Curative Therapies for Hemophilias and Hemoglobinopathies in Adults: Immune, Gene, and Stem Cell Approaches in a Global Context
Abstract
1. Introduction
2. Disease Background
2.1. Hemophilias A and B
2.2. Rare Congenital Coagulation Factor Deficiencies
2.3. Von Willebrand Disease
2.4. Hemoglobinopathies
3. Immune-Based Therapeutics
3.1. Immune Tolerance Induction in Hemophilia (ITI)
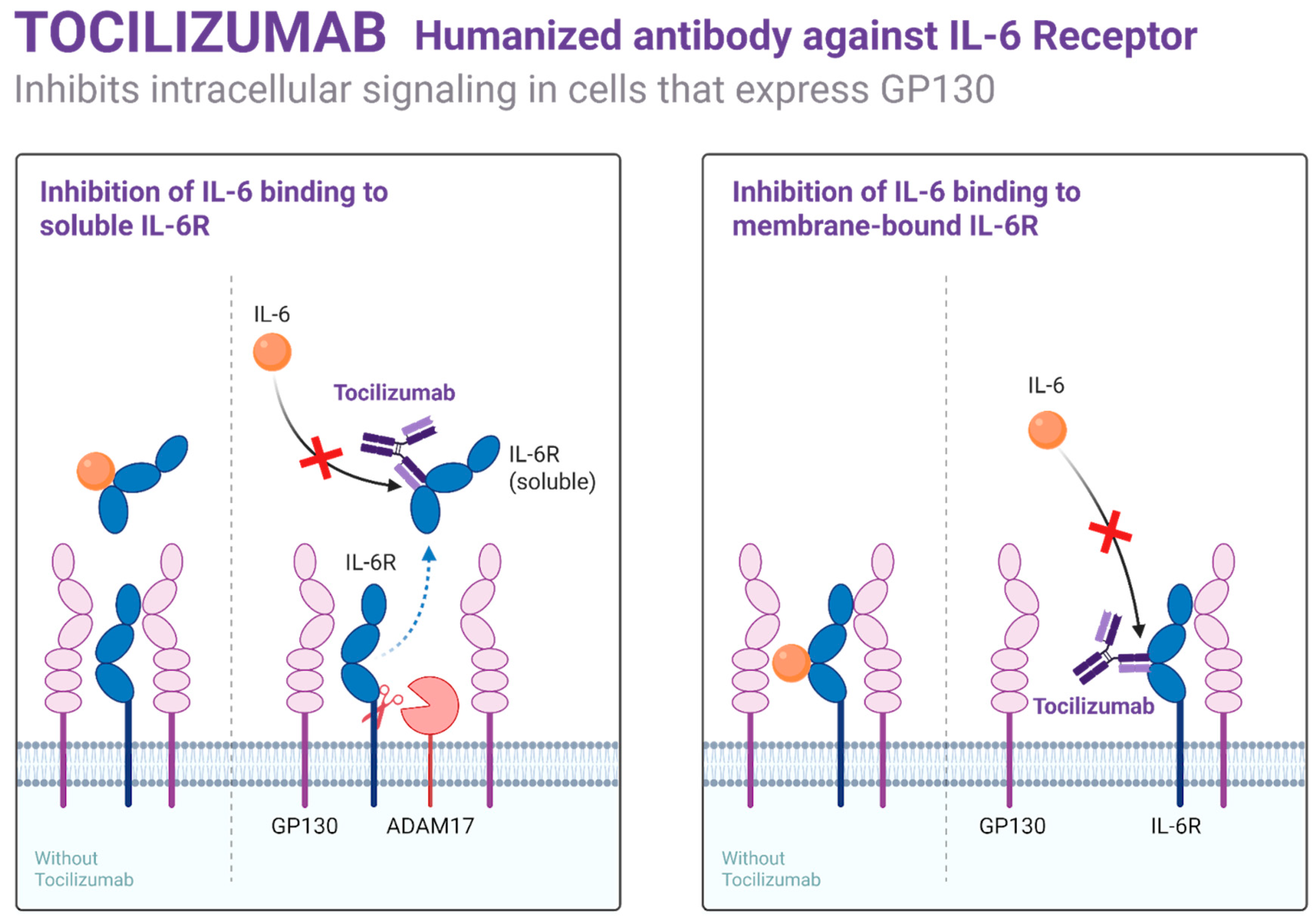
3.2. Monoclonal Antibodies and Bispecific Agents in Hemophilia
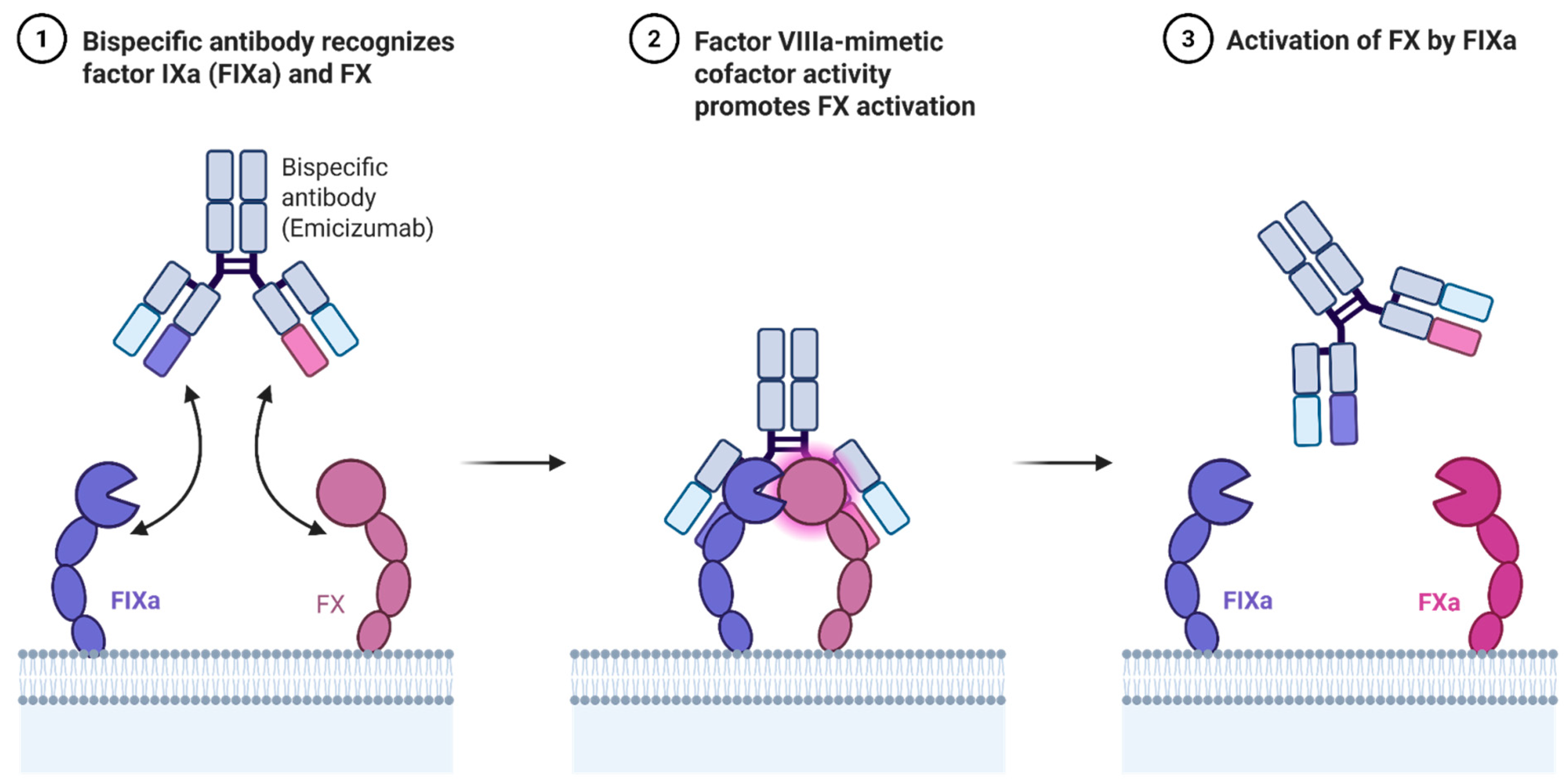
3.3. Emicizumab for Hemophilia A: Mechanism and Indications
3.4. Anti-TFPI and Anti-ATIII Approaches: Fitusiran and Concizumab
3.5. Immune Modulation in Sickle Cell Disease (SCD) and Thalassemia
3.6. Role of Inflammation in Vaso-Occlusion
3.7. Potential of Anti-Adhesion and Anti-Inflammatory Biologics
3.8. Immune Therapy in Acquired Von Willebrand Disease (AVWD)
4. Gene Therapy Approaches
4.1. Viral-Vector-Mediated Gene Addition
4.2. SCD/β-Thalassemia: Lentiviral Vectors with β-Globin or Anti-Sickling Globin
4.3. Genome Editing Technologies: CRISPR-Cas9, Base Editors, and Prime Editing
4.4. Targeting BCL11A Enhancer to Restore Fetal Hemoglobin
4.5. Risks: Off-Target Effects, Durability, Immunogenicity
4.6. Clinical Trials and Real-World Outcomes
4.7. Gene Therapy for Von Willebrand Disease (VWD)
5. Stem-Cell-Based Strategies
5.1. Allogeneic Hematopoietic Stem Cell Transplantation (HSCT)
5.1.1. Applications in Hemophilias and Hemoglobinopathies
5.1.2. Matched Sibling Donor vs. Haploidentical vs. Unrelated Donors
5.1.3. Autologous Gene-Modified HSC Therapy
6. Challenges in Global Implementation
7. Ethical and Societal Considerations
8. Future Directions
9. Market Withdrawal and Systemic Barriers to Access
10. Conclusions
Funding
Conflicts of Interest
References
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Dewi, M.; Temperley, W.H.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013, 381, 142–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castaman, G.; Matino, D. Hemophilia A and B: Molecular and clinical similarities and differences. Haematologica 2019, 104, 1702–1709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rostami, T.; Rad, S.; Rostami, M.R.; Mirhosseini, S.A.; Alemi, H.; Khavandgar, N.; Janbabai, G.; Kiumarsi, A.; Kasaeian, A.; Mousavi, S.A. Hematopoietic Stem Cell Transplantation in Sickle Cell Disease: A Multidimentional Review. Cell Transplant. 2024, 33, 9636897241246351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.A.; Walters, M.C.; Kwiatkowski, J.; Rasko, J.E.J.; Ribeil, J.A.; Hongeng, S.; Magrin, E.; Schiller, G.J.; Payen, E.; Semeraro, M.; et al. Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2018, 378, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Pasi, K.J.; Rangarajan, S.; Mitchell, N.; Lester, W.; Symington, E.; Madan, B.; Laffan, M.; Russell, C.B.; Li, M.; Pierce, G.F.; et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia, A.N. Engl. J. Med. 2020, 382, 29–40. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Sullivan, S.K.; Giermasz, A.; Rasko, J.E.J.; Samelson-Jones, B.J.; Ducore, J.; Cuker, A.; Sullivan, L.M.; Majumdar, S.; Teitel, J.; et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017, 377, 2215–2227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swan, D.; Mahlangu, J.; Thachil, J. Non-factor therapies for bleeding disorders: A primer for the general haematologist. EJHaem 2022, 3, 584–595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fedewa, S.A.; Valentino, L.A.; Koo, A.; Cafuir, L.; Gillespie, T.W.; Buckner, T.W.; Tran, D.Q.; Antun, A.; Kempton, C.L. Global patterns of hemophilia drug trials, hemophilia care, and health care measures. Res. Pract. Thromb. Haemost. 2025, 9, 102714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- John, M.J.; Stonebraker, J.; Jindal, A.; Tootoonchian, E.; Pierce, G.P.; Gouider, E.; Jain, A.; Coffin, D. Global prophylaxis trends in hemophilia: A macroeconomic analysis and its association with world development indicators. Expert Rev. Hematol. 2024, 17, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Konkle, B.A.; Walsh, C.E.; Escobar, M.A.; Josephson, N.C.; Young, G.; von Drygalski, A.; McPhee, S.W.J.; Samulski, R.J.; Bilic, I.; de la Rosa, M.; et al. BAX 335 hemophilia B gene therapy clinical trial results: Potential impact of CpG sequences on gene expression. Blood 2021, 137, 763–774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Callaghan, M.U.; Negrier, C.; Paz-Priel, I.; Chang, T.; Chebon, S.; Lehle, M.; Mahlangu, J.; Young, G.; Kruse-Jarres, R.; Mancuso, M.E.; et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood 2021, 137, 2231–2242, Erratum in Blood 2023, 142, 1329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shima, M.; Amano, K.; Ogawa, Y.; Yoneyama, K.; Ozaki, R.; Kobayashi, R.; Sakaida, E.; Saito, M.; Okamura, T.; Ito, T.; et al. A prospective, multicenter, open-label phase III study of emicizumab prophylaxis in patients with acquired hemophilia A. J. Thromb. Haemost. 2023, 21, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Gualtierotti, R.; Giachi, A.; Bitto, N.; La Mura, V.; Peyvandi, F. Gene therapy in hemophilia: The dawn of a new era. Res. Pract. Thromb. Haemost. 2024, 9, 102640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, D.; Dou, F.; Gao, J. Fitusiran: The first approved siRNA therapy for hemophilia via reducing plasma antithrombin levels. Drug Discov. Ther. 2025, 19, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Mahlangu, J. Anti-tissue factor pathway inhibitors for hemophilia: Are these treatments the answer to overcoming current treatment limitations? Expert Rev. Hematol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, F.; Guillet, B.; Mura, T.; Fournel, A.; Volot, F.; Chambost, H.; Suchon, P.; Frotscher, B.; Biron-Andréani, C.; Marlu, R.; et al. Surgery in rare bleeding disorders: The prospective MARACHI study. Res. Pract. Thromb. Haemost. 2023, 7, 102199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gailani, D. Factor XI as a target for preventing venous thromboembolism. J. Thromb. Haemost. 2022, 20, 550–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shima, M. Current status and future prospects of activated recombinant coagulation factor VIIa, NovoSeven®, in the treatment of haemophilia and rare bleeding disorders. Ann. Hematol. 2024, 103, 2647–2658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dorgalaleh, A.; Bahraini, M.; Shams, M.; Parhizkari, F.; Dabbagh, A.; Naderi, T.; Fallah, A.; Fazeli, A.; Ahmadi, S.E.; Samii, A.; et al. Molecular basis of rare congenital bleeding disorders. Blood Rev. 2023, 59, 101029. [Google Scholar] [CrossRef] [PubMed]
- James, P.D.; Connell, N.T.; Ameer, B.; Di Paola, J.; Eikenboom, J.; Giraud, N.; Haberichter, S.; Jacobs-Pratt, V.; Konkle, B.; McLintock, C.; et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021, 5, 280–300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalvehalli Kashinath, S.; Kouides, P.A. The diagnosis, natural history, and management of von Willebrand disease in women in the age of guidelines. Expert. Rev. Hematol. 2023, 16, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Ragni, M.V. Rapidly loading emicizumab without immunosuppression in acquired haemophilia. Lancet Haematol. 2023, 10, e870–e871. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, C.I.; Hodulik, K.L.; Telen, M.J.; Strouse, J.J. Management of Older Adults with Sickle Cell Disease: Considerations for Current and Emerging Therapies. Drugs Aging 2023, 40, 317–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curtis, S.A.; Betancourt, J.; Kottapalli, N.; Campbell, S.; Minniti, C. Voxelotor use in adults with sickle cell disease in a real-world setting. Am. J. Hematol. 2022, 97, E125–E128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappelli, B.; Gluckman, E.; Corbacioglu, S.; de la Fuente, J.; Abboud, M.R. Hemoglobinopathies (Sickle Cell Disease and Thalassemia). In The EBMT Handbook: Hematopoietic Cell Transplantation and Cellular Therapies, 8th ed.; Sureda, A., Corbacioglu, S., Greco, R., Kröger, N., Carreras, E., Eds.; Springer: Cham, Switzerland, 2024; Chapter 80. [Google Scholar] [PubMed]
- Al Mozain, N.; Elobied, Y.; Al-Omran, A.; Aljaloud, A.; Omair, A.B.; Tuwaim, R.B.; Alkhalifah, S.; Altawil, E.S.; Abraham, S.; Salcedo, L.R.; et al. Comparative study between chronic automated red blood cell exchange and manual exchange transfusion in patients with sickle cell disease: A single center experience from Saudi Arabia. Asian J. Transfus. Sci. 2023, 17, 91–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oomen, I.; Camelo, R.M.; Rezende, S.M.; Voorberg, J.; Mancuso, M.E.; Oldenburg, J.; Carcao, M.; Matino, D.; Lillicrap, D.; Fischer, K.; et al. International Genetic and clinical determinants of the Outcome of Immune Tolerance Induction (GO-ITI) study group. Determinants of successful immune tolerance induction in hemophilia A: Systematic review and meta-analysis. Res. Pract. Thromb. Haemost. 2022, 7, 100020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doshi, B.S.; Witmer, C.M. Recurrence of a high-titre factor VIII inhibitor in a haemophilia A patient on emicizumab prophylaxis. Haemophilia 2021, 27, e551–e553. [Google Scholar] [CrossRef] [PubMed]
- Ljung, R.; Auerswald, G.; Benson, G.; Dolan, G.; Duffy, A.; Hermans, C.; Jiménez-Yuste, V.; Lambert, T.; Morfini, M.; Zupančić-Šalek, S.; et al. Inhibitors in haemophilia A and B: Management of bleeds, inhibitor eradication and strategies for difficult-to-treat patients. Eur. J. Haematol. 2019, 102, 111–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Male, C.; Andersson, N.G.; Rafowicz, A.; Liesner, R.; Kurnik, K.; Fischer, K.; Platokouki, H.; Santagostino, E.; Chambost, H.; Nolan, B.; et al. Inhibitor incidence in an unselected cohort of previously untreated patients with severe haemophilia B: A PedNet study. Haematologica 2021, 106, 123–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deshpande, S.R.; Joseph, K.; Tong, J.; Chen, Y.; Pishko, A.; Cuker, A. Adeno-associated virus-based gene therapy for hemophilia A and B: A systematic review and meta-analysis. Blood Adv. 2024, 8, 5957–5974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermans, C.; Pierce, G.F. Bispecific antibodies mimicking factor VIII in hemophilia A: Converting innovation to an essential medicine. Res. Pract. Thromb. Haemost. 2023, 7, 100173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahlangu, J.; Jiménez-Yuste, V.; Ventriglia, G.; Niggli, M.; Barlera, S.; Hermans, C.; Lehle, M.; Chowdary, P.; Jew, L.; Windyga, J.; et al. Long-term outcomes with emicizumab in hemophilia A without inhibitors: Results from the HAVEN 3 and 4 studies. Res. Pract. Thromb. Haemost. 2024, 8, 102364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chowdary, P.; Angchaisuksiri, P.; Apte, S.; Astermark, J.; Benson, G.; Chan, A.K.C.; Jiménez Yuste, V.; Matsushita, T.; Høgh Nielsen, A.R.; Sathar, J.; et al. Concizumab prophylaxis in people with haemophilia A or haemophilia B without inhibitors (explorer8): A prospective, multicentre, open-label, randomised, phase 3a trial. Lancet Haematol. 2024, 11, e891–e904, Erratum in Lancet Haematol. 2024, 11, e886. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; von Mackensen, S.; Abraham, A.; Castaman, G.; Hampton, K.; Knoebl, P.; Linari, S.; Odgaard-Jensen, J.; Neergaard, J.S.; Stasyshyn, O.; et al. Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: Patient-reported outcome results from the phase 3 explorer7 study. Res. Pract. Thromb. Haemost. 2024, 8, 102476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahagna, A.A.; Annunziata, S.; Torriani, C.; Jannelli, E.; Mascia, B.; Montagna, A.; Mosconi, M.; Mattia, C.; Pasta, G. Perioperative Pain Management in Hemophilic Patient Undergoing Orthopedic Surgery: A Narrative Review. Healthcare 2024, 12, 2007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfrepper, C.; Siecke, A.; Klamroth, R.; Kühnöl, C.; Kentouche, K.; Holzhauer, S.; Fischer, L.; Aumann, V.; Trautmann-Grill, K.; Scholz, U.; et al. Changes in Hemophilia Treatment in the Eastern Part of Germany between 2015 and 2021-Data from the Kompetenznetz Hämorrhagische Diathese Ost (KHDO). Hamostaseologie 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Castaman, G.; Croteau, S.E.; Quon, D.; Lee, L.; Polito, L.; Jiménez-Yuste, V. A literature review of major surgery experience with emicizumab in people with hemophilia A without factor VIII inhibitors. Res. Pract. Thromb. Haemost. 2025, 9, 102693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, G.; Pipe, S.W.; Kenet, G.; Oldenburg, J.; Safavi, M.; Czirok, T.; Nissen, F.; Mahlangu, J. Emicizumab is well tolerated and effective in people with congenital hemophilia A regardless of age, severity of disease, or inhibitor status: A scoping review. Res. Pract. Thromb. Haemost. 2024, 8, 102415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, G.; Kavakli, K.; Klamroth, R.; Matsushita, T.; Peyvandi, F.; Pipe, S.W.; Rangarajan, S.; Shen, M.C.; Srivastava, A.; Sun, J.; et al. Safety and efficacy of a fitusiran antithrombin-based dose regimen in people with hemophilia A or B: The ATLAS-OLE study. Blood 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.D.; Angchaisuksiri, P.; Astermark, J.; Benson, G.; Castaman, G.; Eichler, H.; Jiménez-Yuste, V.; Kavakli, K.; Matsushita, T.; Poulsen, L.H.; et al. Long-term efficacy and safety of subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors. Blood Adv. 2022, 6, 3422–3432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsushita, T.; Shapiro, A.; Abraham, A.; Angchaisuksiri, P.; Castaman, G.; Cepo, K.; d’Oiron, R.; Frei-Jones, M.; Goh, A.S.; Haaning, J.; et al. Phase 3 Trial of Concizumab in Hemophilia with Inhibitors. N. Engl. J. Med. 2023, 389, 783–794. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.; Reiss, U.M.; Hanna, D.; Bolous, N.S. The role of public health in rare diseases: Hemophilia as an example. Front. Public Health 2025, 13, 1450625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colombatti, R.; Hegemann, I.; Medici, M.; Birkegård, C. Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection. J. Clin. Med. 2023, 12, 5538, Erratum in J. Clin. Med. 2024, 13, 2893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.R.; Cançado, R.D.; De Montalembert, M.; Smith, W.R.; Rimawi, H.; Voskaridou, E.; Güvenç, B.; Ataga, K.I.; Keefe, D.; Grosch, K.; et al. Crizanlizumab with or without hydroxyurea in patients with sickle cell disease (STAND): Primary analyses from a placebo-controlled, randomised, double-blind, phase 3 trial. Lancet Haematol. 2025, 12, e248–e257, Erratum in Lancet Haematol. 2025, 12, e329. [Google Scholar] [CrossRef] [PubMed]
- Osunkwo, I.; Manwani, D.; Kanter, J. Current and novel therapies for the prevention of vaso-occlusive crisis in sickle cell disease. Ther. Adv. Hematol. 2020, 11, 2040620720955000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conran, N.; De Paula, E.V. Thromboinflammatory mechanisms in sickle cell disease—Challenging the hemostatic balance. Haematologica 2020, 105, 2380–2390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinto, V.M.; Forni, G.L. Management of Iron Overload in Beta-Thalassemia Patients: Clinical Practice Update Based on Case Series. Int. J. Mol. Sci. 2020, 21, 8771. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frangoul, H.; Locatelli, F.; Eckrich, M.J.; Imren, S.; Li, N.; Xuan, F.; Grupp, S.A. Impact of Different Definitions of Vaso-Occlusion on Efficacy Assessments in Sickle Cell Disease Clinical Trials. Adv. Ther. 2025, 42, 2490–2499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beckman, J.D.; Abdullah, F.; Chen, C.; Kirchner, R.; Rivera-Rodriguez, D.; Kiser, Z.M.; Nguyen, A.; Zhang, P.; Nguyen, J.; Hebbel, R.P.; et al. Endothelial TLR4 Expression Mediates Vaso-Occlusive Crisis in Sickle Cell Disease. Front. Immunol. 2021, 11, 613278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conran, N.; Belcher, J.D. Inflammation in sickle cell disease. Clin. Hemorheol. Microcirc. 2018, 68, 263–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Dossena, S.; Marino, A.; Morabito, R.; Remigante, A. Iron Overload-Related Oxidative Stress Leads to Hyperphosphorylation and Altered Anion Exchanger 1 (Band 3) Function in Erythrocytes from Subjects with β-Thalassemia Minor. Int. J. Mol. Sci. 2025, 26, 1593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, J.; Saraf, S.L.; Gordeuk, V.R. Systematic Review of Crizanlizumab: A New Parenteral Option to Reduce Vaso-occlusive Pain Crises in Patients with Sickle Cell Disease. Pharmacotherapy 2020, 40, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.L.; Koeck, K.; Hottmann, M.; Redfern, A.; Davis, M.; Barth, A.; Geng, X.; Hoppe, C.; Yue, P. A phase 1 study in healthy participants to characterize the safety and pharmacology of inclacumab, a fully human anti-P-selectin antibody, in development for treatment of sickle cell disease. Eur. J. Clin. Pharmacol. 2023, 79, 1219–1228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morikis, V.A.; Hernandez, A.A.; Magnani, J.L.; Sperandio, M.; Simon, S.I. Targeting Neutrophil Adhesive Events to Address Vaso-Occlusive Crisis in Sickle Cell Patients. Front. Immunol. 2021, 12, 663886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franchini, M.; Mannucci, P.M. Acquired von Willebrand syndrome: Focused for hematologists. Haematologica 2020, 105, 2032–2037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leebeek, F.W.G. New Developments in Diagnosis and Management of Acquired Hemophilia and Acquired von Willebrand Syndrome. Hemasphere 2021, 5, e586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mistry, J.; Lowe, G.C.; Lester, W.; Percy, C.L. Sustained good response to rituximab in acquired von Willebrand syndrome. Blood Coagul. Fibrinolysis 2024, 35, 147–149. [Google Scholar] [CrossRef]
- Poza, M.; Íñiguez, R.; Zamanillo, I.; Redondo, S.; Alonso, R.; Martínez-López, J.; Jiménez-Ubieto, A. Ibrutinib effect in acquired von Willebrand syndrome secondary to Waldenström macroglobulinemia. Ther. Adv. Hematol. 2021, 12, 20406207211039326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langer, A.L.; Connell, N.T. Acquired von Willebrand Syndrome. Hematol. Oncol. Clin. North. Am. 2021, 35, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Mingozzi, F.; High, K.A. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu. Rev. Virol. 2017, 4, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.D. Etranacogene dezaparvovec for hemophilia B gene therapy. Ther. Adv. Rare Dis. 2021, 2, 26330040211058896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorovits, B.; Azadeh, M.; Buchlis, G.; Harrison, T.; Havert, M.; Jawa, V.; Long, B.; McNally, J.; Milton, M.; Nelson, R.; et al. Evaluation of the Humoral Response to Adeno-Associated Virus-Based Gene Therapy Modalities Using Total Antibody Assays. AAPS J. 2021, 23, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragni, M.V.; Mead, H.; de Jong, Y.P.; Kaczmarek, R.; Leavitt, A.D.; Long, B.; Nugent, D.J.; Sabatino, D.E.; Fong, S.; von Drygalski, A.; et al. Optimizing liver health before and after gene therapy for hemophilia, A. Blood Adv. 2024, 8, 5203–5212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwiatkowski, J.L.; Walters, M.C.; Hongeng, S.; Yannaki, E.; Kulozik, A.E.; Kunz, J.B.; Sauer, M.G.; Thrasher, A.J.; Thuret, I.; Lal, A.; et al. Betibeglogene autotemcel gene therapy in patients with transfusion-dependent, severe genotype β-thalassaemia (HGB-212): A non-randomised, multicentre, single-arm, open-label, single-dose, phase 3 trial. Lancet 2024, 404, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Locatelli, F.; Sharma, A.; Bhatia, M.; Mapara, M.; Molinari, L.; Wall, D.; Liem, R.I.; Telfer, P.; Shah, A.J.; et al. Exagamglogene Autotemcel for Severe Sickle Cell Disease. N. Engl. J. Med. 2024, 390, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Esrick, E.B.; Lehmann, L.E.; Biffi, A.; Achebe, M.; Brendel, C.; Ciuculescu, M.F.; Daley, H.; MacKinnon, B.; Morris, E.; Federico, A.; et al. Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. N. Engl. J. Med. 2021, 384, 205–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frangoul, H.; Stults, A.; Bruce, K.; Domm, J.; Carroll, C.; Aide, S.; Duckworth, M.; Evans, M.; McManus, M. Best Practices in Gene Therapy for Sickle Cell Disease and Transfusion-dependent β-Thalassemia. Transplant. Cell Ther. 2025, 31, e1–e352. [Google Scholar] [CrossRef] [PubMed]
- Newby, G.A.; Yen, J.S.; Woodard, K.J.; Mayuranathan, T.; Lazzarotto, C.R.; Li, Y.; Sheppard-Tillman, H.; Porter, S.N.; Yao, Y.; Mayberry, K.; et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature 2021, 595, 295–302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; George, A.; Ravi, N.S.; Mohankumar, K.M. CRISPR/Cas based gene editing: Marking a new era in medical science. Mol. Biol. Rep. 2021, 48, 4879–4895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Testa, U.; Leone, G.; Cappellini, M.D. Therapeutic Gene Editing for Hemoglobinopathies. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drysdale, C.M.; Nassehi, T.; Gamer, J.; Yapundich, M.; Tisdale, J.F.; Uchida, N. Hematopoietic-Stem-Cell-Targeted Gene-Addition and Gene-Editing Strategies for β-hemoglobinopathies. Cell Stem Cell 2021, 28, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yuan, Z.; Zhong, S.L.; Xu, H.; Jiang, S. Minimizing the off-target frequency of the CRISPR/Cas9 system via zwitterionic polymer conjugation and peptide fusion. Chem. Sci. 2023, 14, 6375–6382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anguela, X.M.; High, K.A. Hemophilia B and gene therapy: A new chapter with etranacogene dezaparvovec. Blood Adv. 2024, 8, 1796–1803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dever, D.P.; Bak, R.O.; Reinisch, A.; Camarena, J.; Washington, G.; Nicolas, C.E.; Pavel-Dinu, M.; Saxena, N.; Wilkens, A.B.; Mantri, S.; et al. CRISPR/Cas9 β-globin gene correction in sickle cell disease and β-thalassemia. Nat. Rev. Drug Discov. 2021, 20, 389–400. [Google Scholar]
- Shah, J.; Kim, H.; Sivamurthy, K.; Monahan, P.E.; Fries, M. Comprehensive analysis and prediction of long-term durability of factor IX activity following etranacogene dezaparvovec gene therapy in the treatment of hemophilia, B. Curr. Med. Res. Opin. 2023, 39, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Portier, I.; Vanhoorelbeke, K.; Verhenne, S.; Pareyn, I.; Vandeputte, N.; Deckmyn, H.; Goldenberg, D.S.; Samal, H.B.; Singh, M.; Ivics, Z.; et al. High and long-term von Willebrand factor expression after Sleeping Beauty transposon-mediated gene therapy in a mouse model of severe von Willebrand disease. J. Thromb. Haemost. 2018, 16, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Schober, S.; Döring, M.; Lang, P.; Schulte, J.; Olivieri, M.; Icheva, V. Hematopoietic stem cell transplantation in a newborn suffering from severe combined immunodeficiency and severe hemophilia A: A case report and review of the literature. Res. Pract. Thromb. Haemost. 2025, 9, 102842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishnamurti, L.; Neuberg, D.S.; Sullivan, K.M.; Kamani, N.R.; Abraham, A.; Campigotto, F.; Zhang, W.; Dahdoul, T.; De Castro, L.; Parikh, S.; et al. Bone marrow transplantation for adolescents and young adults with sickle cell disease: Results of a prospective multicenter pilot study. Am. J. Hematol. 2019, 94, 446–454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suh, J.K.; Im, H.J.; Kang, S.H.; Kim, H.; Choi, E.S.; Koh, K.N. Treosulfan-based conditioning regimen for allogeneic hematopoietic stem cell transplantation in children with non-malignant diseases. Bone Marrow Transplant. 2022, 57, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, L.J.; Hamerschlak, N.; Rocha, V.; Bonfim, C.; Kerbauy, M.N. Outcomes after Haploidentical Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide: A Systematic Review and Meta-Analysis Comparing Myeloablative with Reduced-Intensity Conditioning Regimens and Bone Marrow with Peripheral Blood Stem Cell Grafts. Transplant. Cell Ther. 2021, 27, e1–e782. [Google Scholar] [CrossRef] [PubMed]
- Rahal, I.; Galambrun, C.; Bertrand, Y.; Garnier, N.; Paillard, C.; Frange, P.; Pondarré, C.; Dalle, J.H.; de Latour, R.P.; Michallet, M.; et al. Late effects after hematopoietic stem cell transplantation for β-thalassemia major: The French national experience. Haematologica 2018, 103, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulas, O.; Caocci, G.; Efficace, F.; Piras, E.; Targhetta, C.; Frau, V.; Barella, S.; Piroddi, A.; Orofino, M.G.; Vacca, A.; et al. Long-term health-related quality of life in patients with β-thalassemia after unrelated hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022, 57, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E.; Cappelli, B.; Bernaudin, F.; Labopin, M.; Volt, F.; Carreras, J.; Pinto Simões, B.; Ferster, A.; Dupont, S.; de la Fuente, J.; et al. Sickle cell disease: An international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood 2017, 129, 1548–1556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kassim, A.A.; de la Fuente, J.; Nur, E.; Wilkerson, K.L.; Alahmari, A.D.; Seber, A.; Bonfim, C.; Simões, B.P.; Alzahrani, M.; Eckrich, M.J.; et al. An international learning collaborative phase 2 trial for haploidentical bone marrow transplant in sickle cell disease. Blood 2024, 143, 2654–2665. [Google Scholar] [CrossRef] [PubMed]
- Tozatto-Maio, K.; Torres, M.A.; Degaide, N.H.S.; Cardoso, J.F.; Volt, F.; Pinto, A.C.S.; Oliveira, D.; Elayoubi, H.; Kashima, S.; Loiseau, P.; et al. HLA-Matched Unrelated Donors for Patients with Sickle Cell Disease: Results of International Donor Searches. Biol. Blood Marrow Transplant. 2020, 26, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Vellaichamy Swaminathan, V.; Ravichandran, N.; Ramanan, K.M.; Meena, S.K.; Varla, H.; Ramakrishnan, B.; Jayakumar, I.; Uppuluri, R.; Raj, R. Augmented immunosuppression and PTCY-based haploidentical hematopoietic stem cell transplantation for thalassemia major. Pediatr. Transplant. 2021, 25, e13893. [Google Scholar] [CrossRef] [PubMed]
- El Cheikh, J.; Bidaoui, G.; Sharrouf, L.; Zahreddine, A.; Massoud, R.; Nehme, R.; Kreidieh, N.; Moukalled, N.; Abou Dalle, I.; Mahfouz, R.; et al. Haploidentical stem cell transplantation with post-transplant cyclophosphamide challenges and outcome from a tertiary care center in Lebanon. Front. Transplant. 2023, 2, 1149393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Z.; Yao, B.; Xie, H.; Su, X. Clinical Progress and Preclinical Insights Into Umbilical Cord Blood Transplantation Improvement. Stem Cells Transl. Med. 2022, 11, 912–926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, R.S.; Rezvani, K. Immune reconstitution post allogeneic transplant and the impact of immune recovery on the risk of infection. Virulence 2016, 7, 901–916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russell, A.L.; Prince, C.; Lundgren, T.S.; Knight, K.A.; Denning, G.; Alexander, J.S.; Zoine, J.T.; Spencer, H.T.; Chandrakasan, S.; Doering, C.B. Non-genotoxic conditioning facilitates hematopoietic stem cell gene therapy for hemophilia A using bioengineered factor VIII. Mol. Ther. Methods Clin. Dev. 2021, 21, 710–727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giommetti, A.; Papanikolaou, E. Advancements in Hematopoietic Stem Cell Gene Therapy: A Journey of Progress for Viral Transduction. Cells 2024, 13, 1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, F.; Li, H.; Wu, X.; Liu, W.; Zhang, F.; Tang, D.; Chen, Y.; Wang, W.; Chi, Y.; Zheng, J.; et al. Safety and activity of an engineered, liver-tropic adeno-associated virus vector expressing a hyperactive Padua factor IX administered with prophylactic glucocorticoids in patients with haemophilia B: A single-centre, single-arm, phase 1, pilot trial. Lancet Haematol. 2022, 9, e504–e513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, D. Big stride in gene therapy for hemophilia B in China. Blood Sci. 2023, 5, 138–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Cho, H.; Han, J.W.; Kim, A.Y.; Park, S.; Lee, M.; Cho, S.; Baik, D.; Kang, H.Y. Cost-utility analysis of emicizumab prophylaxis in haemophilia A patients with factor VIII inhibitors in Korea. Haemophilia 2021, 27, e12–e21. [Google Scholar] [CrossRef] [PubMed]
- Wainstock, D.; Katz, A. Advancing rare disease policy in Latin America: A call to action. Lancet Reg. Health Am. 2023, 18, 100434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brito, M.; Ginete, C.; Ofakunrin, A.; Diaku-Akinwumi, I.; Inusa, B.P.D. Treating sickle cell disease in resource-limited sub-Saharan Africa: Recent strategies and recommendations in addressing the gaps for the provision of evidence-based management. Expert. Rev. Hematol. 2025, 18, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Z.; Chen, M.; Huang, H. Current status and trends in thalassemia burden across South, East and Southeast Asia, 1990-2021 a systematic analysis for the global burden of disease study 2021. BMC Public Health 2024, 24, 3472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koplin, J.J.; Gyngell, C.; Savulescu, J. Germline gene editing and the precautionary principle. Bioethics 2020, 34, 49–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desine, S.; Hollister, B.M.; Abdallah, K.E.; Persaud, A.; Hull, S.C.; Bonham, V.L. The Meaning of Informed Consent: Genome Editing Clinical Trials for Sickle Cell Disease. AJOB Empir. Bioeth. 2020, 11, 195–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miesbach, W.; Klamroth, R.; Oldenburg, J.; Tiede, A. Gene Therapy for Hemophilia-Opportunities and Risks. Dtsch. Arztebl. Int. 2022, 119, 887–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheah, P.Y.; Jatupornpimol, N.; Hanboonkunupakarn, B.; Khirikoekkong, N.; Jittamala, P.; Pukrittayakamee, S.; Day, N.P.J.; Parker, M.; Bull, S. Challenges arising when seeking broad consent for health research data sharing: A qualitative study of perspectives in Thailand. BMC Med. Ethics 2018, 19, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marshall, P.; Royal, C.D.M.; Chadwick, R. Translational Science, DNA Commercialization, and Informed Consent: The Need for Specific Terminology, Insights from a Review of H3Africa Projects. Public Health Genom. 2022, 25, 112–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munung, N.S.; Nnodu, O.E.; Moru, P.O.; Kalu, A.A.; Impouma, B.; Treadwell, M.J.; Wonkam, A. Looking ahead: Ethical and social challenges of somatic gene therapy for sickle cell disease in Africa. Gene Ther. 2024, 31, 202–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Author Correction: Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2022, 40, 1690, Erratum in Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R. Synthetic Biology-Based Approaches to Investigate Host–Pathogen Interactions. SynBio 2025, 3, 4. [Google Scholar] [CrossRef]
- Danaeifar, M.; Najafi, A. Artificial Intelligence and Computational Biology in Gene Therapy: A Review. Biochem. Genet. 2025, 63, 960–983. [Google Scholar] [CrossRef] [PubMed]
- Drahos, J.; Boateng-Kuffour, A.; Calvert, M.; Levine, L.; Dongha, N.; Li, N.; Pakbaz, Z.; Shah, F.T.; Ainsworth, N.; Martin, A.P. Health-related quality of life and economic impacts in adults with transfusion-dependent β-thalassemia: Findings from a prospective longitudinal real-world study. Qual. Life Res. 2025, 34, 2005–2017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, Y.; Bhat, D.; Sridevi, P.; Surti, S.B.; Ranjit, M.; Sarmah, J.; Sudhakar, G.; Babu, B.V. Sickle cell disease in Indian tribal population: Findings of a multi-centre Indian SCD registry. Blood Cells Mol. Dis. 2024, 109, 102873. [Google Scholar] [CrossRef] [PubMed]
- Noori, T.; Ghazisaeedi, M.; Aliabad, G.M.; Mehdipour, Y.; Mehraeen, E.; Conte, R.; Safdari, R. International Comparison of Thalassemia Registries: Challenges and Opportunities. Acta Inform. Med. 2019, 27, 58–63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farmakis, D.; Porter, J.; Taher, A.; Domenica Cappellini, M.; Angastiniotis, M.; Eleftheriou, A. 2021 Thalassaemia International Federation Guidelines for the Management of Transfusion-dependent Thalassemia. Hemasphere 2022, 6, e732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Region | Approved Gene Therapies | Immune Therapies Available | Key Implementation Challenges |
|---|---|---|---|
| Europe | ✔ Roctavian (Hem A), Hemgenix (Hem B), Casgevy (SCD/β-thal), Zynteglo (β-thal) | ✔ Emicizumab, concizumab, crizanlizumab | High cost, national reimbursement delays, infrastructure limitations |
| Asia | ✔ BBM-H901 (Hem B—China only) | ✔ Emicizumab in Japan, China, Korea; limited crizanlizumab use | Uneven regulatory progress, cost barriers, limited transplant centers |
| South America | ✖ No approved gene therapies | ✔ Emicizumab in Brazil, Argentina; limited crizanlizumab | Lack of reimbursement, delayed regulatory access, trial-dependent access |
| Antarctica | ✖ None | ✖ None | No healthcare infrastructure |
| Oceania | ✔ Roctavian, Hemgenix, Casgevy (special access) | ✔ Emicizumab, crizanlizumab | Geographic disparity, rural access limitations |
| Africa | ✖ None | ✔ Limited emicizumab, crizanlizumab via trials/donation | High disease burden, no gene therapy access, weak transplant capacity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangolo, A.; Amoozgar, B.; Zhang, L.; Gill, S.; Lushimba Milolo, D.; Ngindu Kankonde, J.; Mbuyi Batakamuna, C.; Tassan, R.; Cho, C.; Bukasa-Kakamba, J.; et al. Curative Therapies for Hemophilias and Hemoglobinopathies in Adults: Immune, Gene, and Stem Cell Approaches in a Global Context. Biomedicines 2025, 13, 2022. https://doi.org/10.3390/biomedicines13082022
Bangolo A, Amoozgar B, Zhang L, Gill S, Lushimba Milolo D, Ngindu Kankonde J, Mbuyi Batakamuna C, Tassan R, Cho C, Bukasa-Kakamba J, et al. Curative Therapies for Hemophilias and Hemoglobinopathies in Adults: Immune, Gene, and Stem Cell Approaches in a Global Context. Biomedicines. 2025; 13(8):2022. https://doi.org/10.3390/biomedicines13082022
Chicago/Turabian StyleBangolo, Ayrton, Behzad Amoozgar, Lili Zhang, Sarvarinder Gill, Daniel Lushimba Milolo, Justin Ngindu Kankonde, Claude Mbuyi Batakamuna, Robert Tassan, Christina Cho, John Bukasa-Kakamba, and et al. 2025. "Curative Therapies for Hemophilias and Hemoglobinopathies in Adults: Immune, Gene, and Stem Cell Approaches in a Global Context" Biomedicines 13, no. 8: 2022. https://doi.org/10.3390/biomedicines13082022
APA StyleBangolo, A., Amoozgar, B., Zhang, L., Gill, S., Lushimba Milolo, D., Ngindu Kankonde, J., Mbuyi Batakamuna, C., Tassan, R., Cho, C., Bukasa-Kakamba, J., & Mowatt-Pesce, K. (2025). Curative Therapies for Hemophilias and Hemoglobinopathies in Adults: Immune, Gene, and Stem Cell Approaches in a Global Context. Biomedicines, 13(8), 2022. https://doi.org/10.3390/biomedicines13082022







