Acceleration-Dependent Effects of Vibrotactile Gamma Stimulation on Cognitive Recovery and Cholinergic Function in a Scopolamine-Induced Neurotoxicity Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Reverse Transcription Followed by Quantitative PCR
2.3. Animals
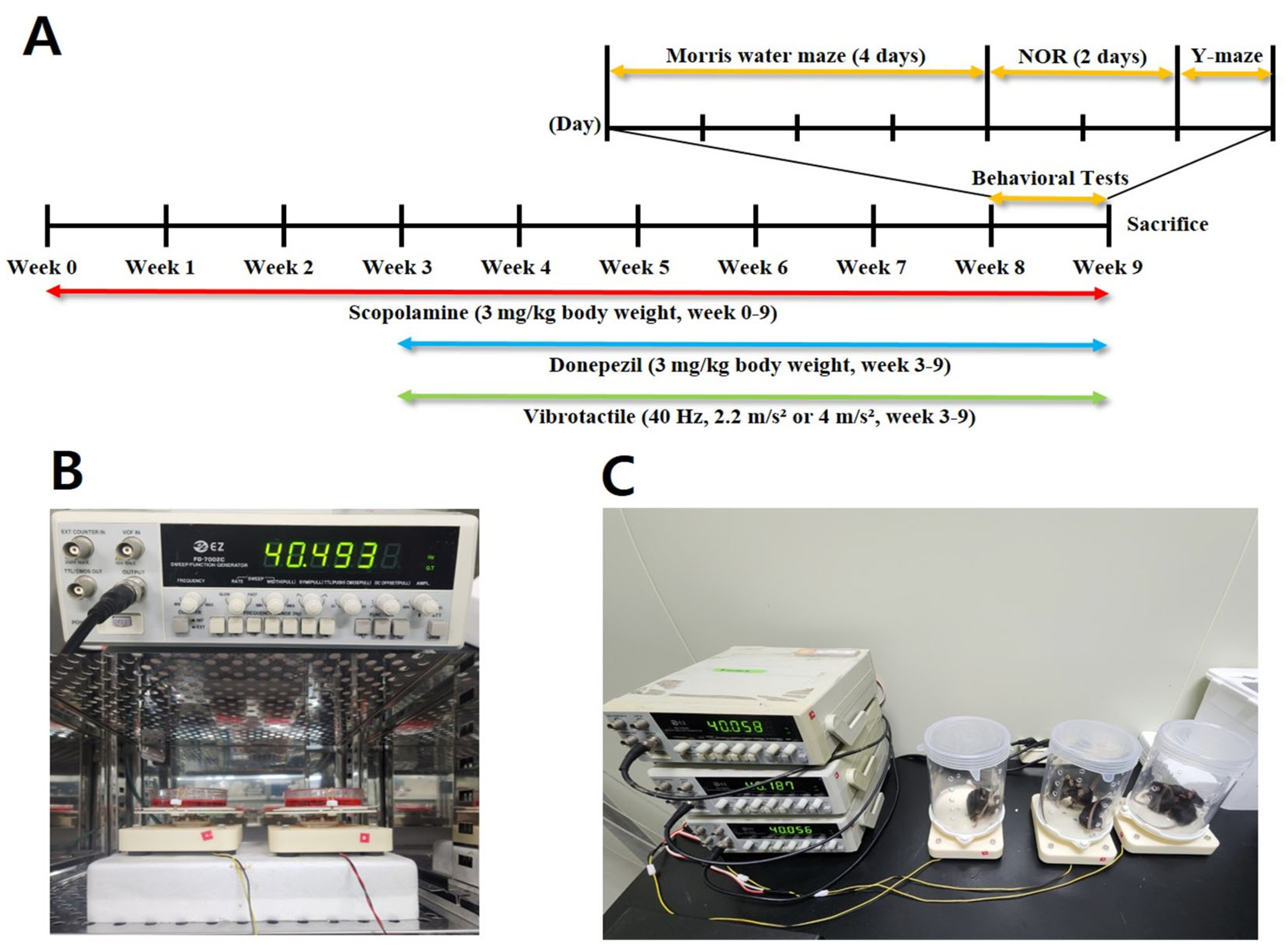
2.4. Experimental Design
2.5. Vibrotactile Stimulation
2.6. Behavior Test
2.6.1. Morris Water Maze
2.6.2. Novel Object Recognition Test
2.6.3. Y-Maze Test
2.7. Lipid Peroxidation
2.8. AChE Activity
2.9. Western Blotting
2.10. Immunohistochemical Analyses
2.11. Statistical Analysis
3. Results
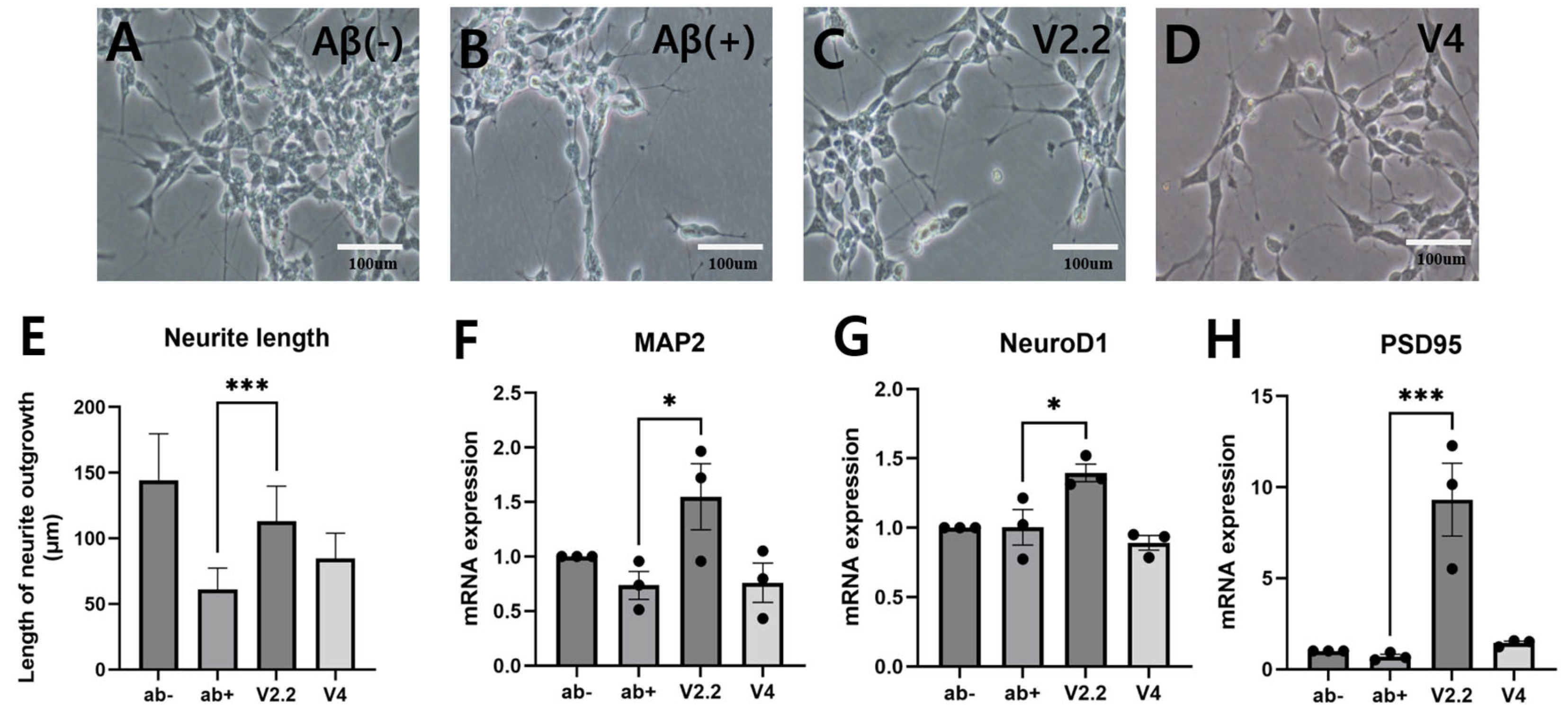
3.1. Effects of Vibrotactile Stimulation on Aβ-Induced Neural Damage In Vitro
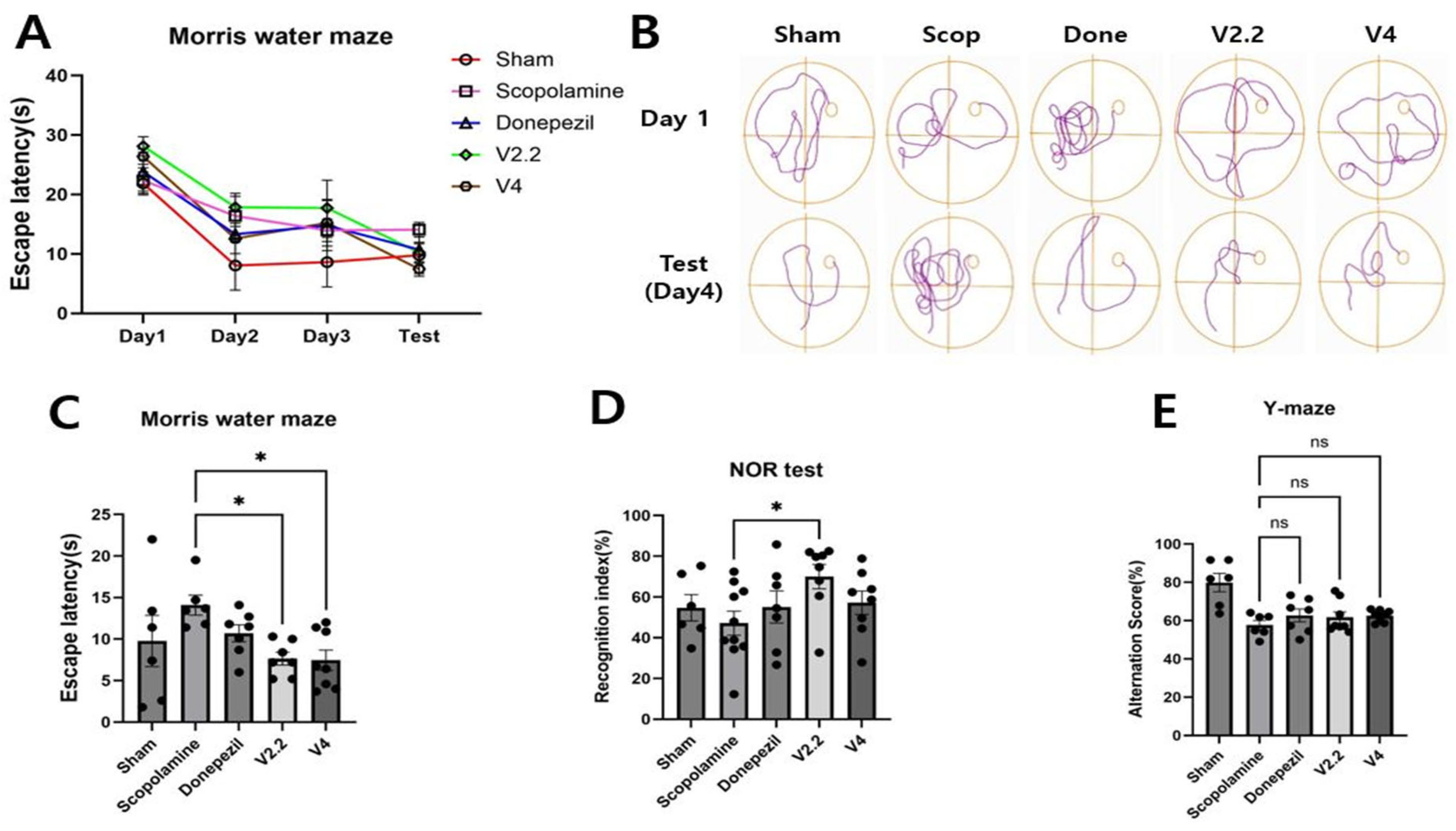
3.2. Effects of Vibrotactile Stimulation on Cognitive Function in a Scopolamine-Induced Alzheimer’s Disease Model
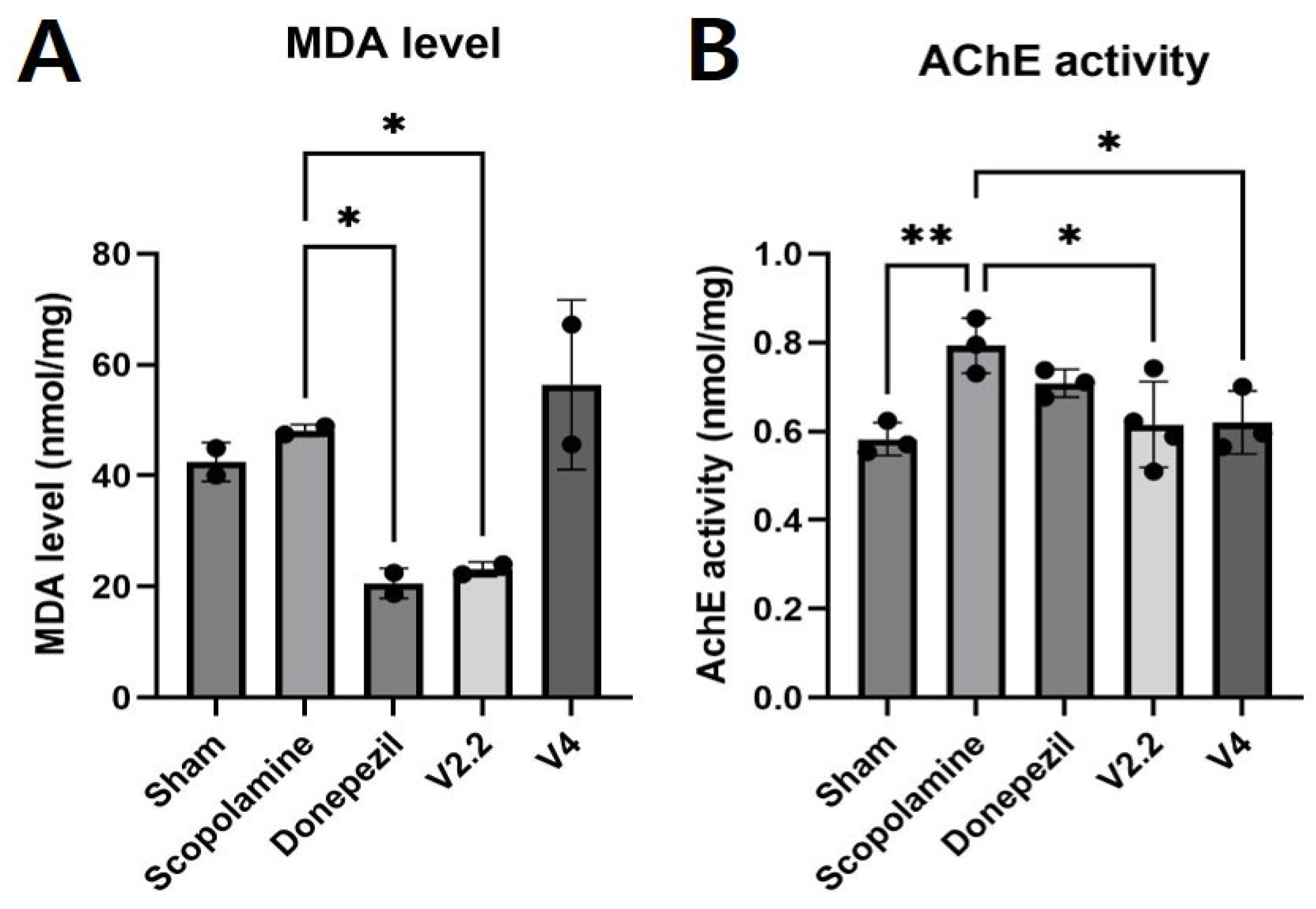
3.3. Biochemical Analysis of Oxidative Stress and Cholinergic Function in the Brain Following Scopolamine and Vibrotactile Stimulation
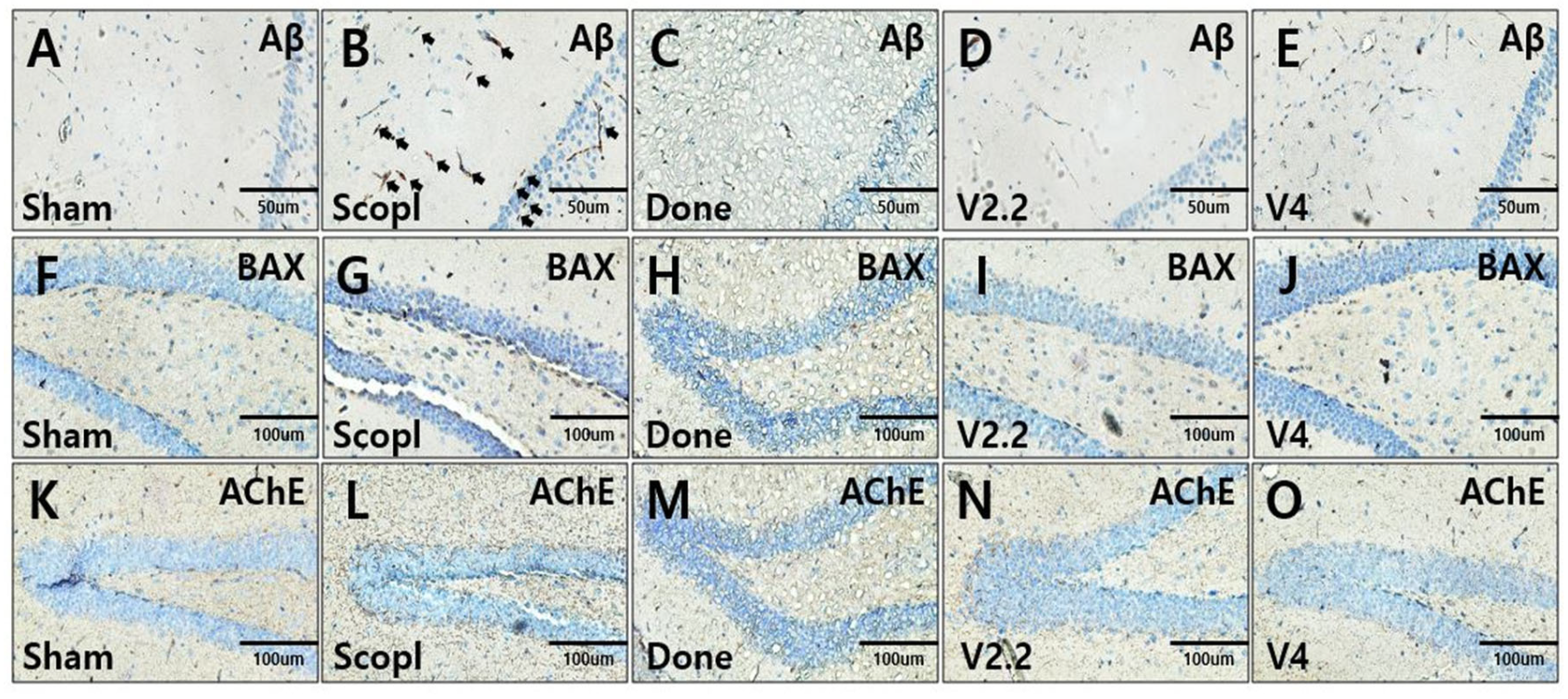
3.4. Immunohistochemical Analysis of Aβ, BAX, and AChE Expression in the Hippocampus of a Scopolamine-Induced AD Mouse Model Following Vibrotactile Stimulation
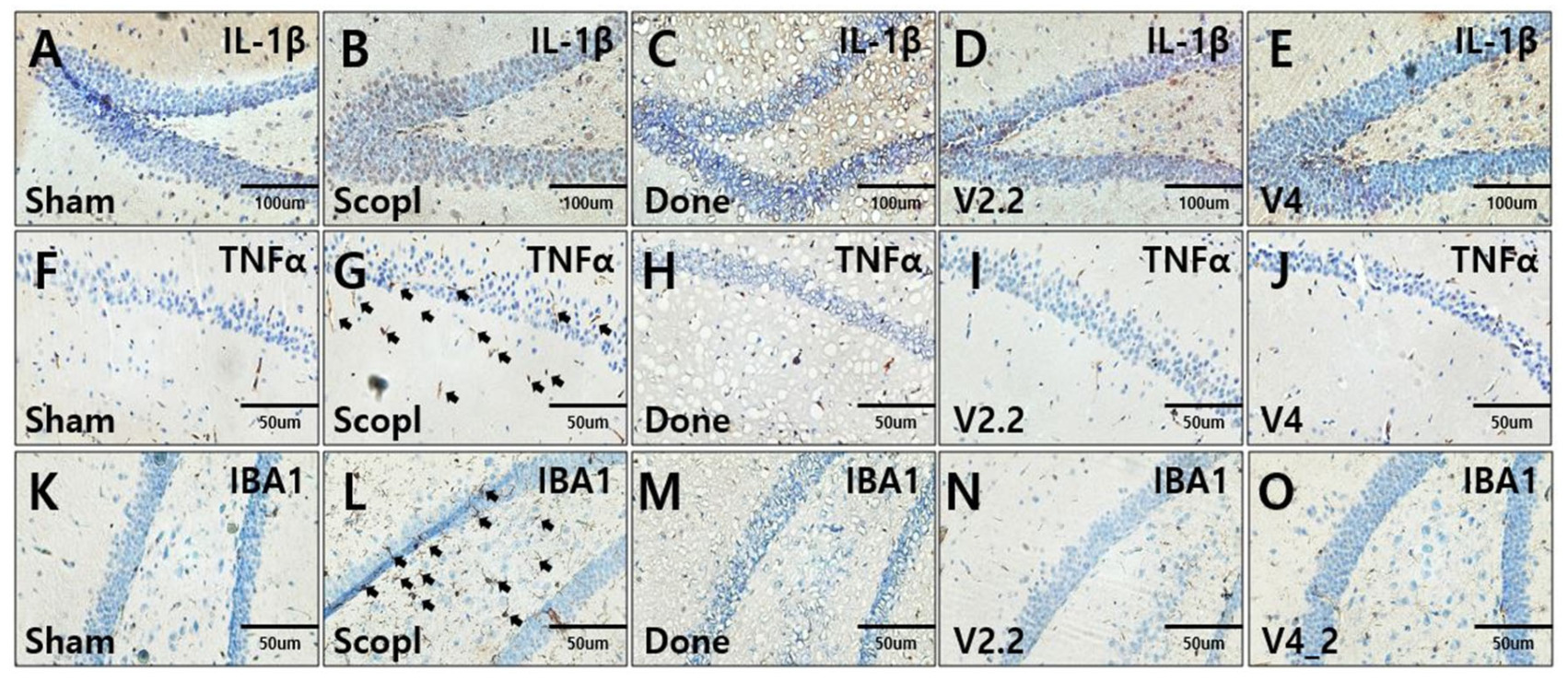
3.5. Immunohistochemical Analysis of Neuroinflammation in a Scopolamine-Induced Alzheimer’s Disease Mouse Model Following Vibrotactile Stimulation
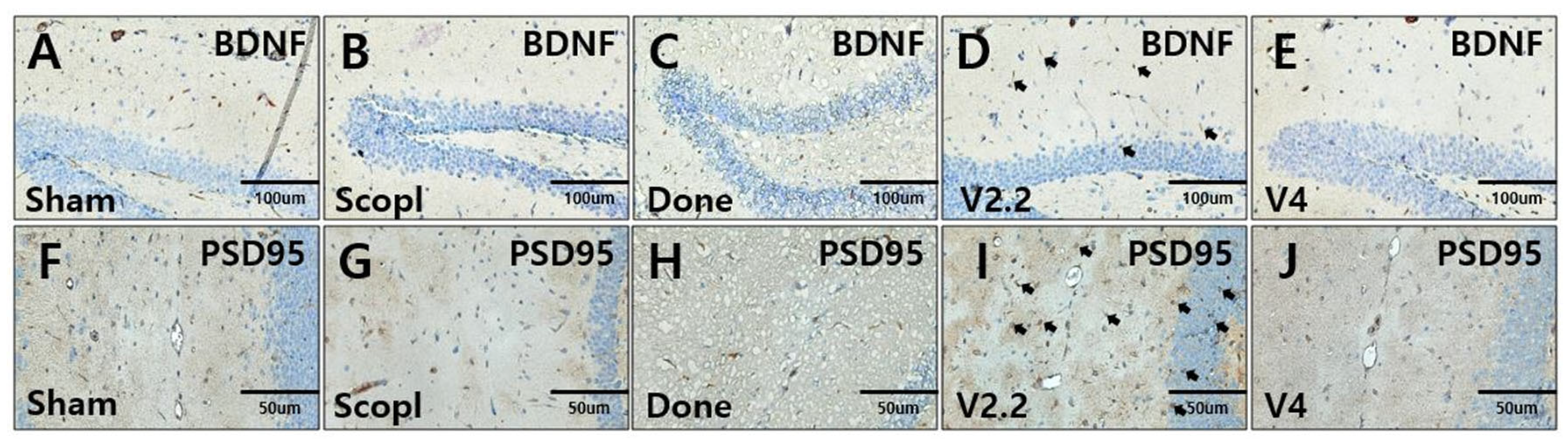
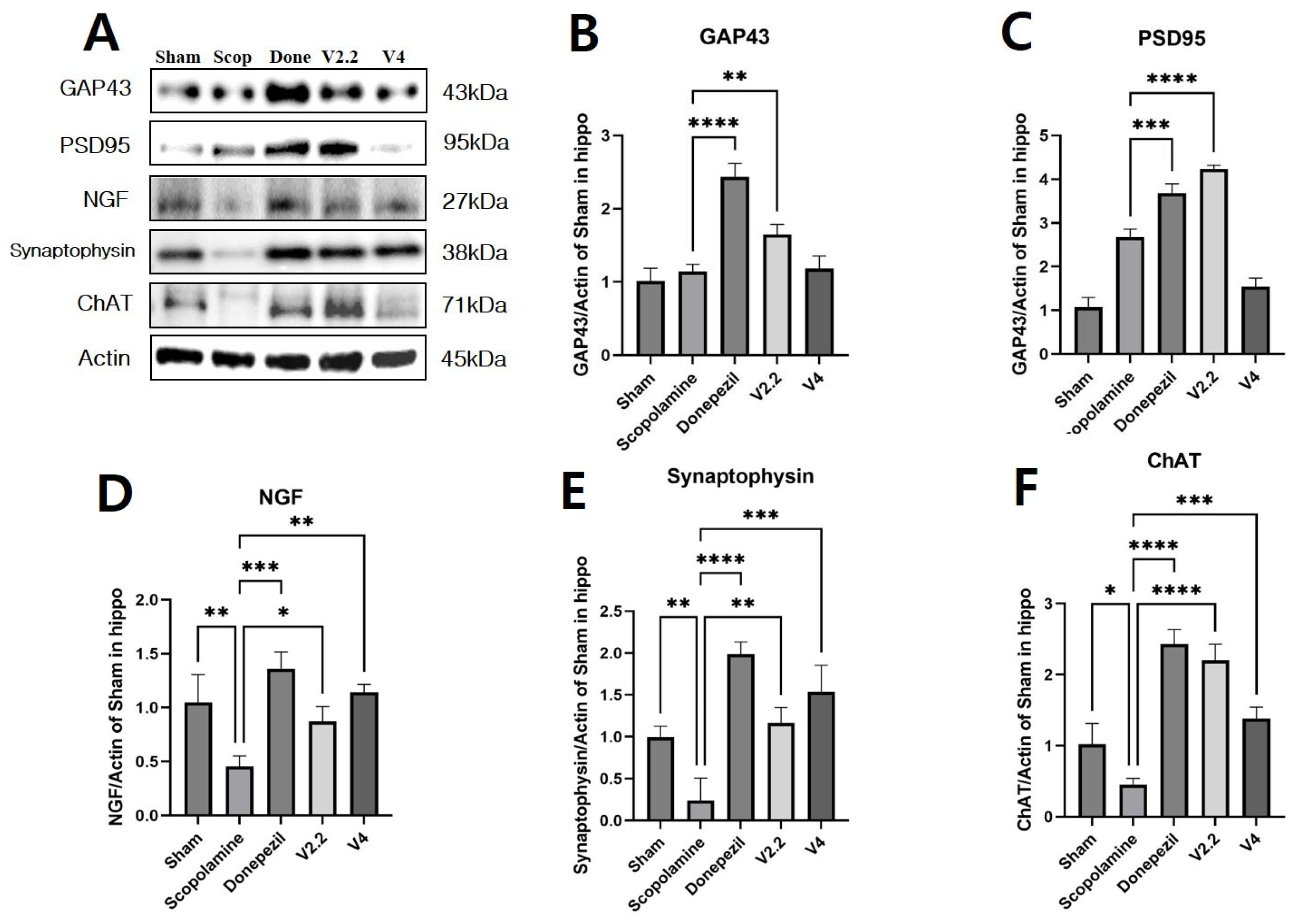
3.6. Immunohistochemical Analysis of Neural Recovery in a Scopolamine-Induced Alzheimer’s Disease Mouse Model Following Vibrotactile Stimulation
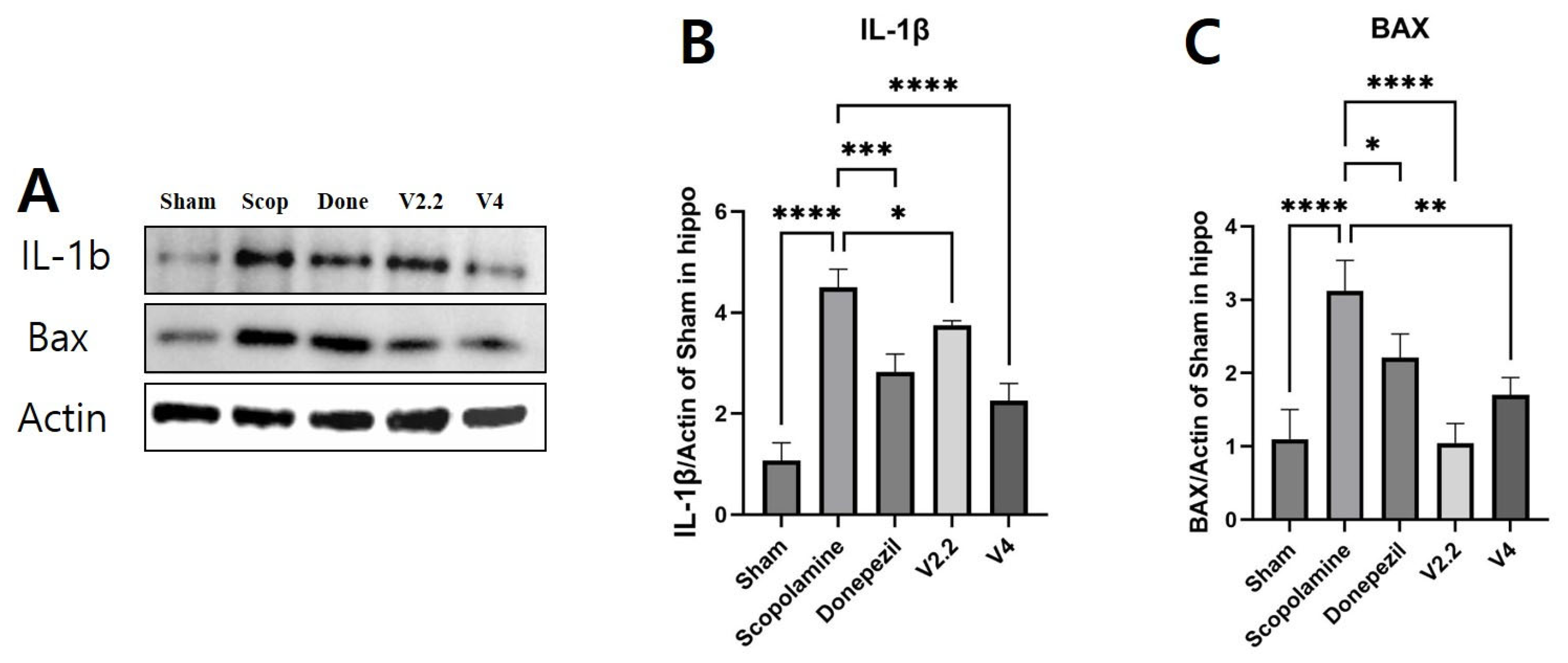
3.7. Effects of Vibrotactile Stimulation on Neuroinflammation and Apoptosis-Related Proteins in the Hippocampus of a Scopolamine-Induced AD Mouse Model
3.8. Effects of Vibrotactile Stimulation on Cholinergic Function in the Hippocampus of a Scopolamine-Induced AD Mouse Model
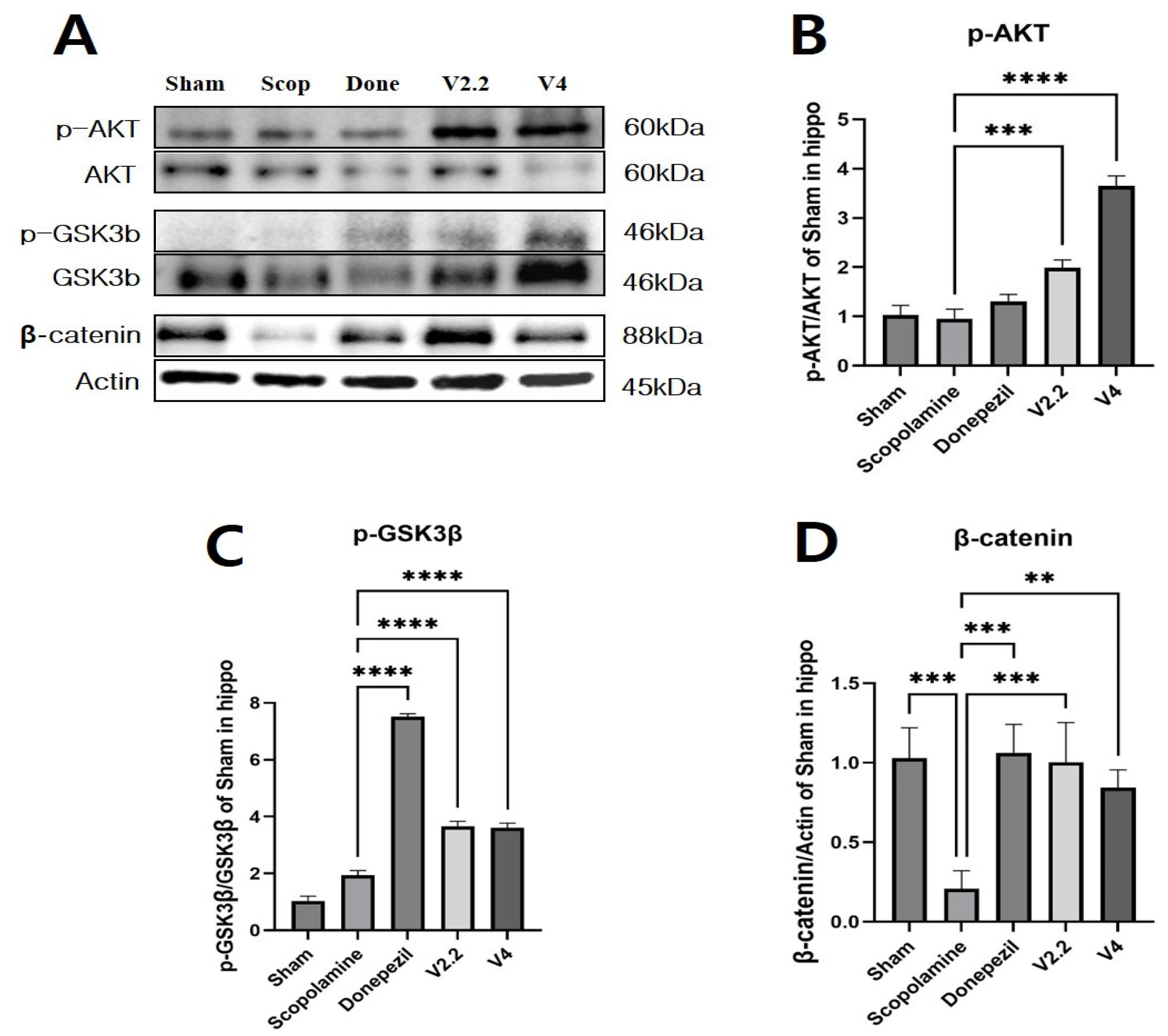
3.9. Effects of Vibrotactile Stimulation on the AKT/GSK3β/β-Catenin Pathway in the Hippocampus of a Scopolamine-Induced AD Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement. 2017, 3, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Goverdhan, P.; Sravanthi, A.; Mamatha, T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int. J. Alzheimers Dis. 2012, 2012, 974013. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.Y.; Xia, Z.; Xie, Q.; Ge, X.X.; Zhang, W.W.; Sun, J.; Jiang, P.; Wang, H.; Le, W.D.; Qiu, Z.B.; et al. Meserine, a novel carbamate AChE inhibitor, ameliorates scopolamine-induced dementia and alleviates amyloidogenesis of APP/PS1 transgenic mice. CNS Neurosci. Ther. 2014, 20, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sadegh Malvajerd, S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Sharif Zadeh, M.; Akbari Javar, H.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef]
- Sintov, A.C. AmyloLipid Nanovesicles: A self-assembled lipid-modified starch hybrid system constructed for direct nose-to-brain delivery of curcumin. Int. J. Pharm. 2020, 588, 119725. [Google Scholar] [CrossRef]
- Pariyar, R.; Yoon, C.S.; Svay, T.; Kim, D.S.; Cho, H.K.; Kim, S.Y.; Oh, H.; Kim, Y.C.; Kim, J.; Lee, H.S.; et al. Vitis labruscana leaf extract ameliorates scopolamine-induced impairments with activation of Akt, ERK and CREB in mice. Phytomedicine 2017, 36, 8–17. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Jung, J.M.; Kim, J.M.; Cai, M.; Liu, X.; Hong, J.G.; Lee, C.H.; Lee, K.R.; Ryu, J.H. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur. J. Pharmacol. 2012, 676, 64–70. [Google Scholar] [CrossRef]
- Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 2006, Cd005593. [Google Scholar] [CrossRef]
- Budzynska, B.; Boguszewska-Czubara, A.; Kruk-Slomka, M.; Skalicka-Wozniak, K.; Michalak, A.; Musik, I.; Biala, G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology 2015, 232, 931–942. [Google Scholar] [CrossRef]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr. Neurosci. 2022, 25, 485–501. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, Y.; Gao, X. Potential role of hippocampal neurogenesis in spinal cord injury induced post-trauma depression. Neural Regen. Res. 2024, 19, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Q.; Xu, Z.P.; Zhang, S.; Cao, X.S.; Liu, T.S. Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. Behav. Brain Res. 2009, 199, 197–202. [Google Scholar] [CrossRef]
- Massa, S.M.; Yang, T.; Xie, Y.; Shi, J.; Bilgen, M.; Joyce, J.N.; Nehama, D.; Rajadas, J.; Longo, F.M. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J. Clin. Investig. 2010, 120, 1774–1785. [Google Scholar] [CrossRef]
- Yin, G.; Li, L.Y.; Qu, M.; Luo, H.B.; Wang, J.Z.; Zhou, X.W. Upregulation of AKT attenuates amyloid-β-induced cell apoptosis. J. Alzheimers Dis. 2011, 25, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Cohen, P.; Alessi, D.R. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 1997, 416, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Q.; Liu, E.J.; Zhao, L.; Song, J.; Liu, X.A.; Ren, Q.G.; Jiang, X.; Zeng, J.; Yang, Y.T.; et al. Activation of GSK-3 disrupts cholinergic homoeostasis in nucleus basalis of Meynert and frontal cortex of rats. J. Cell. Mol. Med. 2017, 21, 3515–3528. [Google Scholar] [CrossRef]
- Saha, S.; Pal, D.; Nimse, S.B. Recent Advances in the Discovery of GSK-3 Inhibitors from Synthetic Origin in the Treatment of Neurological Disorders. Curr. Drug Targets 2021, 22, 1437–1462. [Google Scholar] [CrossRef]
- Sinha, A.; Tamboli, R.S.; Seth, B.; Kanhed, A.M.; Tiwari, S.K.; Agarwal, S.; Nair, S.; Giridhar, R.; Chaturvedi, R.K.; Yadav, M.R. Neuroprotective Role of Novel Triazine Derivatives by Activating Wnt/β Catenin Signaling Pathway in Rodent Models of Alzheimer’s Disease. Mol. Neurobiol. 2015, 52, 638–652. [Google Scholar] [CrossRef]
- Jia, L.; Piña-Crespo, J.; Li, Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 2019, 12, 104. [Google Scholar] [CrossRef]
- Griffiths, B.J.; Jensen, O. Gamma oscillations and episodic memory. Trends Neurosci. 2023, 46, 832–846. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.; Chen, R.; Wang, Q.; Zeng, T.; Hu, J.; Yan, C.; Xiao, J.; Xia, X. Whole-body vibration elicits 40 Hz cortical gamma oscillations and ameliorates age-related cognitive impairment through hippocampal astrocyte synapses in male rats. Biogerontology 2024, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Benussi, A.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Brattini, C.; Datta, A.; Thomas, C.; Santarnecchi, E.; Pascual-Leone, A.; Borroni, B. Exposure to gamma tACS in Alzheimer’s disease: A randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul. 2021, 14, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, W.; Li, S.; Li, Y.; Yuan, Y.; Huang, L.; Cao, T.; Fan, L.; Chen, J.; Wang, J.; et al. Transcranial Alternating Current Stimulation Improves Memory Function in Alzheimer’s Mice by Ameliorating Abnormal Gamma Oscillation. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2060–2068. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Guo, M.; Lei, Q.; He, L.; Li, Z. Acoustic stimulation during sleep improves cognition and ameliorates Alzheimer’s disease pathology in APP/PS1 mice. Exp. Gerontol. 2023, 182, 112299. [Google Scholar] [CrossRef] [PubMed]
- Martorell, A.J.; Paulson, A.L.; Suk, H.J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e222. [Google Scholar] [CrossRef]
- Adaikkan, C.; Middleton, S.J.; Marco, A.; Pao, P.C.; Mathys, H.; Kim, D.N.; Gao, F.; Young, J.Z.; Suk, H.J.; Boyden, E.S.; et al. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron 2019, 102, 929–943.e928. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Suk, H.-J.; Buie, N.; Xu, G.; Banerjee, A.; Boyden, E.S.; Tsai, L.-H. Vibrotactile stimulation at gamma frequency mitigates pathology related to neurodegeneration and improves motor function. Front. Aging Neurosci. 2023, 15, 1129510. [Google Scholar] [CrossRef]
- Penasso, H.; Petersen, F.; Peternell, G. Vascular and Neural Response to Focal Vibration, Sensory Feedback, and Piezo Ion Channel Signaling. J. Vasc. Dis. 2023, 2, 42–90. [Google Scholar] [CrossRef]
- Sapozhnikov, G.; Ranganathan, R.; Wang, S.; Song, Y.-Q. Mechanosensitive apoptosis in Alzheimer’s disease. Aging Neurodegener. Dis. 2025, 5, 8. [Google Scholar] [CrossRef]
- Kodama, A.; Suzuki, Y.; Sakuraba, K.; Kume, Y.; Ota, H. The Effect of Deep Micro Vibrotactile Stimulation on Cognitive Function of Mild Cognitive Impairment and Mild Dementia. Int. J. Environ. Res. Public Health 2022, 19, 3803. [Google Scholar] [CrossRef] [PubMed]
- Mimenza-Alvarado, A.J.; Aguilar-Navarro, S.G.; Abarca-Jiménez, I.E.; Vázquez-Villaseñor, I.; Luna-Umanzor, D.I.; Dorard, C.; Villafuerte, G. Low intensity gamma-frequency TMS safely modulates gamma oscillations in probable mild Alzheimer’s dementia: A randomized 2 × 2 crossover pilot study. Front. Neurol. 2025, 16, 1566476. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, H.B. The effects of whole body vibration exercise intervention on electroencephalogram activation and cognitive function in women with senile dementia. J. Exerc. Rehabil. 2018, 14, 586–591. [Google Scholar] [CrossRef]
- Odano, I.; Maeyatsu, F.; Asari, M.; Yamaguchi, S.; Miura, T.; Taki, Y. Whole-body vibration exercise and training increase regional CBF in mild cognitive impairment with enhanced cognitive function. Ann. Nucl. Med. 2022, 36, 82–94. [Google Scholar] [CrossRef]
- Etter, G.; van der Veldt, S.; Manseau, F.; Zarrinkoub, I.; Trillaud-Doppia, E.; Williams, S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat. Commun. 2019, 10, 5322. [Google Scholar] [CrossRef]
- Wilson, C.A.; Fouda, S.; Sakata, S. Effects of optogenetic stimulation of basal forebrain parvalbumin neurons on Alzheimer’s disease pathology. Sci. Rep. 2020, 10, 15456. [Google Scholar] [CrossRef]
- Traikapi, A.; Konstantinou, N. Gamma Oscillations in Alzheimer’s Disease and Their Potential Therapeutic Role. Front. Syst. Neurosci. 2021, 15, 782399. [Google Scholar] [CrossRef]
- Wu, C.-S.; Lin, T.-X.; Lo, Y.-H.; Ke, S.-C.; Sahu, P.P.; Tseng, P. Single-session gamma sensory stimulation entrains real-time electroencephalography but does not enhance perception, attention, short-term memory, or long-term memory. J. Alzheimer’s Dis. Rep. 2025, 9, 25424823241311927. [Google Scholar] [CrossRef]
- Ismail, R.; Hansen, A.K.; Parbo, P.; Brændgaard, H.; Gottrup, H.; Brooks, D.J.; Borghammer, P. The Effect of 40-Hz Light Therapy on Amyloid Load in Patients with Prodromal and Clinical Alzheimer’s Disease. Int. J. Alzheimers Dis. 2018, 2018, 6852303. [Google Scholar] [CrossRef]
- Yang, Y.L.; Lai, T.W. Chronic Visual Stimulation with LED Light Flickering at 24, 40, or 80 Hz Failed to Reduce Amyloid β Load in the 5XFAD Alzheimer’s Disease Mouse Model. eNeuro 2023, 10, ENEURO.0189-23.2023. [Google Scholar] [CrossRef] [PubMed]
- Oroszi, T.; Geerts, E.; Rajadhyaksha, R.; Nyakas, C.; van Heuvelen, M.J.G.; van der Zee, E.A. Whole-body vibration ameliorates glial pathological changes in the hippocampus of hAPP transgenic mice, but does not affect plaque load. Behav. Brain Funct. 2023, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, G.; Arauz, Y.L.A.; van Heuvelen, M.J.G.; Kortholt, A.; Oroszi, T.; van der Zee, E.A. The effects of whole-body vibration therapy on immune and brain functioning: Current insights in the underlying cellular and molecular mechanisms. Front. Neurol. 2024, 15, 1422152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhou, G.S.; Tan, Y.J.; Tao, H.J.; Chen, J.Q.; Pu, Z.J.; Ma, J.Y.; She, W.; Kang, A.; et al. Studies of the Anti-amnesic Effects and Mechanisms of Single and Combined Use of Donepezil and Ginkgo Ketoester Tablet on Scopolamine-Induced Memory Impairment in Mice. Oxidative Med. Cell. Longev. 2019, 2019, 8636835. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Wei, Y.; Liu, W.; Huang, F.; Shi, H.; Zhang, B.; Wu, X.; Wang, C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol. 2015, 768, 96–107. [Google Scholar] [CrossRef]
- Hoang, T.H.X.; Ho, D.V.; Van Phan, K.; Le, Q.V.; Raal, A.; Nguyen, H.T. Effects of Hippeastrum reticulatum on memory, spatial learning and object recognition in a scopolamine-induced animal model of Alzheimer’s disease. Pharm. Biol. 2020, 58, 1107–1113. [Google Scholar] [CrossRef]
- Pattanashetti, L.A.; Taranalli, A.D.; Parvatrao, V.; Malabade, R.H.; Kumar, D. Evaluation of neuroprotective effect of quercetin with donepezil in scopolamine-induced amnesia in rats. Indian. J. Pharmacol. 2017, 49, 60–64. [Google Scholar] [CrossRef]
- Park, H.-J.; Nam, M.-H.; Park, J.-H.; Lee, J.-M.; Hong, H.-S.; Kim, T.-W.; Lee, I.-H.; Shin, C.-H.; Lee, S.-H.; Seo, Y.-K. Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine. Biomedicines 2024, 12, 2475. [Google Scholar] [CrossRef]
- Hughes, J.R. Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy Behav. 2008, 13, 25–31. [Google Scholar] [CrossRef]
- van Vugt, M.K.; Schulze-Bonhage, A.; Litt, B.; Brandt, A.; Kahana, M.J. Hippocampal gamma oscillations increase with memory load. J. Neurosci. 2010, 30, 2694–2699. [Google Scholar] [CrossRef]
- Griffiths, B.J.; Parish, G.; Roux, F.; Michelmann, S.; van der Plas, M.; Kolibius, L.D.; Chelvarajah, R.; Rollings, D.T.; Sawlani, V.; Hamer, H.; et al. Directional coupling of slow and fast hippocampal gamma with neocortical alpha/beta oscillations in human episodic memory. Proc. Natl. Acad. Sci. USA 2019, 116, 21834–21842. [Google Scholar] [CrossRef]
- Buzsáki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef] [PubMed]
- Colgin, L.L.; Moser, E.I. Gamma oscillations in the hippocampus. Physiology 2010, 25, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Penttonen, M.; Kamondi, A.; Acsády, L.; Buzsáki, G. Gamma frequency oscillation in the hippocampus of the rat: Intracellular analysis in vivo. Eur. J. Neurosci. 1998, 10, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Mably, A.J.; Gereke, B.J.; Jones, D.T.; Colgin, L.L. Impairments in spatial representations and rhythmic coordination of place cells in the 3xTg mouse model of Alzheimer’s disease. Hippocampus 2017, 27, 378–392. [Google Scholar] [CrossRef]
- Goutagny, R.; Gu, N.; Cavanagh, C.; Jackson, J.; Chabot, J.G.; Quirion, R.; Krantic, S.; Williams, S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2013, 37, 1896–1902. [Google Scholar] [CrossRef]
- Ross, B.; Jamali, S.; Miyazaki, T.; Fujioka, T. Synchronization of β and γ oscillations in the somatosensory evoked neuromagnetic steady-state response. Exp. Neurol. 2013, 245, 40–51. [Google Scholar] [CrossRef]
- Chan, D.; Suk, H.J.; Jackson, B.L.; Milman, N.P.; Stark, D.; Klerman, E.B.; Kitchener, E.; Fernandez Avalos, V.S.; de Weck, G.; Banerjee, A.; et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: Results of feasibility and pilot studies. PLoS ONE 2022, 17, e0278412. [Google Scholar] [CrossRef]
- Chan, D.; Suk, H.J.; Jackson, B.; Milman, N.P.; Stark, D.; Beach, S.D.; Tsai, L.H. Induction of specific brain oscillations may restore neural circuits and be used for the treatment of Alzheimer’s disease. J. Intern. Med. 2021, 290, 993–1009. [Google Scholar] [CrossRef]
- Park, S.S.; Park, H.S.; Kim, C.J.; Kang, H.S.; Kim, D.H.; Baek, S.S.; Kim, T.W. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 62. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Targett, I.L.; Crompton, L.A.; Conway, M.E.; Craig, T.J. Differentiation of SH-SY5Y neuroblastoma cells using retinoic acid and BDNF: A model for neuronal and synaptic differentiation in neurodegeneration. In Vitro Cell. Dev. Biol. Anim. 2024, 60, 1058–1067. [Google Scholar] [CrossRef]
- de Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Chien, C.-F.; Huang, L.-C.; Chuu, C.-P.; Chang, H.-W.; Hour, T.-C.; Yang, Y.-H. White Light Stimulation at Gamma Frequency to Modify the Aβ42 and tau Proteins in SH-SY5Y Cells. Am. J. Alzheimer’s Dis. Other Dement. 2024, 39, 15333175241236251. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, K.H.; Hwang, Y.R.; Park, H.R.; Kim, H.J.; Kim, H.G.; Kim, H.R. Exposure to RF-EMF Alters Postsynaptic Structure and Hinders Neurite Outgrowth in Developing Hippocampal Neurons of Early Postnatal Mice. Int. J. Mol. Sci. 2021, 22, 5340. [Google Scholar] [CrossRef] [PubMed]
- Leone, L.; Fusco, S.; Mastrodonato, A.; Piacentini, R.; Barbati, S.A.; Zaffina, S.; Pani, G.; Podda, M.V.; Grassi, C. Epigenetic modulation of adult hippocampal neurogenesis by extremely low-frequency electromagnetic fields. Mol. Neurobiol. 2014, 49, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, A.; Aslan, M.A.; Sinen, O.; Munzuroglu, M.; Derin, N.; Parlak, H.; Bulbul, M.; Agar, A. Effects of adropin on learning and memory in rats tested in the Morris water maze. Hippocampus 2022, 32, 253–263. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimers Dis. 2020, 2020, 6372059. [Google Scholar] [CrossRef]
- Botton, P.H.; Costa, M.S.; Ardais, A.P.; Mioranzza, S.; Souza, D.O.; da Rocha, J.B.; Porciúncula, L.O. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav. Brain Res. 2010, 214, 254–259. [Google Scholar] [CrossRef]
- Can, M.V.; Tran, A.H.; Pham, D.M.; Dinh, B.Q.; Le, Q.V.; Nguyen, B.V.; Nguyen, M.T.T.; Nguyen, H.X.; Nguyen, N.T.; Nishijo, H. Willughbeia cochinchinensis prevents scopolamine-induced deficits in memory, spatial learning, and object recognition in rodents. J. Ethnopharmacol. 2018, 214, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, W.-B.; Ren, X.; Li, Y.-F.; Li, W.; Hang, C.-H.; Wang, Y.-H. Whole Body Vibration Attenuates Brain Damage and Neuroinflammation Following Experimental Traumatic Brain Injury. Front. Cell Dev. Biol. 2022, 10, 847859. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Li, X.; Zheng, K.; Chen, H.; Li, W. Ginsenoside Re Regulates Oxidative Stress through the PI3K/Akt/Nrf2 Signaling Pathway in Mice with Scopolamine-Induced Memory Impairments. Curr. Issues Mol. Biol. 2024, 46, 11359–11374. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef]
- Hancianu, M.; Cioanca, O.; Mihasan, M.; Hritcu, L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine 2013, 20, 446–452. [Google Scholar] [CrossRef]
- Tasset, I.; Medina, F.J.; Jimena, I.; Agüera, E.; Gascón, F.; Feijóo, M.; Sánchez-López, F.; Luque, E.; Peña, J.; Drucker-Colín, R.; et al. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington’s disease rat model: Effects on neurotrophic factors and neuronal density. Neuroscience 2012, 209, 54–63. [Google Scholar] [CrossRef]
- Túnez, I.; Drucker-Colín, R.; Jimena, I.; Medina, F.J.; Muñoz Mdel, C.; Peña, J.; Montilla, P. Transcranial magnetic stimulation attenuates cell loss and oxidative damage in the striatum induced in the 3-nitropropionic model of Huntington’s disease. J. Neurochem. 2006, 97, 619–630. [Google Scholar] [CrossRef]
- Reid, G.A.; Chilukuri, N.; Darvesh, S. Butyrylcholinesterase and the cholinergic system. Neuroscience 2013, 234, 53–68. [Google Scholar] [CrossRef] [PubMed]
- El-Marasy, S.A.; Abd-Elsalam, R.M.; Ahmed-Farid, O.A. Ameliorative Effect of Silymarin on Scopolamine-induced Dementia in Rats. Open Access Maced. J. Med. Sci. 2018, 6, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liao, Y.; Zhang, Y.; Lu, H.; Huang, Z.; Huang, F.; Zhang, Z.; Dong, Y.; Wang, Z.; Huang, Y. Levistilide A ameliorates neuroinflammation via inhibiting JAK2/STAT3 signaling for neuroprotection and cognitive improvement in scopolamine-induced Alzheimer’s disease mouse model. Int. Immunopharmacol. 2023, 124, 110783. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Riazi, G.H.; Abbasi, S.; Dadras, A.; Ghalandari, B.; Seidkhani, H.; Modaresi, S.M.S.; Masoudian, N.; Amani, A.; Ahmadian, S. Synaptosomal acetylcholinesterase activity variation pattern in the presence of electromagnetic fields. Int. J. Biol. Macromol. 2014, 65, 8–15. [Google Scholar] [CrossRef]
- Heesterbeek, M.; Jentsch, M.; Roemers, P.; Keijser, J.N.; Tóth, K.; Nyakas, C.; Schoemaker, R.; Heuvelen, M.; Van der Zee, E. Whole Body Vibration Enhances Choline Acetyltransferase-Immunoreactivity in Cortex and Amygdale. J. Neurol. Transl. Neurosci. 2017, 5, 1079. [Google Scholar]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef]
- Xu, T.; Gu, J.; Li, C.; Guo, X.; Tu, J.; Zhang, D.; Sun, W.; Kong, X. Low-intensity pulsed ultrasound suppresses proliferation and promotes apoptosis via p38 MAPK signaling in rat visceral preadipocytes. Am. J. Transl. Res. 2018, 10, 948–956. [Google Scholar]
- Morse, P.T.; Arroum, T.; Wan, J.; Pham, L.; Vaishnav, A.; Bell, J.; Pavelich, L.; Malek, M.H.; Sanderson, T.H.; Edwards, B.F.P.; et al. Phosphorylations and Acetylations of Cytochrome c Control Mitochondrial Respiration, Mitochondrial Membrane Potential, Energy, ROS, and Apoptosis. Cells 2024, 13, 493. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Y.; Ding, J.; Sun, N.; Lin, S. Antarctic krill peptides improve scopolamine-induced memory impairment in mice. Food Biosci. 2022, 49, 101987. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Gaber, R.; Zrieq, R.; Hafez, T.A.; Mubaraki, M.A.; Abdel Moneim, A.E. Myristica fragrans seed extract reverses scopolamine-induced cortical injury via stimulation of HO-1 expression in male rats. Environ. Sci. Pollut. Res. 2020, 27, 12395–12404. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Griffin, S.; Munch, G.; Pasinetti, G.M. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer’s disease brain exists. J. Alzheimers Dis. 2002, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Demirci, K.; Nazıroğlu, M.; Övey, İ.S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Yerraguravagari, B.; Penchikala, N.P.; Kolusu, A.S.; Ganesh, G.S.; Konduri, P.; Nemmani, K.V.S.; Samudrala, P.K. Montelukast Ameliorates Scopolamine-induced Alzheimer’s Disease: Role on Cholinergic Neurotransmission, Antioxidant Defence System, Neuroinflammation and Expression of BDNF. CNS Neurol. Disord. Drug Targets 2024, 23, 1040–1055. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Faria, M.C.; Gonçalves, G.S.; Rocha, N.P.; Moraes, E.N.; Bicalho, M.A.; Gualberto Cintra, M.T.; de Paula, J.J.; de Miranda, L.F.J.R.; de Souza Ferreira, A.C.; Teixeira, A.L.; et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. J. Psychiatr. Res. 2014, 53, 166–172. [Google Scholar] [CrossRef]
- Cameron, B.; Landreth, G.E. Inflammation, microglia, and alzheimer’s disease. Neurobiol. Dis. 2010, 37, 503–509. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef]
- Wan, M.; Sun, S.; Di, X.; Zhao, M.; Lu, F.; Zhang, Z.; Li, Y. Icariin improves learning and memory function in Aβ(1-42)-induced AD mice through regulation of the BDNF-TrκB signaling pathway. J. Ethnopharmacol. 2024, 318, 117029. [Google Scholar] [CrossRef]
- Tamura, H.; Shiosaka, S.; Morikawa, S. Trophic modulation of gamma oscillations: The key role of processing protease for Neuregulin-1 and BDNF precursors. Neurochem. Int. 2018, 119, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Wang, L.; Liu, H.; Zhang, Y.; Shen, W. Post-stroke cognitive impairment and synaptic plasticity: A review about the mechanisms and Chinese herbal drugs strategies. Front. Neurosci. 2023, 17, 1123817. [Google Scholar] [CrossRef] [PubMed]
- Falone, S.; Grossi, M.R.; Cinque, B.; D’Angelo, B.; Tettamanti, E.; Cimini, A.; Di Ilio, C.; Amicarelli, F. Fifty hertz extremely low-frequency electromagnetic field causes changes in redox and differentiative status in neuroblastoma cells. Int. J. Biochem. Cell Biol. 2007, 39, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, Y.; Niu, B.; Shi, Z.; Li, J.; Ma, Z.; Wang, J.; Gong, P.; Zheng, A.; Zhang, F.; et al. Polymorphic variation in CHAT gene modulates general cognitive ability: An association study with random student cohort. Neurosci. Lett. 2016, 617, 122–126. [Google Scholar] [CrossRef]
- Mengel-From, J.; Christensen, K.; Thinggaard, M.; McGue, M.; Christiansen, L. Genetic variants in the choline acetyltransferase (ChAT) gene are modestly associated with normal cognitive function in the elderly. Genes Brain Behav. 2011, 10, 876–882. [Google Scholar] [CrossRef]
- Honer, W.G.; Barr, A.M.; Sawada, K.; Thornton, A.E.; Morris, M.C.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl. Psychiatry 2012, 2, e114. [Google Scholar] [CrossRef]
- Head, E.; Corrada, M.M.; Kahle-Wrobleski, K.; Kim, R.C.; Sarsoza, F.; Goodus, M.; Kawas, C.H. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol. Aging 2009, 30, 1125–1134. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Y.; Zhu, X.; Xu, H.; Cai, P.; Xia, R.; Mao, L.; Zhao, B.-Q.; Fan, W. Extremely low-frequency electromagnetic fields enhance the proliferation and differentiation of neural progenitor cells cultured from ischemic brains. NeuroReport 2015, 26, 896–902. [Google Scholar] [CrossRef]
- Nakao, K.; Singh, M.; Sapkota, K.; Hagler, B.C.; Hunter, R.N.; Raman, C.; Hablitz, J.J.; Nakazawa, K. GSK3β inhibition restores cortical gamma oscillation and cognitive behavior in a mouse model of NMDA receptor hypofunction relevant to schizophrenia. Neuropsychopharmacology 2020, 45, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Mastrodonato, A.; Barbati, S.A.; Leone, L.; Colussi, C.; Gironi, K.; Rinaudo, M.; Piacentini, R.; Denny, C.A.; Grassi, C. Olfactory memory is enhanced in mice exposed to extremely low-frequency electromagnetic fields via Wnt/β-catenin dependent modulation of subventricular zone neurogenesis. Sci. Rep. 2018, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Riquelme, M.A.; Hua, R.; Zhang, J.; Acosta, F.M.; Gu, S.; Jiang, J.X. Mechanosensitive piezo1 calcium channel activates connexin 43 hemichannels through PI3K signaling pathway in bone. Cell Biosci. 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Zhao, X.; Li, J.; Chen, Y. Piezo channels in the urinary system. Exp. Mol. Med. 2022, 54, 697–710. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Zhang, D.; Men, H.; Huo, L.; Geng, Q.; Wang, S.; Gao, Y.; Zhang, W.; Zhang, Y.; et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int. J. Oncol. 2019, 55, 629–644. [Google Scholar] [CrossRef]
- Katiyar, P.; Singh Rathore, A.; Banerjee, S.; Nathani, S.; Zahra, W.; Singh, S.P.; Sircar, D.; Roy, P. Wheatgrass extract imparts neuroprotective actions against scopolamine-induced amnesia in mice. Food Funct. 2022, 13, 8474–8488. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein Ameliorates Scopolamine-Induced Amnesia in Mice Through the Regulation of the Cholinergic Neurotransmission, Antioxidant System and the ERK/CREB/BDNF Signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Pak, M.E.; Yang, H.J.; Li, W.; Kim, J.K.; Go, Y. Yuk-Gunja-Tang attenuates neuronal death and memory impairment via ERK/CREB/BDNF signaling in the hippocampi of experimental Alzheimer’s disease model. Front. Pharmacol. 2022, 13, 1014840. [Google Scholar] [CrossRef]
- Ikiz, E.D.; Hascup, E.R.; Bae, C.; Hascup, K.N. Microglial Piezo1 mechanosensitive channel as a therapeutic target in Alzheimer’s disease. Front. Cell. Neurosci. 2024, 18, 1423410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-W.; Park, H.-J.; Nam, M.-H.; Lee, I.-H.; Chen, Z.-Y.; Yun, H.-D.; Seo, Y.-K. Acceleration-Dependent Effects of Vibrotactile Gamma Stimulation on Cognitive Recovery and Cholinergic Function in a Scopolamine-Induced Neurotoxicity Mouse Model. Biomedicines 2025, 13, 2031. https://doi.org/10.3390/biomedicines13082031
Kim T-W, Park H-J, Nam M-H, Lee I-H, Chen Z-Y, Yun H-D, Seo Y-K. Acceleration-Dependent Effects of Vibrotactile Gamma Stimulation on Cognitive Recovery and Cholinergic Function in a Scopolamine-Induced Neurotoxicity Mouse Model. Biomedicines. 2025; 13(8):2031. https://doi.org/10.3390/biomedicines13082031
Chicago/Turabian StyleKim, Tae-Woo, Hee-Jung Park, Myeong-Hyun Nam, In-Ho Lee, Zu-Yu Chen, Hee-Deok Yun, and Young-Kwon Seo. 2025. "Acceleration-Dependent Effects of Vibrotactile Gamma Stimulation on Cognitive Recovery and Cholinergic Function in a Scopolamine-Induced Neurotoxicity Mouse Model" Biomedicines 13, no. 8: 2031. https://doi.org/10.3390/biomedicines13082031
APA StyleKim, T.-W., Park, H.-J., Nam, M.-H., Lee, I.-H., Chen, Z.-Y., Yun, H.-D., & Seo, Y.-K. (2025). Acceleration-Dependent Effects of Vibrotactile Gamma Stimulation on Cognitive Recovery and Cholinergic Function in a Scopolamine-Induced Neurotoxicity Mouse Model. Biomedicines, 13(8), 2031. https://doi.org/10.3390/biomedicines13082031







