Questionnaire (FIQR): Validation and psychometric properties. Arthritis Res. Ther. 2009, 11, R120. [Google Scholar] [CrossRef]
- Bennett, R.M.; Bushmakin, A.G.; Cappelleri, J.C.; Zlateva, G.; Sadosky, A.B. Minimal Clinically Important Difference in the Fibromyalgia Impact Questionnaire. J. Rheumatol. 2009, 36, 1304–1311. [Google Scholar] [CrossRef]
- Alexandra Kredlow, M.; Fenster, R.J.; Laurent, E.S.; Ressler, K.J.; Phelps, E.A. Prefrontal cortex, amygdala, and threat processing: Implications for PTSD. Neuropsychopharmacology 2022, 47, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Pitts, B.L.; Eisenberg, M.L.; Bailey, H.R.; Zacks, J.M. PTSD is associated with impaired event processing and memory for everyday events. Cogn. Res. 2022, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Lacefield, K.; Samph, S.P.; Orbon, S.; Otis, J. Integrated intervention for comorbid posttraumatic stress disorder and fibromyalgia: A pilot study of women veterans. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Lumley, M.A.; Cohen, J.L.; Stout, R.L.; Neely, L.C.; Sander, L.M.; Burger, A.J. An emotional exposure-based treatment of traumatic stress for people with chronic pain: Preliminary results for fibromyalgia syndrome. Psychother. Theory Res. Pract. Train. 2008, 45, 165–172. [Google Scholar] [CrossRef]
- Beck, A.T. Cognitive therapy: Nature and relation to behavior therapy. Behav. Ther. 1970, 1, 184–200. [Google Scholar] [CrossRef]
- Disner, S.G.; Beevers, C.G.; Haigh, E.A.P.; Beck, A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011, 12, 467–477. [Google Scholar] [CrossRef]
- Kindt, M.; Soeter, M.; Vervliet, B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat. Neurosci. 2009, 12, 256–258. [Google Scholar] [CrossRef]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406, 722–726. [Google Scholar] [CrossRef]
- Schiller, D.; Phelps, E.A. Does Reconsolidation Occur in Humans? Front. Behav. Neurosci. 2011, 5, 24. [Google Scholar] [CrossRef]
- Clauw, D.J. Fibromyalgia: A Clinical Review. JAMA 2014, 311, 1547. [Google Scholar] [CrossRef]
- Martinez-Lavin, M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res. Ther. 2007, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Roullet, P.; Vaiva, G.; Véry, E.; Bourcier, A.; Yrondi, A.; Dupuch, L.; Lamy, P.; Thalamas, C.; Jasse, L.; El Hage, W.; et al. Traumatic memory reactivation with or without propranolol for PTSD and comorbid MD symptoms: A randomised clinical trial. Neuropsychopharmacology 2021, 46, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Thierrée, S.; Richa, S.; Brunet, A.; Egreteau, L.; Roig, Q.; Clarys, D.; El-Hage, W. Trauma reactivation under propranolol among traumatized Syrian refugee children: Preliminary evidence regarding efficacy. Eur. J. Psychotraumatol. 2020, 11, 1733248. [Google Scholar] [CrossRef] [PubMed]
- Yule, A. Integrating Treatment for Co-Occurring Mental Health Conditions. Alcohol Res. Curr. Rev. 2019, 40, 61–73. [Google Scholar] [CrossRef]
- Grewal, H.; Zhuang, C.; Iqbal, M.; Ur Rehman, B.A.; Norton, J.; Vernon, C.M.; Deol, S.; Brose, S.W. Integrative approach for women with fibromyalgia in a Veterans Affairs Medical Center: An observational study. Medicine 2023, 102, e36285. [Google Scholar] [CrossRef]
- Borst, M.; Moeyaert, M.; Van Rood, Y. The effect of eye movement desensitization and reprocessing on fibromyalgia: A multiple-baseline experimental case study across ten participants. Neuropsychol. Rehabil. 2024, 34, 1422–1454. [Google Scholar] [CrossRef]
- Goldstein, E.; McDonnell, C.; Atchley, R.; Dorado, K.; Bedford, C.; Brown, R.L.; Zgierska, A.E. The Impact of Psychological Interventions on Posttraumatic Stress Disorder and Pain Symptoms: A Systematic Review and Meta-Analysis. Clin. J. Pain 2019, 35, 703–712. [Google Scholar] [CrossRef]
- López-Martínez, A.E.; Reyes-Pérez, Á.; Serrano-Ibáñez, E.R.; Esteve, R.; Ramírez-Maestre, C. Chronic pain, posttraumatic stress disorder, and opioid intake: A systematic review. World J. Clin. Cases 2019, 7, 4254–4269. [Google Scholar] [CrossRef]
- Choy, E.H.S. The role of sleep in pain and fibromyalgia. Nat. Rev. Rheumatol. 2015, 11, 513–520. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
| Patient | Types of Trauma | Patient | Types of Trauma |
|---|---|---|---|
| 01 | Father’s suicide | 10 | Adolescent rape |
| 02 | Intrafamilial sexual abuse (sibling) | 11 | Road traffic accident |
| 03 | Spouse’s stroke | 12 | Complicated abortion |
| 04 | Intrafamilial abuse involving a child | 13 | Workplace sexual harassment |
| 05 | Assault by employer | 14 | Adult rape |
| 06 | Death threats from ex-partner | 15 | Death of one’s baby at birth |
| 07 | Domestic violence | 16 | Workplace harassment |
| 08 | Violence from father | 17 | Childhood rape |
| 09 | Childhood rape by an uncle |
| Baseline (T0) | 3 Month Follow-Up (T3) | p-Value | |
|---|---|---|---|
| FIQ score | 65.2 ± 12.8 | 53.5 ± 18.3 | 0.018 |
| PCL-5 score | 50.1 ± 9.5 | 25.9 ± 14.2 | <0.001 |
| RSES score | 21.2 ± 5.3 | 24.6 ± 5.6 | 0.032 |
| MADRS score | 15.5 ± 6.6 | 8.6 ± 4.3 | <0.001 |
| BDI score | 17 ± 3.5 | 12 ± 6.1 | 0.003 |
| CGI-S score | 4.8 ± 0.5 | 3.9 ± 0.9 | 0.002 |
| CGI-I | 2.0 ± 0.9 | ||
| SF-36 | |||
| Physical Functioning | 47.2 ± 14.6 | 53.9 ± 19.2 | 0.088 |
| Role-Physical | 15.6 ± 27.2 | 25 ± 31 | 0.466 |
| Bodily Pain | 29.1 ± 14.8 | 34.3 ± 14.9 | 0.146 |
| General Health | 36 ± 15.4 | 42.7 ± 20.4 | 0.047 |
| Physical Health Component | 23.8 ± 1 | 24.2 ± 1.2 | 0.169 |
| Vitality | 17.2 ± 11.8 | 30.7 ± 24.9 | 0.023 |
| Social Functioning | 37.5 ± 20.4 | 55.4 ± 20.6 | 0.008 |
| Role-Emotional | 14.6 ± 29.7 | 47.6 ± 42.8 | 0.026 |
| Mental Health | 59.5 ± 9.5 | 63.4 ± 4.4 | 0.183 |
| Mental Health Component | 22.2 ± 0.9 | 22.9 ± 0.7 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Harika Germaneau, G.; Rannou, D.; Charrier, E.; El Fairouqi, Y.; Brunet, A.; Doolub, D.; Langbour, N.; Raviart, I.; Wassouf, I.; Jaafari, N. Impact of Post-Traumatic Stress Disorder Management Through Reconsolidation Therapy on Fibromyalgia Syndrome: A Pilot Study. Biomedicines 2026, 14, 190. https://doi.org/10.3390/biomedicines14010190
Harika Germaneau G, Rannou D, Charrier E, El Fairouqi Y, Brunet A, Doolub D, Langbour N, Raviart I, Wassouf I, Jaafari N. Impact of Post-Traumatic Stress Disorder Management Through Reconsolidation Therapy on Fibromyalgia Syndrome: A Pilot Study. Biomedicines. 2026; 14(1):190. https://doi.org/10.3390/biomedicines14010190
Chicago/Turabian StyleHarika Germaneau, Ghina, Delphine Rannou, Elodie Charrier, Yassir El Fairouqi, Alain Brunet, Damien Doolub, Nicolas Langbour, Isabelle Raviart, Issa Wassouf, and Nemat Jaafari. 2026. "Impact of Post-Traumatic Stress Disorder Management Through Reconsolidation Therapy on Fibromyalgia Syndrome: A Pilot Study" Biomedicines 14, no. 1: 190. https://doi.org/10.3390/biomedicines14010190
APA StyleHarika Germaneau, G., Rannou, D., Charrier, E., El Fairouqi, Y., Brunet, A., Doolub, D., Langbour, N., Raviart, I., Wassouf, I., & Jaafari, N. (2026). Impact of Post-Traumatic Stress Disorder Management Through Reconsolidation Therapy on Fibromyalgia Syndrome: A Pilot Study. Biomedicines, 14(1), 190. https://doi.org/10.3390/biomedicines14010190






