From Microbial Switches to Metabolic Sensors: Rewiring the Gut–Brain Kynurenine Circuit
Abstract
1. Introduction
2. Microbiota-Driven Modulation of Indoleamine 2,3-Dioxygenase 1 (IDO1) and Tryptophan 2,3-Dioxygenase (TDO) Signaling
2.1. Literature Review: Microbial Metabolites as Modulators of Intestinal Integrity and Systemic Disease
2.2. Research Gaps: Gaps in Dosing Strategies, Longitudinal Efficacy, and Mechanistic Insights
2.3. Time-Stamped Isotope Tracing in Gnotobiotic Mice Can Tag Flux Through Indoleamine 2,3-Dioxygenase 1 (IDO1) Versus Tryptophan 2,3-Dioxygenase (TDO)
2.4. Single-Cell Proteomics in Intestinal Organoids Could Reveal Which Epithelial or Immune Subsets Sense Each “Metabokine”
2.5. Synthetic Consortia with Inducible Kynurenine (KYN) Operons Would Let Us Dial Metabolite Output Like a Volume Knob
2.6. Molecular Mechanisms Linking Gut Microbiota to Kynurenine (KYN) Pathway Enzymes
3. Kynurenine (KYN) Metabolic Pathway “Checkpoints” in the Brain’s Cellular Grid
3.1. Literature Review: Mapping Kynurenine (KYN) Dynamics Across Neurovascular and Immune Landscapes
3.2. Research Gaps: Mapping, Monitoring, and Modulating Kynurenine (KYN) Checkpoints Across Systems
3.3. Clustered Regularly Interspaced Short Palindromic Repeats Interference (CRISPRi) “Zip-Codes” Delivered by Adeno-Associated Virus (AAV) Can Silence Kynurenine 3-Monooxygenase (KMO) or Kynureninase (KYNU) Only in Perivascular Endothelium and Watch Downstream Glutamatergic Sync Crash—or Not
3.4. Light-Addressable Riboswitches Could Let Us Pulse Kynurenine (KYN) Enzymes in Astrocytes and Read Real-Time Calcium Waves
4. Sex and the Circadian City: Hidden Modifiers
4.1. Literature Review: Circadian Misalignment (CM), Mood Vulnerability, and Emerging Chronotherapeutics
4.2. Research Gaps: Timing, Sex, and Biomarker Integration for Precision Kynurenine (KYN) Intervention
4.3. Multi-Time-Point Plasma Kynurenine (KYN) Profiles Stratified by Sex and Hormonal Phase
4.4. Wearable Light Exposure + Metabolite Logging to See If Circadian Misalignment (CM) Exaggerates the Quinolinic Acid (QA) Spike
4.5. Adaptive Trial Designs That Randomize Dose Timing Rather than Just Dose Size
5. Microbiota Engineering as a Precision Switch
5.1. Literature Review: Microbiota-Targeted Strategies for Modulating Mood and Inflammation
5.2. Research Gaps: Live Biotherapeutic Products (LBPs) Against Multi-Drug Resistant Enteric Pathogens: Research Gaps
5.3. Designer Strains with Kill Switches and Inducible Kynurenine Aminotransferase (KAT) Expression
5.4. Encapsulated “Post-Biotics” (e.g., Stabilized Kynurenic Acid (KYNA)) to Bypass Colonization
5.5. Cloud-Linked Stool Metabolomics Dashboards to Guide Weekly Probiotic Titration
6. Intervention 2.0: Dual Inhibitors, Exercise, and Real-Time Biosensing
6.1. Literature Review: Dual Inhibition and Kynurenine (KYN) Modulation
6.2. Research Gaps: Adaptive Dose Timing and Real-Time Monitoring
6.3. Crosstalk Between Kynurenine (KYN) Pathway Modulation and Broader Metabolic Networks
6.4. Phase-Ib “Smart Protocols”: Micro-Dosed Dual Inhibitors Guided by Saliva Kynurenic Acid (KYNA) Sensors
6.5. Conceptual and Translational Limitations
6.6. Artificial Intelligence (AI)-Driven Feedback Loops That Auto-Adjust Evening Treadmill Sessions or Probiotic Cocktails Based on Morning Kynurenine (KYN)/Tryptophan (Trp) Slope: AI-Driven KYN/Trp Feedback Loops
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| AD | Alzheimer’s disease |
| AhR | ary hydrocarbon receptor |
| AI | artificial intelligence |
| BBB | blood–brain barrier |
| CM | circadian misalignment |
| COVID-19 | coronavirus disease 2019 |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| CRISPRi | clustered regularly interspaced short palindromic repeats interference |
| IDO1 | indoleamine 2,3-dioxygenase 1 |
| KMO | kynurenine 3-monooxygenase |
| KYN | kynurenine |
| KYNU | kynureninase |
| KYNA | kynurenic acid |
| KAT | kynurenine aminotransferase |
| LBPs | live biotherapeutic products |
| LC-MS | liquid chromatography–mass spectrometry |
| NAD | nicotinamide adenine dinucleotide |
| QA | quinolinic acid |
| SCFAs | short-chain fatty acids |
| TDO | tryptophan 2,3-dioxygenase |
| TLR | Toll-like receptor |
| Trp | tryptophan |
| ZIM3 | zinc finger protein 3 |
References
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cavanaugh, C.R.; Hornby, P.J. Emerging effects of tryptophan pathway metabolites and intestinal microbiota on metabolism and intestinal function. Amino Acids 2022, 54, 57–70. [Google Scholar] [CrossRef]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, D.; Yu, J.; Song, X.-J.; Li, X.; Cui, Y.-L. Tryptophan metabolism disorder-triggered diseases, mechanisms, and therapeutic strategies: A scientometric review. Nutrients 2024, 16, 3380. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S.; Giménez-Llort, L.; Chen, C.; Hepsomali, P.; Avenanti, A.; Vécsei, L. Innovation at the Intersection: Emerging Translational Research in Neurology and Psychiatry. Cells 2024, 13, 790. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13, 1045. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Sujino, T.; Kanai, T. The tryptophan metabolic pathway of the microbiome and host cells in health and disease. Int. Immunol. 2024, 36, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Cellini, B.; Zelante, T.; Dindo, M.; Bellet, M.M.; Renga, G.; Romani, L.; Costantini, C. Pyridoxal 5′-phosphate-dependent enzymes at the crossroads of host–microbe tryptophan metabolism. Int. J. Mol. Sci. 2020, 21, 5823. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining Roles: A Paradigm Shift in Tryptophan-Kynurenine Metabolism for Innovative Clinical Applications. Int. J. Mol. Sci. 2024, 25, 12767. [Google Scholar] [CrossRef]
- Vandereyken, K.; Sifrim, A.; Thienpont, B.; Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 2023, 24, 494–515. [Google Scholar] [CrossRef] [PubMed]
- Bressan, D.; Battistoni, G.; Hannon, G.J. The dawn of spatial omics. Science 2023, 381, eabq4964. [Google Scholar] [CrossRef] [PubMed]
- Hrovatin, K.; Fischer, D.S.; Theis, F.J. Toward modeling metabolic state from single-cell transcriptomics. Mol. Metab. 2022, 57, 101396. [Google Scholar] [CrossRef]
- Guan, D.; Lazar, M.A. Interconnections between circadian clocks and metabolism. J. Clin. Investig. 2021, 131, e148278. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex differences in sleep, circadian rhythms, and metabolism: Implications for precision medicine. Sleep Med. Rev. 2024, 75, 101926. [Google Scholar] [CrossRef]
- Lévi, F.A.; Okyar, A.; Hadadi, E.; Innominato, P.F.; Ballesta, A. Circadian regulation of drug responses: Toward sex-specific and personalized chronotherapy. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Weger, M.; Weger, B.D.; Gachon, F. Understanding circadian dynamics: Current progress and future directions for chronobiology in drug discovery. Expert Opin. Drug Discov. 2023, 18, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Truong, T.; Saxton, A.J.; Boekweg, H.; Payne, S.H.; Van Ry, P.M.; Kelly, R.T. HyperSCP: Combining isotopic and isobaric labeling for higher throughput single-cell proteomics. Anal. Chem. 2023, 95, 8020–8027. [Google Scholar] [CrossRef]
- Fernández-García, J.; Altea-Manzano, P.; Pranzini, E.; Fendt, S.-M. Stable isotopes for tracing mammalian-cell metabolism in vivo. Trends Biochem. Sci. 2020, 45, 185–201. [Google Scholar] [CrossRef]
- Rottinghaus, A.G.; Ferreiro, A.; Fishbein, S.R.; Dantas, G.; Moon, T.S. Genetically stable CRISPR-based kill switches for engineered microbes. Nat. Commun. 2022, 13, 672. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, M. A Bayesian Dynamic Model-Based Adaptive Design for Oncology Dose Optimization in Phase I/II Clinical Trials. Pharm. Stat. 2025, 24, e2451. [Google Scholar] [CrossRef]
- Joisten, N.; Kummerhoff, F.; Koliamitra, C.; Schenk, A.; Walzik, D.; Hardt, L.; Knoop, A.; Thevis, M.; Kiesl, D.; Metcalfe, A.J.; et al. Exercise and the Kynurenine pathway: Current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc. Immunol. Rev. 2020, 26, 24–42. [Google Scholar] [PubMed]
- Jankovskaja, S.; Engblom, J.; Rezeli, M.; Marko-Varga, G.; Ruzgas, T.; Björklund, S. Non-invasive skin sampling of tryptophan/kynurenine ratio in vitro towards a skin cancer biomarker. Sci. Rep. 2021, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.P.; Jesus, A.M.C.; Cotrim, M.D. Adaptive Design: A Review of the Technical, Statistical, and Regulatory Aspects of Implementation in a Clinical Trial. Ther. Innov. Regul. Sci. 2020, 54, 246–258. [Google Scholar] [CrossRef]
- Ma, N.; He, T.; Johnston, L.J.; Ma, X. Host–microbiome interactions: The aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes 2020, 11, 1203–1219. [Google Scholar] [CrossRef]
- Colucci Cante, R.; Nigro, F.; Passannanti, F.; Lentini, G.; Gallo, M.; Nigro, R.; Budelli, A.L. Gut health benefits and associated systemic effects provided by functional components from the fermentation of natural matrices. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13356. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 1773. [Google Scholar] [CrossRef] [PubMed]
- Hand, T.W.; Vujkovic-Cvijin, I.; Ridaura, V.K.; Belkaid, Y. Linking the Microbiota, Chronic Disease, and the Immune System. Trends Endocrinol. Metab. 2016, 27, 831–843. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. 2015, 20, 1116–1143. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Mayengbam, S.; Chleilat, F.; Reimer, R.A. Dietary Vitamin B6 Deficiency Impairs Gut Microbiota and Host and Microbial Metabolites in Rats. Biomedicines 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Shi, H.; Xiao, J.; Song, X.; Luo, Z.; Ma, X.; Shi, L.; Wei, H.; Li, J. Pomegranate Juice Supplemented with Inulin Modulates Gut Microbiota and Promotes the Production of Microbiota-Associated Metabolites in Overweight/Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Agric. Food Chem. 2024, 72, 14663–14677. [Google Scholar] [CrossRef] [PubMed]
- Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheault, D.; Leyrolle, Q.; Bindels, L.B.; Gomes da Silveira Cauduro, C.; Mulders, M.; Zamariola, G.; Azzi, A.S.; et al. Link between gut microbiota and health outcomes in inulin-treated obese patients: Lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin. Nutr. 2020, 39, 3618–3628. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [Google Scholar] [CrossRef]
- Wang, L.; Qin, N.; Shi, L.; Liu, R.; Zhu, T. Gut Microbiota and Tryptophan Metabolism in Pathogenesis of Ischemic Stroke: A Potential Role for Food Homologous Plants. Mol. Nutr. Food Res. 2024, 68, e2400639. [Google Scholar] [CrossRef]
- Gupta, S.K.; Vyavahare, S.; Duchesne Blanes, I.L.; Berger, F.; Isales, C.; Fulzele, S. Microbiota-derived tryptophan metabolism: Impacts on health, aging, and disease. Exp. Gerontol. 2023, 183, 112319. [Google Scholar] [CrossRef] [PubMed]
- Meghani, S.; Frishkopf, M.; Park, T.; Montgomery, C.L.; Norris, C.; Papathanassoglou, E. Music-based interventions and theoretical mechanisms in post-ICU survivors: A critical narrative synthesis. Intensive Crit. Care Nurs. 2025, 86, 103777. [Google Scholar] [CrossRef]
- Dimitriadis, T.; Della Porta, D.; Perschl, J.; Evers, A.W.M.; Magee, W.L.; Schaefer, R.S. Motivation and music interventions in adults: A systematic review. Neuropsychol. Rehabil. 2024, 34, 649–678. [Google Scholar] [CrossRef]
- Kuuse, A.K.; Paulander, A.S.; Eulau, L. Characteristics and impacts of live music interventions on health and wellbeing for children, families, and health care professionals in paediatric hospitals: A scoping review. Int. J. Qual. Stud. Health Well Being 2023, 18, 2180859. [Google Scholar] [CrossRef]
- Pakdeesatitwara, N.; Clark, I.; Tamplin, J. A mixed-studies systematic review of self-administered music interventions (SAMIs) for psychological wellbeing in people with chronic health conditions: Meta-analysis and narrative summary. Patient Educ. Couns. 2024, 118, 108006. [Google Scholar] [CrossRef]
- Chang, E.X.; Brooker, J.; Hiscock, R.; O’Callaghan, C. Music-based intervention impacts for people with eating disorders: A narrative synthesis systematic review. J. Music Ther. 2023, 60, 202–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fan, Y.; Zhang, G.; Cai, S.; Ma, Y.; Yang, L.; Wang, Y.; Yu, H.; Qiao, S.; Zeng, X. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome 2024, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Wang, Y.N.; Feng, H.Y.; Guo, Z.Y.; Li, X.; Nie, X.L.; Zhao, Y.Y. Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling. Free Radic. Biol. Med. 2022, 184, 30–41. [Google Scholar] [CrossRef]
- Zheng, D.; Ratiner, K.; Elinav, E. Circadian Influences of Diet on the Microbiome and Immunity. Trends Immunol. 2020, 41, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Frith, M.; Harris, D.; Leone, V.A. Mediators of Host-Microbe Circadian Rhythms in Immunity and Metabolism. Biology 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Chang, E.B. Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol. Metab. 2020, 31, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut microbiota, kynurenine pathway and mental disorders—Review. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, S.; Ji, X.; Wu, J.; Meng, J.; Gao, J.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef]
- Aslamkhan, A.G.; Xu, Q.; Loughlin, A.; Vu, H.; Pacchione, S.; Bhatt, B.; Garfinkel, I.; Styring, T.G.; LaFranco-Scheuch, L.; Pearson, K.; et al. Characterization of indoleamine-2,3-dioxygenase 1, tryptophan-2,3-dioxygenase, and Ido1/Tdo2 knockout mice. Toxicol. Appl. Pharmacol. 2020, 406, 115216. [Google Scholar] [CrossRef]
- Peyraud, F.; Guegan, J.P.; Bodet, D.; Cousin, S.; Bessede, A.; Italiano, A. Targeting Tryptophan Catabolism in Cancer Immunotherapy Era: Challenges and Perspectives. Front. Immunol. 2022, 13, 807271. [Google Scholar] [CrossRef]
- Platten, M.; Friedrich, M.; Wainwright, D.A.; Panitz, V.; Opitz, C.A. Tryptophan metabolism in brain tumors—IDO and beyond. Curr. Opin. Immunol. 2021, 70, 57–66. [Google Scholar] [CrossRef]
- Yu, L.; Lu, J.; Du, W. Tryptophan metabolism in digestive system tumors: Unraveling the pathways and implications. Cell Commun. Signal. 2024, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Tijono, S.M.; Palmer, B.D.; Tomek, P.; Flanagan, J.U.; Henare, K.; Gamage, S.; Braun, L.; Ching, L.M. Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2. Pharmaceuticals 2022, 15, 1090. [Google Scholar] [CrossRef]
- Naing, A.; Eder, J.P.; Piha-Paul, S.A.; Gimmi, C.; Hussey, E.; Zhang, S.; Hildebrand, V.; Hosagrahara, V.; Habermehl, C.; Moisan, J.; et al. Preclinical investigations and a first-in-human phase I trial of M4112, the first dual inhibitor of indoleamine 2,3-dioxygenase 1 and tryptophan 2,3-dioxygenase 2, in patients with advanced solid tumors. J. Immunother. Cancer 2020, 8, e000870. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Spector, S.A.; Theodoropoulos, G.; Nguyen, D.J.M.; Kim, E.Y.; Garcia, A.; Savaraj, N.; Lim, D.C.; Paul, A.; Feun, L.G.; et al. Dual inhibition of IDO1/TDO2 enhances anti-tumor immunity in platinum-resistant non-small cell lung cancer. Cancer Metab. 2023, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J. Neurochem. 2025, 169, e70075. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front. Biosci. 2025, 30, 25706. [Google Scholar] [CrossRef]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals 2025, 18, 607. [Google Scholar] [CrossRef]
- Du, L.; Xing, Z.; Tao, B.; Li, T.; Yang, D.; Li, W.; Zheng, Y.; Kuang, C.; Yang, Q. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn-AhR-AQP4 signaling pathway. Signal Transduct. Target. Ther. 2020, 5, 10. [Google Scholar] [CrossRef]
- Capochiani de Iudicibus, R.; Tomek, P.; Palmer, B.D.; Tijono, S.M.; Flanagan, J.U.; Ching, L.M. Parallel discovery of selective and dual inhibitors of tryptophan dioxygenases IDO1 and TDO2 with a newly-modified enzymatic assay. Bioorg. Med. Chem. 2021, 39, 116160. [Google Scholar] [CrossRef] [PubMed]
- MacCannell, A.D.; Roberts, L.D. Metabokines in the regulation of systemic energy metabolism. Curr. Opin. Pharmacol. 2022, 67, 102286. [Google Scholar] [CrossRef]
- Mund, A.; Brunner, A.D.; Mann, M. Unbiased spatial proteomics with single-cell resolution in tissues. Mol. Cell 2022, 82, 2335–2349. [Google Scholar] [CrossRef]
- Petelski, A.A.; Emmott, E.; Leduc, A.; Huffman, R.G.; Specht, H.; Perlman, D.H.; Slavov, N. Multiplexed single-cell proteomics using SCoPE2. Nat. Protoc. 2021, 16, 5398–5425. [Google Scholar] [CrossRef]
- Mansuri, M.S.; Williams, K.; Nairn, A.C. Uncovering biology by single-cell proteomics. Commun. Biol. 2023, 6, 381. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Jung, S.B.; Chang, J.Y.; Shong, M. Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress. Endocrinol. Metab. 2021, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ai, Y.; Xie, R.; Xiong, J.; Wang, Y.; Liang, Q. Organoids/organs-on-a-chip: New frontiers of intestinal pathophysiological models. Lab Chip 2023, 23, 1192–1212. [Google Scholar] [CrossRef]
- Kip, A.M.; Soons, Z.; Mohren, R.; Duivenvoorden, A.A.M.; Röth, A.A.J.; Cillero-Pastor, B.; Neumann, U.P.; Dejong, C.H.C.; Heeren, R.M.A.; Olde Damink, S.W.M.; et al. Proteomics analysis of human intestinal organoids during hypoxia and reoxygenation as a model to study ischemia-reperfusion injury. Cell Death Dis. 2021, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.; Krause, F.N.; Moran, A.; MacCannell, A.D.V.; Scragg, J.L.; McNally, B.D.; Boateng, E.; Murfitt, S.A.; Virtue, S.; Wright, J.; et al. Brown and beige adipose tissue regulate systemic metabolism through a metabolite interorgan signaling axis. Nat. Commun. 2021, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Lu, V.B.; Bany Bakar, R.; Miedzybrodzka, E.; Davison, A.; Goldspink, D.; Reimann, F.; Gribble, F.M. Single-cell transcriptomics of human organoid-derived enteroendocrine cell populations from the small intestine. J. Physiol. 2024. early review. [Google Scholar] [CrossRef]
- James, K.R.; Elmentaite, R.; Teichmann, S.A.; Hold, G.L. Redefining intestinal immunity with single-cell transcriptomics. Mucosal Immunol. 2022, 15, 531–541. [Google Scholar] [CrossRef]
- Burclaff, J.; Bliton, R.J.; Breau, K.A.; Ok, M.T.; Gomez-Martinez, I.; Ranek, J.S.; Bhatt, A.P.; Purvis, J.E.; Woosley, J.T.; Magness, S.T. A Proximal-to-Distal Survey of Healthy Adult Human Small Intestine and Colon Epithelium by Single-Cell Transcriptomics. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1554–1589. [Google Scholar] [CrossRef]
- Labib, M.; Kelley, S.O. Single-cell analysis targeting the proteome. Nat. Rev. Chem. 2020, 4, 143–158. [Google Scholar] [CrossRef]
- Schoof, E.M.; Furtwängler, B.; Üresin, N.; Rapin, N.; Savickas, S.; Gentil, C.; Lechman, E.; Keller, U.A.D.; Dick, J.E.; Porse, B.T. Quantitative single-cell proteomics as a tool to characterize cellular hierarchies. Nat. Commun. 2021, 12, 3341. [Google Scholar] [CrossRef]
- Petrosius, V.; Aragon-Fernandez, P.; Üresin, N.; Kovacs, G.; Phlairaharn, T.; Furtwängler, B.; Op De Beeck, J.; Skovbakke, S.L.; Goletz, S.; Thomsen, S.F.; et al. Exploration of cell state heterogeneity using single-cell proteomics through sensitivity-tailored data-independent acquisition. Nat. Commun. 2023, 14, 5910. [Google Scholar] [CrossRef]
- Taylor, M.J.; Lukowski, J.K.; Anderton, C.R. Spatially Resolved Mass Spectrometry at the Single Cell: Recent Innovations in Proteomics and Metabolomics. J. Am. Soc. Mass Spectrom. 2021, 32, 872–894. [Google Scholar] [CrossRef]
- Gebreyesus, S.T.; Siyal, A.A.; Kitata, R.B.; Chen, E.S.; Enkhbayar, B.; Angata, T.; Lin, K.I.; Chen, Y.J.; Tu, H.L. Streamlined single-cell proteomics by an integrated microfluidic chip and data-independent acquisition mass spectrometry. Nat. Commun. 2022, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, S.; Bai, Y.; Chen, H.; Liao, G.; Mukherjee, N.; Vazquez, G.; McIlwain, D.R.; Tzankov, A.; Lee, I.T.; et al. Robust single-cell matching and multimodal analysis using shared and distinct features. Nat. Methods 2023, 20, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Kelly, R.T. Single-cell Proteomics: Progress and Prospects. Mol. Cell. Proteomics. 2020, 19, 1739–1748. [Google Scholar] [CrossRef]
- Truong, T.; Kelly, R.T. What’s new in single-cell proteomics. Curr. Opin. Biotechnol. 2024, 86, 103077. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia. Biotechnol. Adv. 2020, 40, 107500. [Google Scholar] [CrossRef]
- Hampton, T. From the Literature. Circulation 2020, 142, 1491–1493. [Google Scholar] [CrossRef]
- Benninghaus, L.; Schwardmann, L.S.; Jilg, T.; Wendisch, V.F. Establishment of synthetic microbial consortia with Corynebacterium glutamicum and Pseudomonas putida: Design, construction, and application to production of γ-glutamylisopropylamide and L-theanine. Microb. Biotechnol. 2024, 17, e14400. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Darlington, A.P.S.; South, E.J.; Chen, H.H.; Jiang, W.; Ledesma-Amaro, R. A molecular toolkit of cross-feeding strains for engineering synthetic yeast communities. Nat. Microbiol. 2024, 9, 848–863. [Google Scholar] [CrossRef]
- Gasparek, M.; Steel, H.; Papachristodoulou, A. Deciphering mechanisms of production of natural compounds using inducer-producer microbial consortia. Biotechnol. Adv. 2023, 64, 108117. [Google Scholar] [CrossRef] [PubMed]
- Alnahhas, R.N.; Sadeghpour, M.; Chen, Y.; Frey, A.A.; Ott, W.; Josić, K.; Bennett, M.R. Majority sensing in synthetic microbial consortia. Nat. Commun. 2020, 11, 3659. [Google Scholar] [CrossRef]
- Bodapati, S.; Daley, T.P.; Lin, X.; Zou, J.; Qi, L.S. A benchmark of algorithms for the analysis of pooled CRISPR screens. Genome Biol. 2020, 21, 62. [Google Scholar] [CrossRef]
- Sun, P.; Wang, M.; Liu, Y.X.; Li, L.; Chai, X.; Zheng, W.; Chen, S.; Zhu, X.; Zhao, S. High-fat diet-disturbed gut microbiota-colonocyte interactions contribute to dysregulating peripheral tryptophan-kynurenine metabolism. Microbiome 2023, 11, 154. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1863134. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef]
- Prukpitikul, P.; Sirivarasai, J.; Sutjarit, N. The molecular mechanisms underlying gut microbiota-miRNA interaction in metabolic disorders. Benef. Microbes 2024, 15, 83–96. [Google Scholar] [CrossRef]
- Huang, Z.; Yao, Q.; Ma, S.; Zhou, J.; Wang, X.; Meng, Q.; Liu, Y.; Yu, Z.; Chen, X. The synergistic role of gut microbiota and RNA in metabolic diseases: Mechanisms and therapeutic insights. Front. Microbiol. 2025, 16, 1504395. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Yang, S.; Lu, J.; Jin, X.; Wu, M. Diet-gut microbiota-epigenetics in metabolic diseases: From mechanisms to therapeutics. Biomed. Pharmacother. 2022, 153, 113290. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Cabatbat, J.J.T.; Martin, T.L.P.; Kim, H.; Kim, S.; Sung, J.; Ghim, C.M.; Kim, P.J. Large-scale metabolic interaction network of the mouse and human gut microbiota. Sci. Data 2020, 7, 204. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Jiang, J.; Chen, Z. Metabolic checkpoints and novel approaches for immunotherapy against cancer. Int. J. Cancer 2022, 150, 195–207. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, Z.; Zhu, Y.; Liang, C.; Chen, Z.; Zhang, J.; Leng, L. Multi-omics analysis reveals GAPDH posttranscriptional regulation of IFN-γ and PHGDH as a metabolic checkpoint of microglia polarization. Brain Behav. Immun. 2024, 117, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: New insights and therapeutic implications. Cell Death Dis. 2023, 14, 586. [Google Scholar] [CrossRef]
- Liu, M.; Hong, L.; Sridhar, S.; Jaynes, P.; Tipgomut, C.; Poon, L.; De Mel, S.; Lee, J.S.X.; Ng, S.-B.; Tan, C.L. Spatial-Resolved Transcriptomics Reveals Immune Landscape Variations in Primary Central Nervous System Lymphoma (PCNSL) and Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2024, 144, 3004. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural Transm. 2024, 131, 1367–1387. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.A.; Gabr, M. Small molecule immunomodulators as next-generation therapeutics for glioblastoma. Cancers 2024, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 484. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Patel, K.; Jackson, C.M. The potential for immune checkpoint modulators in cerebrovascular injury and inflammation. Expert Opin. Ther. Targets 2021, 25, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Fazio, C.D.; Borgomaneri, S.; Avenanti, A. Cortisol Imbalance and Fear Learning in PTSD: Therapeutic Approaches to Control Abnormal Fear Responses. Curr. Neuropharmacol. 2025, 23, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Di Fazio, C.; Mazzà, M.; Tamietto, M.; Avenanti, A. Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. Int. J. Mol. Sci. 2024, 25, 864. [Google Scholar] [CrossRef]
- Tortora, F.; Hadipour, A.L.; Battaglia, S.; Falzone, A.; Avenanti, A.; Vicario, C.M. The Role of Serotonin in Fear Learning and Memory: A Systematic Review of Human Studies. Brain Sci. 2023, 13, 1197. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune influencers in action: Metabolites and enzymes of the tryptophan-kynurenine metabolic pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic tryptophan homeostasis. Front. Mol. Biosci. 2022, 9, 897929. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2–KYN–AhR pathway for cancer immunotherapy—Challenges and opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a ‘reflex’ feature of neuroimmune communication: Sensor and effector functions for the indoleamine-2,3-dioxygenase kynurenine pathway. J. Neurochem. 2024, 168, 3333–3357. [Google Scholar] [CrossRef]
- Labadie, B.W.; Bao, R.; Luke, J.J. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan–kynurenine–aryl hydrocarbon axis. Clin. Cancer Res. 2019, 25, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; von Knebel Doeberitz, N.; Oezen, I.; Wick, W.; Ochs, K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front. Immunol. 2015, 5, 673. [Google Scholar] [CrossRef]
- Triplett, T.A.; Garrison, K.C.; Marshall, N.; Donkor, M.; Blazeck, J.; Lamb, C.; Qerqez, A.; Dekker, J.D.; Tanno, Y.; Lu, W.-C. Reversal of indoleamine 2,3-dioxygenase–mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat. Biotechnol. 2018, 36, 758–764. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 2023, 44, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.L.; Frei, A.L.; Koessler, T.; Berger, M.D.; Dawson, H.; Michielin, O.; Zlobec, I. The current landscape of spatial biomarkers for prediction of response to immune checkpoint inhibition. NPJ Precis. Oncol. 2024, 8, 178. [Google Scholar] [CrossRef]
- Kim, M.; Tomek, P. Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO. Front. Immunol. 2021, 12, 636081. [Google Scholar] [CrossRef]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef]
- Ala, M. The footprint of kynurenine pathway in every cancer: A new target for chemotherapy. Eur. J. Pharmacol. 2021, 896, 173921. [Google Scholar] [CrossRef]
- Zeitler, L.; Murray, P.J. IL4i1 and IDO1: Oxidases that control a tryptophan metabolic nexus in cancer. J. Biol. Chem. 2023, 299, 104827. [Google Scholar] [CrossRef] [PubMed]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Di Fazio, C.; Borgomaneri, S. The role of pre-supplementary motor cortex in action control with emotional stimuli: A repetitive transcranial magnetic stimulation study. Ann. N. Y. Acad. Sci. 2024, 1536, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Fullana, M.A.; di Pellegrino, G.; Borgomaneri, S. ‘Nip it in the bud’: Low-frequency rTMS of the prefrontal cortex disrupts threat memory consolidation in humans. Behav. Res. Ther. 2024, 178, 104548. [Google Scholar] [CrossRef]
- Tanaka, M.; He, Z.; Han, S.; Battaglia, S. Editorial: Noninvasive brain stimulation: A promising approach to study and improve emotion regulation. Front. Behav. Neurosci. 2025, 19, 1633936. [Google Scholar] [CrossRef]
- Krolak, T.; Chan, K.Y.; Kaplan, L.; Huang, Q.; Wu, J.; Zheng, Q.; Kozareva, V.; Beddow, T.; Tobey, I.G.; Pacouret, S.; et al. A High-Efficiency AAV for Endothelial Cell Transduction Throughout the Central Nervous System. Nat. Cardiovasc. Res. 2022, 1, 389–400. [Google Scholar] [CrossRef]
- Gleichman, A.J.; Kawaguchi, R.; Sofroniew, M.V.; Carmichael, S.T. A toolbox of astrocyte-specific, serotype-independent adeno-associated viral vectors using microRNA targeting sequences. Nat. Commun. 2023, 14, 7426. [Google Scholar] [CrossRef]
- Alerasool, N.; Segal, D.; Lee, H.; Taipale, M. An efficient KRAB domain for CRISPRi applications in human cells. Nat. Methods 2020, 17, 1093–1096. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef]
- Chen, X.; Wolfe, D.A.; Bindu, D.S.; Zhang, M.; Taskin, N.; Goertsen, D.; Shay, T.F.; Sullivan, E.E.; Huang, S.F.; Ravindra Kumar, S.; et al. Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates. Nat. Commun. 2023, 14, 3345. [Google Scholar] [CrossRef]
- Bolanos-Palmieri, P.; Kotb, A.; Schenk, H.; Bähre, H.; Schroder, P.; Schiffer, M. MO006 Changes in the Kynurenine Pathway Lead to Alterations in NAD Balance and Bioenergetics Parameters in Glomerular Cells In Vitro and Contribute to Proteinuria in a Zebrafish Model. Nephrol. Dial. Transplant. 2021, 36, gfab079.002. [Google Scholar] [CrossRef]
- Kampmann, M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem. Biol. 2018, 13, 406–416. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in Cognitive Impairment and Mood Disorders for Experimental, Clinical and Translational Neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Späth, M.R.; Hoyer-Allo, K.J.R.; Seufert, L.; Höhne, M.; Lucas, C.; Bock, T.; Isermann, L.; Brodesser, S.; Lackmann, J.W.; Kiefer, K.; et al. Organ Protection by Caloric Restriction Depends on Activation of the De Novo NAD+ Synthesis Pathway. J. Am. Soc. Nephrol. 2023, 34, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Altermatt, M.; Dobreva, T.; Chen, S.; Wang, A.; Thomson, M.; Gradinaru, V. Deep Parallel Characterization of AAV Tropism and AAV-Mediated Transcriptional Changes via Single-Cell RNA Sequencing. Front. Immunol. 2021, 12, 730825. [Google Scholar] [CrossRef]
- Börner, K.; Kienle, E.; Huang, L.Y.; Weinmann, J.; Sacher, A.; Bayer, P.; Stüllein, C.; Fakhiri, J.; Zimmermann, L.; Westhaus, A.; et al. Pre-arrayed Pan-AAV Peptide Display Libraries for Rapid Single-Round Screening. Mol. Ther. 2020, 28, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, K.M.; Cantor, J.R. Uncovering the Conditionally Essential Roles of NAD Kinases in Human Cells. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Walsh, S.; Gardner, L.; Deiters, A.; Williams, G.J. Intracellular light-activation of riboswitch activity. Chembiochem 2014, 15, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Borrachero-Conejo, A.I.; Adams, W.R.; Saracino, E.; Mola, M.G.; Wang, M.; Posati, T.; Formaggio, F.; De Bellis, M.; Frigeri, A.; Caprini, M.; et al. Stimulation of water and calcium dynamics in astrocytes with pulsed infrared light. FASEB J. 2020, 34, 6539–6553. [Google Scholar] [CrossRef]
- Spennato, D.; Leone, J.; Gundhardt, C.; Varnavski, O.; Fabbri, R.; Caprini, M.; Zamboni, R.; Benfenati, V.; Goodson, T., III. Investigations of Astrocyte Calcium Signaling and Imaging with Classical and Nonclassical Light. J. Phys. Chem. B 2024, 128, 7966–7977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rózsa, M.; Liang, Y.; Bushey, D.; Wei, Z.; Zheng, J.; Reep, D.; Broussard, G.J.; Tsang, A.; Tsegaye, G.; et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature 2023, 615, 884–891. [Google Scholar] [CrossRef]
- Qiao, L.; Niu, L.; Wang, M.; Wang, Z.; Kong, D.; Yu, G.; Ye, H. A sensitive red/far-red photoswitch for controllable gene therapy in mouse models of metabolic diseases. Nat. Commun. 2024, 15, 10310. [Google Scholar] [CrossRef]
- Shemetov, A.A.; Monakhov, M.V.; Zhang, Q.; Canton-Josh, J.E.; Kumar, M.; Chen, M.; Matlashov, M.E.; Li, X.; Yang, W.; Nie, L.; et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat. Biotechnol. 2021, 39, 368–377. [Google Scholar] [CrossRef]
- Lohr, C.; Beiersdorfer, A.; Fischer, T.; Hirnet, D.; Rotermund, N.; Sauer, J.; Schulz, K.; Gee, C.E. Using Genetically Encoded Calcium Indicators to Study Astrocyte Physiology: A Field Guide. Front. Cell. Neurosci. 2021, 15, 690147. [Google Scholar] [CrossRef]
- Gorzo, K.A.; Gordon, G.R. Photonics tools begin to clarify astrocyte calcium transients. Neurophotonics 2022, 9, 021907. [Google Scholar] [CrossRef]
- Walton, J.C.; Bumgarner, J.R.; Nelson, R.J. Sex Differences in Circadian Rhythms. Cold Spring Harb. Perspect. Biol. 2022, 14, a039107. [Google Scholar] [CrossRef]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From Pathways to Potential Therapies. Cells 2024, 13, 1098. [Google Scholar] [CrossRef]
- Minbay, M.; Khan, A.; Ghasemi, A.R.; Ingram, K.K.; Ay, A.A. Sex-specific associations between circadian-related genes and depression in UK Biobank participants highlight links to glucose metabolism, inflammation and neuroplasticity pathways. Psychiatry Res. 2024, 337, 115948. [Google Scholar] [CrossRef]
- Abo, S.M.; Layton, A.T. Modeling the circadian regulation of the immune system: Sexually dimorphic effects of shift work. PLoS Comput. Biol. 2021, 17, e1008514. [Google Scholar] [CrossRef]
- Logan, R.W.; Xue, X.; Ketchesin, K.D.; Hoffman, G.; Roussos, P.; Tseng, G.; McClung, C.A.; Seney, M.L. Sex Differences in Molecular Rhythms in the Human Cortex. Biol. Psychiatry 2022, 91, 152–162. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Christofides, K.; Weissleder, C.; Huang, X.F.; Shannon Weickert, C.; Lim, C.K.; Newell, K.A. Sex- and suicide-specific alterations in the kynurenine pathway in the anterior cingulate cortex in major depression. Neuropsychopharmacology 2024, 49, 584–592. [Google Scholar] [CrossRef]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024, 164, 105791. [Google Scholar] [CrossRef]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 2014, 35, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv. Clin. Exp. Med. 2024, 33, 1295–1301. [Google Scholar] [CrossRef]
- Wu, F.; Langer, P.; Shim, J.; Fleisch, E.; Barata, F. Comparative Efficacy of Commercial Wearables for Circadian Rhythm Home Monitoring from Activity, Heart Rate, and Core Body Temperature. IEEE J. Biomed. Health Inform. 2025, 29, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Lightman, S.L.; Conway-Campbell, B.L. Circadian and ultradian rhythms: Clinical implications. J. Intern. Med. 2024, 296, 121–138. [Google Scholar] [CrossRef]
- Steele, T.A.; St Louis, E.K.; Videnovic, A.; Auger, R.R. Circadian Rhythm Sleep-Wake Disorders: A Contemporary Review of Neurobiology, Treatment, and Dysregulation in Neurodegenerative Disease. Neurotherapeutics 2021, 18, 53–74. [Google Scholar] [CrossRef]
- Walker, W.H., II; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian rhythm disruption and mental health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Takada, K.; Shimokawa, M.; Mizuki, F.; Takamori, S.; Takenaka, T.; Miura, N.; Shikada, Y.; Yoshizumi, T. Association between sex and outcomes in patients with non-small-cell lung cancer receiving combination chemoimmunotherapy as a first-line therapy: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Med. Res. 2022, 27, 157. [Google Scholar] [CrossRef] [PubMed]
- Karaboué, A.; Innominato, P.F.; Wreglesworth, N.I.; Duchemann, B.; Adam, R.; Lévi, F.A. Why does circadian timing of administration matter for immune checkpoint inhibitors’ efficacy? Br. J. Cancer 2024, 131, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S.; Koyanagi, S.; Matsunaga, N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacol. Ther. 2019, 202, 72–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zeng, Q.; Gül, Z.M.; Wang, S.; Pick, R.; Cheng, P.; Bill, R.; Wu, Y.; Naulaerts, S.; Barnoud, C.; et al. Circadian tumor infiltration and function of CD8+ T cells dictate immunotherapy efficacy. Cell 2024, 187, 2690–2702.e17. [Google Scholar] [CrossRef]
- Ye, Y.; Xiang, Y.; Ozguc, F.M.; Kim, Y.; Liu, C.J.; Park, P.K.; Hu, Q.; Diao, L.; Lou, Y.; Lin, C.; et al. The Genomic Landscape and Pharmacogenomic Interactions of Clock Genes in Cancer Chronotherapy. Cell Syst. 2018, 6, 314–328.e2. [Google Scholar] [CrossRef]
- Ephraim, A.; Leatheng, C.; Lu, Z.E.; Xia, X.; Pirruccello, J.P.; Marotti, J.D.; MacKenzie, T.; Chamberlin, M.D. Association of plasma kynurenine (KYN) with plasma osteopontin (OPN) in patients with locally invasive breast cancer. J. Clin. Oncol. 2024, 42, e12551. [Google Scholar] [CrossRef]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Chantrapanichkul, P.; Stevenson, M.O.; Suppakitjanusant, P.; Goodman, M.; Tangpricha, V. Serum Hormone Concentrations in Transgender Individuals Receiving Gender-Affirming Hormone Therapy: A Longitudinal Retrospective Cohort Study. Endocr. Pr. 2021, 27, 27–33. [Google Scholar] [CrossRef]
- Kervezee, L.; Cermakian, N.; Boivin, D.B. Individual metabolomic signatures of circadian misalignment during simulated night shifts in humans. PLoS Biol. 2019, 17, e3000303. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Skornyakov, E.; Chowdhury, N.R.; Gajula, R.P.; Middleton, B.; Satterfield, B.C.; Porter, K.I.; Van Dongen, H.P.A.; Gaddameedhi, S. Separation of circadian- and behavior-driven metabolite rhythms in humans provides a window on peripheral oscillators and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 7825–7830. [Google Scholar] [CrossRef]
- Whittaker, D.S.; Akhmetova, L.; Carlin, D.; Romero, H.; Welsh, D.K.; Colwell, C.S.; Desplats, P. Circadian modulation by time-restricted feeding rescues brain pathology and improves memory in mouse models of Alzheimer’s disease. Cell Metab. 2023, 35, 1704–1721.e6. [Google Scholar] [CrossRef]
- Cheng, P.; Walch, O.; Huang, Y.; Mayer, C.; Sagong, C.; Cuamatzi Castelan, A.; Burgess, H.J.; Roth, T.; Forger, D.B.; Drake, C.L. Predicting circadian misalignment with wearable technology: Validation of wrist-worn actigraphy and photometry in night shift workers. Sleep 2021, 44, zsaa180. [Google Scholar] [CrossRef]
- Emens, J.S.; Burgess, H.J. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med. Clin. 2015, 10, 435–453. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the Circadian System in Shift Work and Its Health Impact. J. Biol. Rhythms. 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, R.; Chen, X.; Jiang, L.; Wang, F. Optimizing dose-schedule regimens with Bayesian adaptive designs: Opportunities and challenges. Front. Pharmacol. 2023, 14, 1261312. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, Y.; Liu, S. A Bayesian pharmacokinetics integrated phase I-II design to optimize dose-schedule regimes. Biostatistics 2024, 26, kxae034. [Google Scholar] [CrossRef]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Lévi, F.A. Systems Chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef]
- Dong, D.; Yang, D.; Lin, L.; Wang, S.; Wu, B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem. Pharmacol. 2020, 178, 114045. [Google Scholar] [CrossRef]
- Jiang, L.; Li, R.; Yan, F.; Yap, T.A.; Yuan, Y. Shotgun: A Bayesian seamless phase I-II design to accelerate the development of targeted therapies and immunotherapy. Contemp. Clin. Trials 2021, 104, 106338. [Google Scholar] [CrossRef]
- Wei, N.; Diekman, C.O. Dosing Time of Day Impacts the Safety of Antiarrhythmic Drugs in a Computational Model of Cardiac Electrophysiology. J. Biol. Rhythm. 2025, 40, 301–310. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Z.; Duraj-Thatte, A.M.; Ebert, M.P.; Zhang, F.; Burgermeister, E.; Liu, X.; Scott, B.M.; Li, G.; Zuo, T. Engineering the gut microbiome. Nat. Rev. Bioeng. 2023, 1, 665–679. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Mousa, W.K.; Al Ali, A. The Gut Microbiome Advances Precision Medicine and Diagnostics for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2024, 25, 11259. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Orešič, M.; Bertram, H.C. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods 2018, 149, 3–12. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Chen, T.; Wang, X.; Zhang, X. Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice. Mol. Neurobiol. 2024, 61, 821–834. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Yamamura, R.; Okubo, R.; Katsumata, N.; Odamaki, T.; Hashimoto, N.; Kusumi, I.; Xiao, J.; Matsuoka, Y.J. Lipid and Energy Metabolism of the Gut Microbiota Is Associated with the Response to Probiotic Bifidobacterium breve Strain for Anxiety and Depressive Symptoms in Schizophrenia. J. Pers. Med. 2021, 11, 987. [Google Scholar] [CrossRef]
- Li, C.; Su, Z.; Chen, Z.; Cao, J.; Liu, X.; Xu, F. Lactobacillus reuteri strain 8008 attenuated the aggravation of depressive-like behavior induced by CUMS in high-fat diet-fed mice through regulating the gut microbiota. Front. Pharmacol. 2023, 14, 1149185. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Wu, P.; Yang, S.; Xue, W.; Xu, B.; Zhang, S.; Tang, B.; Xu, D. Akkermansia muciniphila: A promising probiotic against inflammation and metabolic disorders. Virulence 2024, 15, 2375555. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhao, J.; Li, L.; Wang, Y.; Chen, F.; Li, Y.; Cheng, R.; He, F.; Ze, X.; et al. Effects of Bifidobacterium breve 207-1 on regulating lifestyle behaviors and mental wellness in healthy adults based on the microbiome-gut-brain axis: A randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2024, 63, 2567–2585. [Google Scholar] [CrossRef]
- Liu, J.Y.; Lin, T.L.; Chiu, C.Y.; Hsieh, P.F.; Lin, Y.T.; Lai, L.Y.; Wang, J.T. Decolonization of carbapenem-resistant Klebsiella pneumoniae from the intestinal microbiota of model mice by phages targeting two surface structures. Front. Microbiol. 2022, 13, 877074. [Google Scholar] [CrossRef] [PubMed]
- Medlock, G.; Felix, C.; Alsharif, W.; Cornacchione, L.; Schinn, M.; Watson, A.; Bedard-Shurtleff, S.; Norman, J.; Faith, J.; Kuijper, E.J. 2521. VE707, a defined live biotherapeutic product for prevention of infection by multidrug-resistant gram-negative bacteria. Open Forum Infect. Dis. 2023, 10, ofad500.2139. [Google Scholar] [CrossRef]
- Tavoukjian, V. Faecal microbiota transplantation for the decolonization of antibiotic-resistant bacteria in the gut: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 102, 174–188. [Google Scholar] [CrossRef]
- Macareño-Castro, J.; Solano-Salazar, A.; Dong, L.T.; Mohiuddin, M.; Espinoza, J.L. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. J. Infect. 2022, 84, 749–759. [Google Scholar] [CrossRef]
- Mortzfeld, B.M.; Palmer, J.D.; Bhattarai, S.K.; Dupre, H.L.; Mercado-Lubio, R.; Silby, M.W.; Bang, C.; McCormick, B.A.; Bucci, V. Microcin MccI47 selectively inhibits enteric bacteria and reduces carbapenem-resistant Klebsiella pneumoniae colonization in vivo when administered via an engineered live biotherapeutic. Gut Microbes 2022, 14, 2127633. [Google Scholar] [CrossRef] [PubMed]
- Osbelt, L.; Wende, M.; Almási, É.; Derksen, E.; Muthukumarasamy, U.; Lesker, T.R.; Galvez, E.J.C.; Pils, M.C.; Schalk, E.; Chhatwal, P.; et al. Klebsiella oxytoca causes colonization resistance against multidrug-resistant K. pneumoniae in the gut via cooperative carbohydrate competition. Cell Host Microbe 2021, 29, 1663–1679. [Google Scholar] [CrossRef]
- Heavey, M.K.; Durmusoglu, D.; Crook, N.; Anselmo, A.C. Discovery and delivery strategies for engineered live biotherapeutic products. Trends Biotechnol. 2022, 40, 354–369. [Google Scholar] [CrossRef]
- Nguyen, N.; Wang, M.; Li, L.; Chan, C.T. A genetic safeguard for eliminating target genes in synthetic probiotics in response to a loss of the permissive signal in a gut environment. bioRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Hartig, A.M.; Dai, W.; Zhang, K.; Kapoor, K.; Rottinghaus, A.G.; Moon, T.S.; Parker, K.M. Influence of Environmental Conditions on the Escape Rates of Biocontained Genetically Engineered Microbes. Environ. Sci. Technol. 2024, 58, 22657–22667. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Jasinskytė, D.; Robert, C.; Semsey, S.; Martínez, V.; Petersen, A.; Brunner, K.; de Santiago Torio, A.; Salazar, A.; Turcu, I.C.; et al. Engineered phage with antibacterial CRISPR-Cas selectively reduce E. coli burden in mice. Nat. Biotechnol. 2024, 42, 265–274. [Google Scholar] [CrossRef]
- Agarwal, S.; Tiwari, P.; Deep, A.; Kidwai, S.; Gupta, S.; Thakur, K.G.; Singh, R. System-Wide Analysis Unravels the Differential Regulation and In Vivo Essentiality of Virulence-Associated Proteins B and C Toxin-Antitoxin Systems of Mycobacterium tuberculosis. J. Infect. Dis. 2018, 217, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Kussell, E. Inferring bacterial recombination rates from large-scale sequencing datasets. Nat. Methods 2019, 16, 199–204. [Google Scholar] [CrossRef]
- Martin, G.; Kolida, S.; Marchesi, J.R.; Want, E.; Sidaway, J.E.; Swann, J.R. In Vitro Modeling of Bile Acid Processing by the Human Fecal Microbiota. Front. Microbiol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, A.; Liu, C.; Cao, X.; Wang, R.; Shu, X.; Wang, L.; Zhang, Y.; Xiang, H.; Li, M. The toxin-antitoxin RNA guards of CRISPR-Cas evolved high specificity through repeat degeneration. Nucleic Acids Res. 2022, 50, 9442–9452. [Google Scholar] [CrossRef]
- Wiechert, J.; Gätgens, C.; Wirtz, A.; Frunzke, J. Inducible Expression Systems Based on Xenogeneic Silencing and Counter-Silencing and Design of a Metabolic Toggle Switch. ACS Synth. Biol. 2020, 9, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Gildemeister, D.; Hein, A.; Schröder, P.; Bachmann, J. Environmental fate and effects assessment of human pharmaceuticals: Lessons learnt from regulatory data. Environ. Sci. Eur. 2021, 33, 68. [Google Scholar] [CrossRef]
- Richard, E.; Darracq, B.; Littner, E.; Vit, C.; Whiteway, C.; Bos, J.; Fournes, F.; Garriss, G.; Conte, V.; Lapaillerie, D.; et al. Cassette recombination dynamics within chromosomal integrons are regulated by toxin-antitoxin systems. Sci. Adv. 2024, 10, eadj3498. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J. Colloid Interface Sci. 2019, 539, 497–503. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Yang, A.; Wang, J.; Xie, M.; Zhang, X.; Huang, D.; Xiong, R.; Pei, D.; Huang, C. Responsive and biocompatible chitosan-phytate microparticles with various morphology for antibacterial activity based on gas-shearing microfluidics. J. Colloid Interface Sci. 2023, 649, 68–75. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Zhou, P.; Luo, Z.; Li, X.; Gao, L. Development of the pH responsive chitosan-alginate based microgel for encapsulation of Jughans regia L. polyphenols under simulated gastrointestinal digestion in vitro. Carbohydr. Polym. 2020, 250, 116917. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Dai, S.; Lian, Z.; Tong, X.; Yang, S.; Chen, Y.; Qi, W.; Peng, X.; Wang, H.; Jiang, L. The Layered Encapsulation of Vitamin B2 and β-Carotene in Multilayer Alginate/Chitosan Gel Microspheres: Improving the Bioaccessibility of Vitamin B2 and β-Carotene. Foods 2021, 11, 20. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Huang, F.; Meng, Y.; Chen, Y.; Wang, J.; Wang, S.; Luo, Y.; Li, J.; Liang, Y. pH-sensitive chitosan/sodium alginate/calcium chloride hydrogel beads for potential oral delivery of rice bran bioactive peptides. Food Chem. 2025, 470, 142618. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ran, H.; Wen, X.; Fu, G.; Zhou, X.; Liu, R.; Pan, T. Probiotics improve symptoms of patients with COVID-19 through gut-lung axis: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1179432. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.A.A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.A.; Vinderola, G.; Gomes Cruz, A.; Sivieri, K.; Costa Antunes, A.E. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci. Technol. 2022, 120, 174–192. [Google Scholar] [CrossRef]
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 2023, 14, 1296447. [Google Scholar] [CrossRef]
- Schaub, A.C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: A randomized controlled trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Horvath, A.; Habisch, H.; Prietl, B.; Pfeifer, V.; Balazs, I.; Kovacs, G.; Foris, V.; John, N.; Kleinschek, D.; Feldbacher, N.; et al. Alteration of the Gut-Lung Axis After Severe COVID-19 Infection and Modulation Through Probiotics: A Randomized, Controlled Pilot Study. Nutrients 2024, 16, 3840. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-analysis of randomized controlled trials of the effects of probiotics on functional constipation in adults. Clin. Nutr. 2020, 39, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, C.S.; Liu, Y.; Zhang, X. Effective Regulation of Gut Microbiota With Probiotics and Prebiotics May Prevent or Alleviate COVID-19 Through the Gut-Lung Axis. Front. Pharmacol. 2022, 13, 895193. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Liang, H.; Li, T.; Fang, X.; Xing, Z.; Zhang, S.; Shi, L.; Li, W.; Guo, L.; Kuang, C.; Liu, H.; et al. IDO1/TDO dual inhibitor RY103 targets KYN-AhR pathway and exhibits preclinical efficacy on pancreatic cancer. Cancer Lett. 2021, 522, 32–43. [Google Scholar] [CrossRef]
- Fan, Q.Z.; Zhou, J.; Zhu, Y.B.; He, L.J.; Miao, D.D.; Zhang, S.P.; Liu, X.P.; Zhang, C. Design, synthesis, and biological evaluation of a novel indoleamine 2,3-dioxigenase 1 (IDO1) and thioredoxin reductase (TrxR) dual inhibitor. Bioorganic Chem. 2020, 105, 104401. [Google Scholar] [CrossRef]
- Xing, Z.; Li, X.; He, Z.N.T.; Fang, X.; Liang, H.; Kuang, C.; Li, A.; Yang, Q. IDO1 Inhibitor RY103 Suppresses Trp-GCN2-Mediated Angiogenesis and Counters Immunosuppression in Glioblastoma. Pharmaceutics 2024, 16, 870. [Google Scholar] [CrossRef] [PubMed]
- Lotz-Jenne, C.; Cren, S.; Joesch, C.; Ackerknecht, S.; Brandes, J.; Moebs, C.; Hartl, D.; Hartrampf, F.; Guerry, P.; Pothier, J. Superiority of dual IDO1/TDO2 inhibition versus IDO1 selective inhibition in reducing immunosuppressive KYN levels in tumors co-expressing IDO1 and TDO2. Cancer Res. 2023, 83, 1849. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Spector, S.; Wu, C.; Nguyen, D.; Theodore, G.; Garcia, A.; Feun, L.; Savaraj, N.; Wangpaichitr, M. Targeting kynurenine pathway using novel IDO/TDO dual inhibitor (AT0174) to modulate tumor microenvironment in platinum resistant non-small cell lung cancer cancer: An immunometabolism compliment markers. Cancer Res. 2022, 82, 2325. [Google Scholar] [CrossRef]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The Impact of C-3 Side Chain Modifications on Kynurenic Acid: A Behavioral Analysis of Its Analogs in the Motor Domain. Int. J. Mol. Sci. 2024, 25, 3394. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson’s Disease Therapeutics. Cells 2025, 14, 973. [Google Scholar] [CrossRef]
- He, X.; He, G.; Chu, Z.; Wu, H.; Wang, J.; Ge, Y.; Shen, H.; Zhang, S.; Shan, J.; Peng, K.; et al. Discovery of the First Potent IDO1/IDO2 Dual Inhibitors: A Promising Strategy for Cancer Immunotherapy. J. Med. Chem. 2021, 64, 17950–17968. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, F.; Steinhauser, M.; Maier, M.E.; Thayer, J.F.; Battaglia, S. Error-related cardiac deceleration: Functional interplay between error-related brain activity and autonomic nervous system in performance monitoring. Neurosci. Biobehav. Rev. 2024, 157, 105542. [Google Scholar] [CrossRef]
- Nazzi, C.; Avenanti, A.; Battaglia, S. The Involvement of Antioxidants in Cognitive Decline and Neurodegeneration: Mens Sana in Corpore Sano. Antioxidants 2024, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Nazzi, C.; Lonsdorf, T.B.; Thayer, J.F. Neuropsychobiology of fear-induced bradycardia in humans: Progress and pitfalls. Mol. Psychiatry 2024, 29, 3826–3840. [Google Scholar] [CrossRef]
- Heyes, M.P. Metabolism and neuropathologic significance of quinolinic acid and kynurenic acid. Biochem. Soc. Trans. 1993, 21, 83–89. [Google Scholar] [CrossRef]
- Damerell, V.; Klaassen-Dekker, N.; Brezina, S.; Ose, J.; Ulvik, A.; van Roekel, E.H.; Holowatyj, A.N.; Baierl, A.; Böhm, J.; Bours, M.J.L.; et al. Circulating tryptophan-kynurenine pathway metabolites are associated with all-cause mortality among patients with stage I-III colorectal cancer. Int. J. Cancer 2025, 156, 552–565. [Google Scholar] [CrossRef]
- Chiu, L.C.; Tang, H.Y.; Fan, C.M.; Lo, C.J.; Hu, H.C.; Kao, K.C.; Cheng, M.L. Kynurenine Pathway of Tryptophan Metabolism Is Associated with Hospital Mortality in Patients with Acute Respiratory Distress Syndrome: A Prospective Cohort Study. Antioxidants 2022, 11, 1884. [Google Scholar] [CrossRef]
- Hoong, C.W.S.; Chua, M.W.J. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology 2021, 162, bqab079. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Euglycemic Ketoacidosis as a Complication of SGLT2 Inhibitor Therapy. Clin. J. Am. Soc. Nephrol. 2021, 16, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Rubin, E.H. Adaptive phase 2/3 designs for oncology drug development—Time to hedge. Contemp. Clin. Trials 2023, 125, 107047. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Merchant, M.; Orr, C.; Chan, J.; Den Otter, D.; Berry, L.; Kasman, I.; Koeppen, H.; Rice, K.; Yang, N.Y.; et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012, 72, 210–219. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Xu, Z.; Yang, B.; He, Q.; Luo, P.; Yan, H.; Yang, X. Development and safety of PI3K inhibitors in cancer. Arch. Toxicol. 2023, 97, 635–650. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, C.; Lv, R.; Qin, Q.; Liu, N.; Yin, W.; Wang, R.; Sun, Y.; Wang, X.; Sun, Y.; et al. Design, synthesis, biological evaluation and pharmacophore model analysis of novel tetrahydropyrrolo[3,4-c]pyrazol derivatives as potential TRKs inhibitors. Eur. J. Med. Chem. 2021, 223, 113627. [Google Scholar] [CrossRef]
- Yan, W.; Lakkaniga, N.R.; Carlomagno, F.; Santoro, M.; McDonald, N.Q.; Lv, F.; Gunaganti, N.; Frett, B.; Li, H.Y. Insights into Current Tropomyosin Receptor Kinase (TRK) Inhibitors: Development and Clinical Application. J. Med. Chem. 2019, 62, 1731–1760. [Google Scholar] [CrossRef]
- Altzerinakou, M.A.; Paoletti, X. An adaptive design for the identification of the optimal dose using joint modeling of continuous repeated biomarker measurements and time-to-toxicity in phase I/II clinical trials in oncology. Stat. Methods Med. Res. 2020, 29, 508–521. [Google Scholar] [CrossRef]
- Pinsker, J.E.; Dassau, E.; Deshpande, S.; Raghinaru, D.; Buckingham, B.A.; Kudva, Y.C.; Laffel, L.M.; Levy, C.J.; Church, M.M.; Desrochers, H.; et al. Outpatient Randomized Crossover Comparison of Zone Model Predictive Control Automated Insulin Delivery with Weekly Data Driven Adaptation Versus Sensor-Augmented Pump: Results from the International Diabetes Closed-Loop Trial 4. Diabetes Technol. Ther. 2022, 24, 635–642. [Google Scholar] [CrossRef]
- Iasonos, A.; O’Quigley, J. Adaptive dose-finding studies: A review of model-guided phase I clinical trials. J. Clin. Oncol. 2014, 32, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.M.; Charleer, S.; Fieuws, S.; De Block, C.; Hilbrands, R.; Van Huffel, L.; Maes, T.; Vanhaverbeke, G.; Dirinck, E.; Myngheer, N.; et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): A 6-month, prospective, multicentre, randomised controlled trial. Lancet 2021, 397, 2275–2283. [Google Scholar] [CrossRef]
- Wang, W.; Huang, C.; Sun, L.; Wu, X.; Xu, L.; Xiao, P. A rapid and reliable targeted LC-MS/MS method for quantitative analysis of the Tryptophan-NAD metabolic network disturbances in tissues and blood of sleep deprivation mice. Anal. Chim. Acta 2024, 1328, 343125. [Google Scholar]
- Marszalek-Grabska, M.; Walczak, K.; Gawel, K.; Wicha-Komsta, K.; Wnorowska, S.; Wnorowski, A.; Turski, W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol. Ther. 2021, 225, 107845. [Google Scholar] [CrossRef]
- Fiore, A.; Zeitler, L.; Russier, M.; Groß, A.; Hiller, M.K.; Parker, J.L.; Stier, L.; Köcher, T.; Newstead, S.; Murray, P.J. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol. Cell 2022, 82, 920–932.e7. [Google Scholar] [CrossRef]
- Salminen, A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res. Rev. 2022, 75, 101573. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.W.D.; Abdulai, R.; Mogemark, M.; Petersen, J.; Thomas, M.J.; Valastro, B.; Westin Eriksson, A. Evolution of PI3Kγ and δ Inhibitors for Inflammatory and Autoimmune Diseases. J. Med. Chem. 2019, 62, 4783–4814. [Google Scholar] [CrossRef] [PubMed]
- Bornaei, M.; Khajehsharifi, H.; Shahrokhian, S.; Sheydaei, O.; Zarnegarian, A. Differential pulse voltammetric quantitation of kynurenic acid in human plasma using carbon-paste electrode modified with metal-organic frameworks. Mater. Chem. Phys. 2023, 295, 127016. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Dwyer, P.J.; Clark, J.; Shi, J.G.; Bowman, K.J.; Scherle, P.A.; Newton, R.C.; Schaub, R.; Maleski, J.; Leopold, L.; et al. First-in-Human Phase I Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2017, 23, 3269–3276. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Wu, D.; Wang, X. Temperature and pH dual-stimuli-responsive phase-change microcapsules for multipurpose applications in smart drug delivery. J. Colloid Interface Sci. 2021, 583, 470–486. [Google Scholar] [CrossRef]
- Lin, R.; Zhou, Y.; Yan, F.; Li, D.; Yuan, Y. BOIN12: Bayesian Optimal Interval Phase I/II Trial Design for Utility-Based Dose Finding in Immunotherapy and Targeted Therapies. JCO Precis. Oncol. 2020, 4, 1393–1402. [Google Scholar] [CrossRef]
- Liu, D.; Xin, Z.; Guo, S.; Li, S.; Cheng, J.; Jiang, H. Blood and Salivary MicroRNAs for Diagnosis of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2021, 79, 1082.e1–1082.e13. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Hare, S.M.; Adhikari, B.M.; Mo, C.; Chen, S.; Wijtenburg, S.A.; Seneviratne, C.; Kane-Gerard, S.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Schwarcz, R.; et al. Tryptophan challenge in individuals with schizophrenia and healthy controls: Acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow. Neuropsychopharmacology 2023, 48, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tong, J.; Zhang, P.; Zhou, Y.; Li, Y.; Tan, S.; Wang, Z.; Yang, F.; Kochunov, P.; Chiappelli, J.; et al. Elevated salivary kynurenic acid levels related to enlarged choroid plexus and severity of clinical phenotypes in treatment-resistant schizophrenia. Brain Behav. Immun. 2022, 106, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Boßlau, T.K.; Wasserfurth, P.; Reichel, T.; Weyh, C.; Palmowski, J.; Nebl, J.; Joisten, N.; Belen, S.; Schenk, A.; Hahn, A.; et al. 12-week combined strength and endurance exercise attenuates CD8+ T-cell differentiation and affects the kynurenine pathway in the elderly: A randomized controlled trial. Immun. Ageing 2023, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Wnorowska, S.; Kurzepa, J.; Parada-Turska, J. Alterations in Kynurenine and NAD+ Salvage Pathways during the Successful Treatment of Inflammatory Bowel Disease Suggest HCAR3 and NNMT as Potential Drug Targets. Int. J. Mol. Sci. 2021, 22, 13497. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef]
- Jamshed, L.; Debnath, A.; Jamshed, S.; Wish, J.V.; Raine, J.C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. Int. J. Mol. Sci. 2022, 23, 6300. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Anesi, A.; Dexpert, S.; Aubert, A.; Beau, C.; Forestier, D.; Ledaguenel, P.; Magne, E.; Mattivi, F.; et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated with Systemic Inflammation. Front. Immunol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Cheng, C.K.; Ye, L.; Wang, Y.; Wang, Y.L.; Xia, Y.; Wong, S.H.; Chen, S.; Huang, Y. Exercised gut microbiota improves vascular and metabolic abnormalities in sedentary diabetic mice through gut-vascular connection. J. Sport Health Sci. 2025, 14, 101026. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14, 1161. [Google Scholar] [CrossRef] [PubMed]
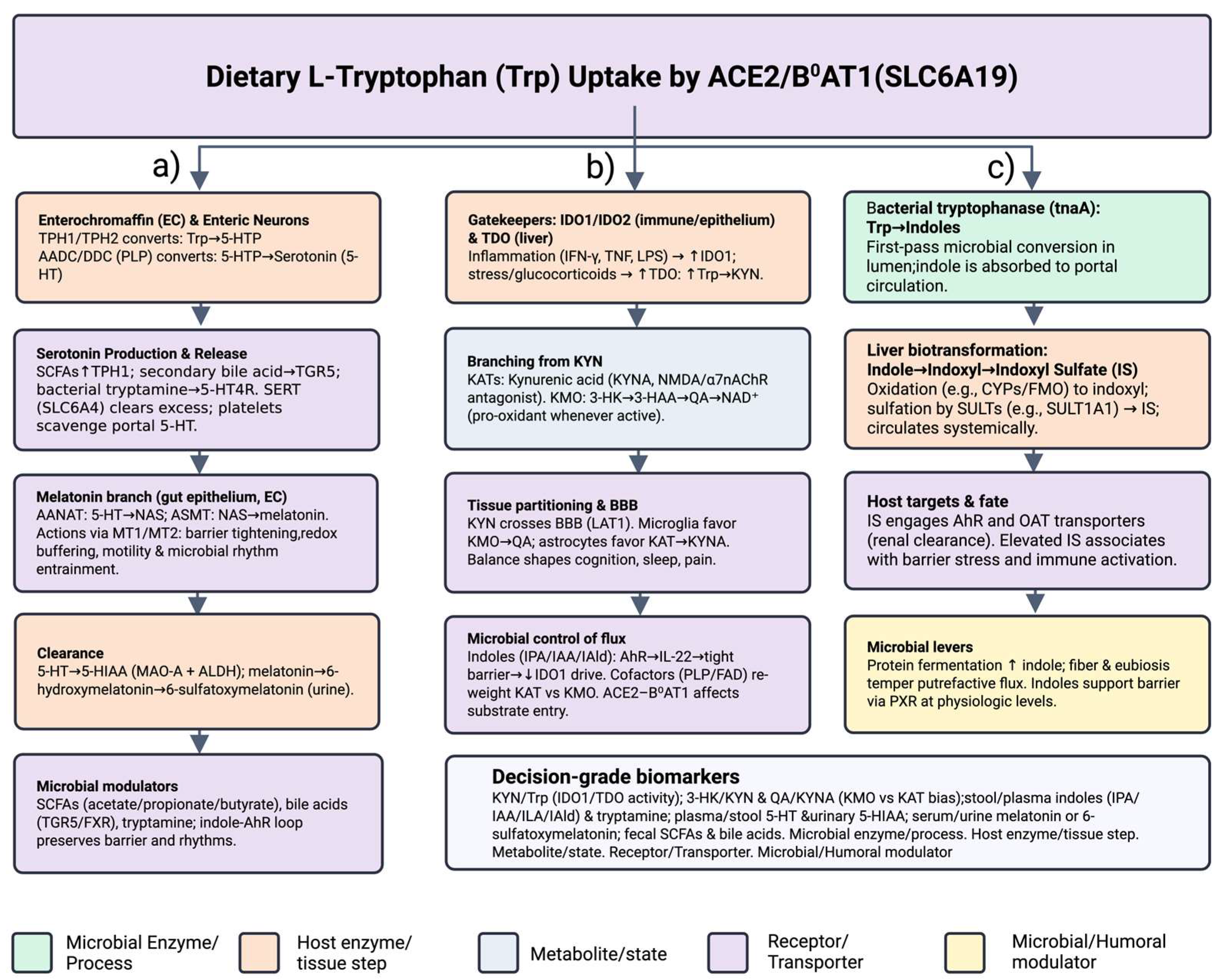
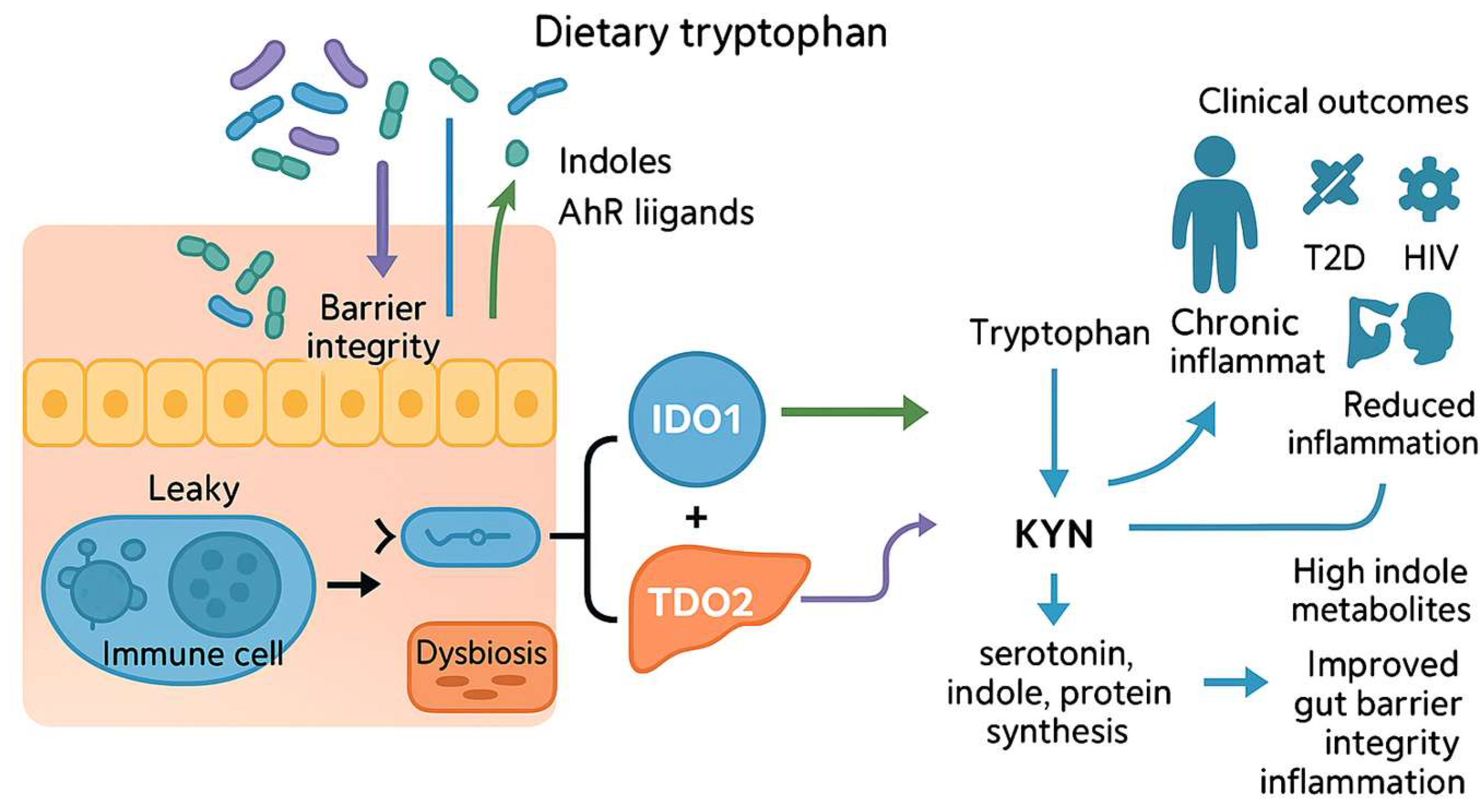
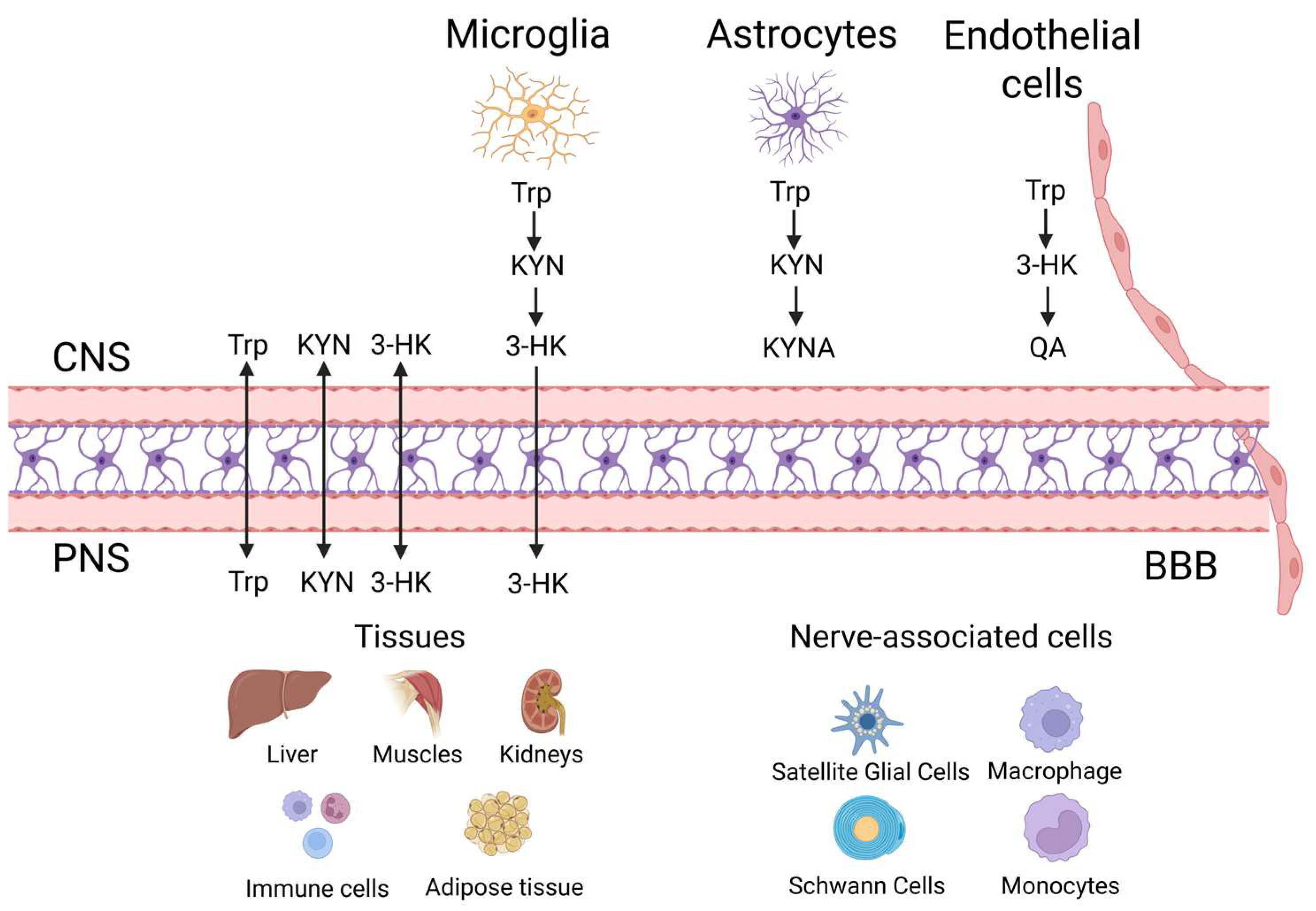



| Category | Description/Core Issue | Implication/Goal | |
|---|---|---|---|
| Translational Challenge | |||
| 1. | Causal Mapping | Thousands of disease associations but no causal framework linking microbiota, host enzymes (IDO1, TDO) and downstream metabolites (KYNA, QA) to physiology | Limits precision design of probiotics, enzyme inhibitors, lifestyle prescriptions |
| 2. | Spatial Resolution | Bulk assays mask cell- and tissue-specific “checkpoints” (astrocytes, microglia, BBB endothelium) | Demands targeted modulation of localized hotspots rather than pathway-wide blockade |
| 3. | Temporal Dynamics | Trp–KYN flux oscillates with circadian rhythms and sex hormones; chronotherapeutic windows under-studied | Missing optimal timing may blunt efficacy or raise toxicity of interventions |
| Key Objective | |||
| 1. | Map Spatial Checkpoints | Chart localized KYN metabolism niches in brain and periphery | Inform cell-type-specific therapeutic targeting |
| 2. | Characterize Sex and Circadian Modifiers | Define how hormones and clocks tilt Trp metabolism toward neurotoxicity or resilience | Enable time- and sex-specific dosing strategies |
| 3. | Develop Microbiota-Based Precision Switches | Engineer probiotic consortia and post-biotics that reroute Trp flux | Provide modular, patient-tailored metabolic control |
| 4. | Outline Integrated “Intervention 2.0” Platform | Combine dual-enzyme inhibitors, exercise, and AI-driven biosensing | Create closed-loop, adaptive therapeutics |
| Experimental Strategy | Mechanistic Lever/Tools | Mechanistic Lever/Tools | Expected Outcome/ Advantage |
|---|---|---|---|
| Endothelial CRISPRi “zip-code” AAV-targeted knock-down of KMO or KYNU in perivascular endothelium |
|
| Precise, vessel-restricted suppression of 3-HK/QA; dampened excitotoxicity and immune escape with minimal systemic off-target effects |
| Light-addressable riboswitch control in astrocytes in milliseconds, reversible tuning of KMO/KYNU translation |
|
| Real-time, non-invasive “dimmer switch” for KP activity with built-in metabolic read-outs; ideal for dissecting causal links between KYN flux and neural circuitry |
| Circadian/Sex-Specific Gap | Why It Matters | Biomarker-Guided Next Step | Anticipated Pay-Off |
|---|---|---|---|
| Absence of chronopharmacology trials for IDO1/TDO, KMO or KAT inhibitors | Optimal dosing windows are unknown; schedules may blunt efficacy or raise toxicity | Launch Bayesian adaptive trials that co-randomize dose and clock time, using real-time KYN/QA read-outs as decision boundaries | Evidence-based chrono-dosing algorithms, reduced off-target effects |
| Inadequate stratification by circadian phase and sex | Female-specific PK/PD and toxicity signals vanish when averaged | Embed wearable-derived chronotype + hormonal phase into inclusion criteria; pre-specify sex-by-time interaction models | Sex-aware precision medicine; higher treatment tolerability |
| Undefined mechanistic links between clock genes, hormones and KYN enzyme activity | Surrogate biomarkers risk misinterpretation without pathway context | Overlay 24 h cortisol/melatonin rhythms onto multi-time-point KYN, QA, KYNA panels; apply mixed-effects chronobiology models | Mechanistic targets for combination therapy; validated biomarkers |
| Wearable metrics (light, sleep) not integrated into study design | Zeitgebers that modulate KYN flux are ignored | Trigger capillary micro-sampling when lux-derived phase-angle deviation crosses threshold (“biomarker-in-the-loop”) | Personalized sampling and dosing windows; lower noise in endpoints |
| Sex- and light-cycle biases in pre-clinical models | Male-only, fixed-light studies limit translation | Use sex-balanced rodents under rotating light cycles; validate with humanized microbiome models | Higher translational validity of pre-clinical findings |
| Lack of validated rapid biomarkers to couple KYN swings to outcomes | Real-time dose adjustment impossible | Develop saliva/finger-stick electrochemical strips for KYN/Trp/QA; calibrate against plasma and microdialysate | Closed-loop dose titration; faster early-phase trials |
| CM Focus: QA spikes during night-shift work | Neurotoxic burden may rise, especially in vulnerable chron otypes | Pilot cross-over study: shift workers + hourly capillary sampling + light and activity trackers Model QA vs. lux-derived phase angle (mixed-effects) Overlay cortisol and melatonin to disentangle stress vs. circadian drivers Test timed blue-light blockers, melatonin, or time-restricted feeding | Identifies high-risk chronotypes and intervention windows; informs occupational health policies |
| Adaptive Gap/ Focus Area | Actionable Strategy and Tool Kit | Key Operational Step(s) | Intended Pay-Off |
|---|---|---|---|
| Dose–Time Randomization | Bayesian hierarchical designs that co-randomize dose level + clock time |
| Evidence-based chrono-dosing rules; smaller, faster trials |
| Sensor–Data Pipeline | Validated software bridges from CGM/lactate/KYN sensors and electronic TMF |
| Seamless biomarker ingestion; regulatory-ready data fidelity |
| Biomarker Validation | Rapid KYN/Trp/QA saliva or finger-stick electrochemical strips |
| Closed-loop dosing feasible at point of care |
| Safety Governance | Rules for rapid dose–time shifts in outpatient settings |
| Protects patients while enabling flexible chrono-titration |
| Patient-Centric Metrics | PROMs tuned to circadian toxicity (fatigue, cognition) |
| Holistic tolerability; improves adherence |
| Pilot Implementation | First-in-human chrono-trials for drugs with known chronotoxicities |
| Proof-of-concept that algorithm-guided timing beats fixed BID regimens |
| Development Challenge/Key Insight | Why It Matters | Precision Strategy or Next Step | |
|---|---|---|---|
| 1. |
| Long-term decolonization or mood relief can fade. |
|
| 2. |
| Regulatory roadblock; patient safety. |
|
| 3. |
| Batch-to-batch variability in metabolite output undermines reproducibility. |
|
| 4. |
| Missing optimal dosing windows weakens efficacy. |
|
| 5. |
| Jurisdictional differences delay global rollout. |
|
| 6. |
| One-size-fits-all combinations underperform. |
|
| 7. |
| Long-term decolonization or mood relief can fade. |
|
| Innovation Track | Key Development Step | Implementation Path/Intended Pay-Off |
|---|---|---|
| Encapsulated Post-Biotics (e.g., KYNA) |
|
|
| Adaptive Probiotic Titration |
|
|
| AI-Driven Gut–Brain Feedback Loops |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut–Brain Kynurenine Circuit. Biomedicines 2025, 13, 2020. https://doi.org/10.3390/biomedicines13082020
Tanaka M, Vécsei L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut–Brain Kynurenine Circuit. Biomedicines. 2025; 13(8):2020. https://doi.org/10.3390/biomedicines13082020
Chicago/Turabian StyleTanaka, Masaru, and László Vécsei. 2025. "From Microbial Switches to Metabolic Sensors: Rewiring the Gut–Brain Kynurenine Circuit" Biomedicines 13, no. 8: 2020. https://doi.org/10.3390/biomedicines13082020
APA StyleTanaka, M., & Vécsei, L. (2025). From Microbial Switches to Metabolic Sensors: Rewiring the Gut–Brain Kynurenine Circuit. Biomedicines, 13(8), 2020. https://doi.org/10.3390/biomedicines13082020








