Beyond the Skin: Exploring the Gut–Skin Axis in Chronic Spontaneous Urticaria and Other Inflammatory Skin Diseases
Abstract
1. Introduction
2. Systemic Immune Regulation Beyond Classical IgE-Mediated Hypersensitivity and the Microbiome
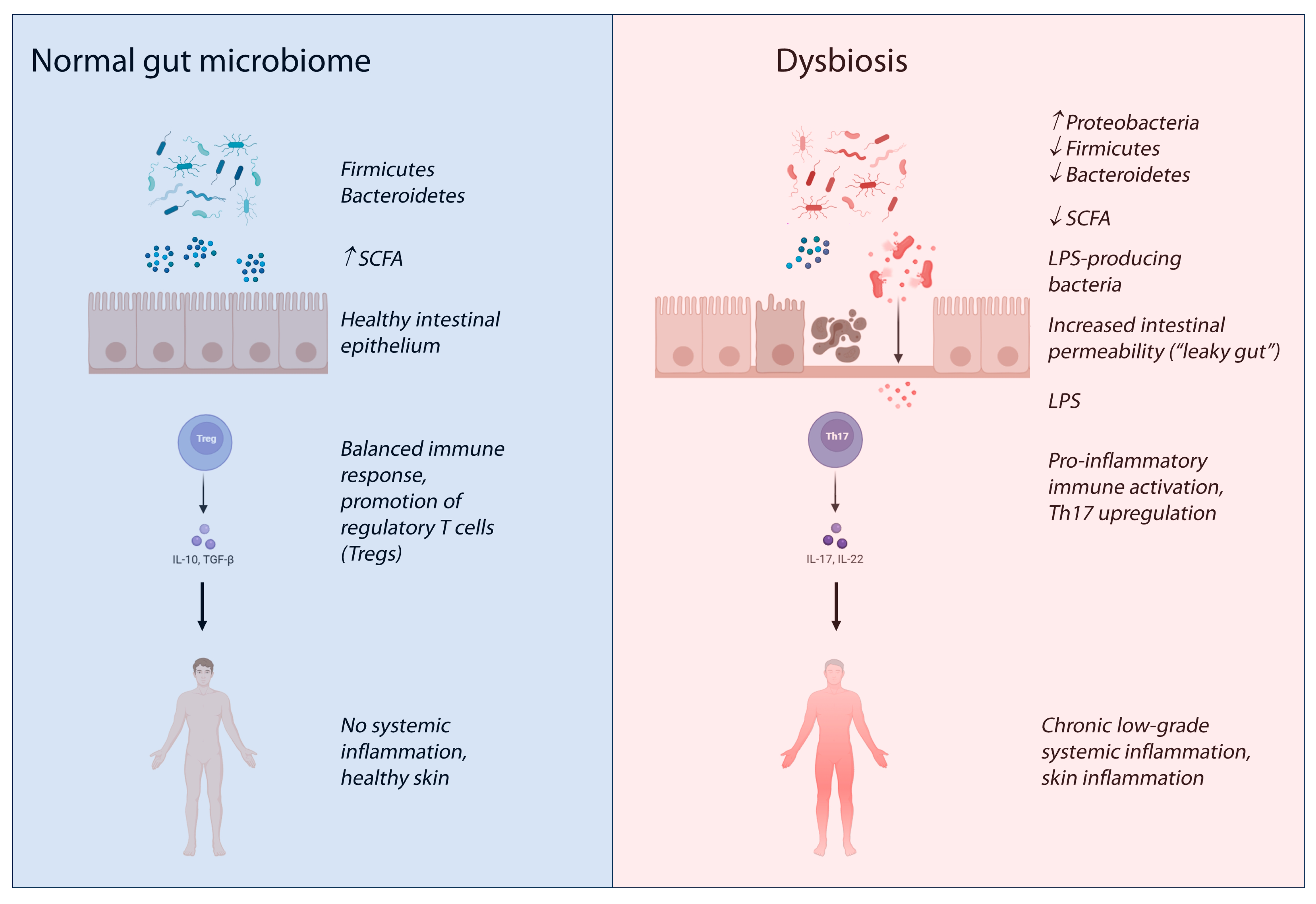
3. The Gut–Skin Axis in Inflammatory Skin Diseases
3.1. Atopic Dermatitis and Gut Dysbiosis
3.2. Psoriasis and Gut Dysbiosis
3.3. Rosacea and Gut Dysbiosis
3.4. Acne and Gut Dysbiosis
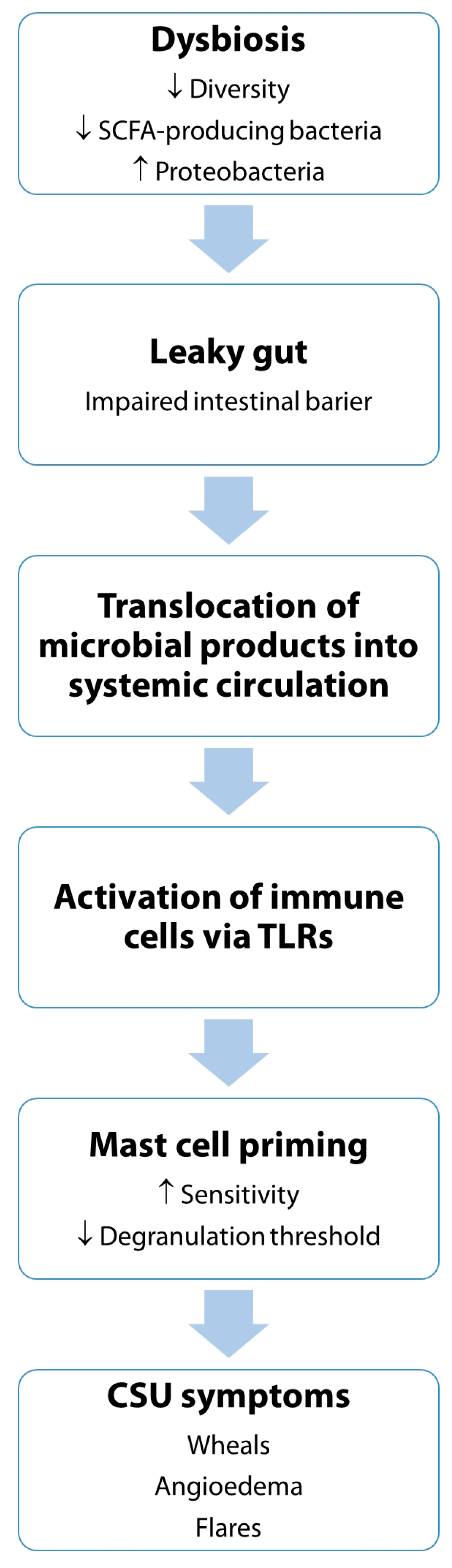
4. Current Evidence Linking the Microbiome to Chronic Spontaneous Urticaria
- Heterogeneity: findings across studies can be inconsistent due to differences in geographical location, dietary habits, sample size, methodology (e.g., 16S rRNA gene sequencing vs. metagenomics), disease duration, severity, and medication use.
- Causality vs. association: most studies establish associations, not direct causation. Mendelian randomization studies are emerging to explore causal links.
- Individual variation: the “normal” gut microbiome itself has significant inter-individual variation, making it challenging to define a universal “dysbiotic” profile.
4.1. Evidence from Animal Studies: Gut Microbiota Modulation Reduces Mast Cell Hyperreactivity
- Enhanced production of SCFAs such as butyrate and acetate, which stabilize mast cells and promote Treg differentiation [116]
- Suppression of pro-inflammatory cytokines like IL-6, IL-17, and TNF [117]
- Inhibition of TLR signaling, which is critical for microbial sensing by immune cells, including mast cells [118]
- Modulation of gut epithelial barrier function, thereby limiting systemic exposure to microbial antigens and endotoxins [119].
4.2. Impact of Probiotics, Prebiotics, Fecal Microbiota Transplantation and Diet in CSU
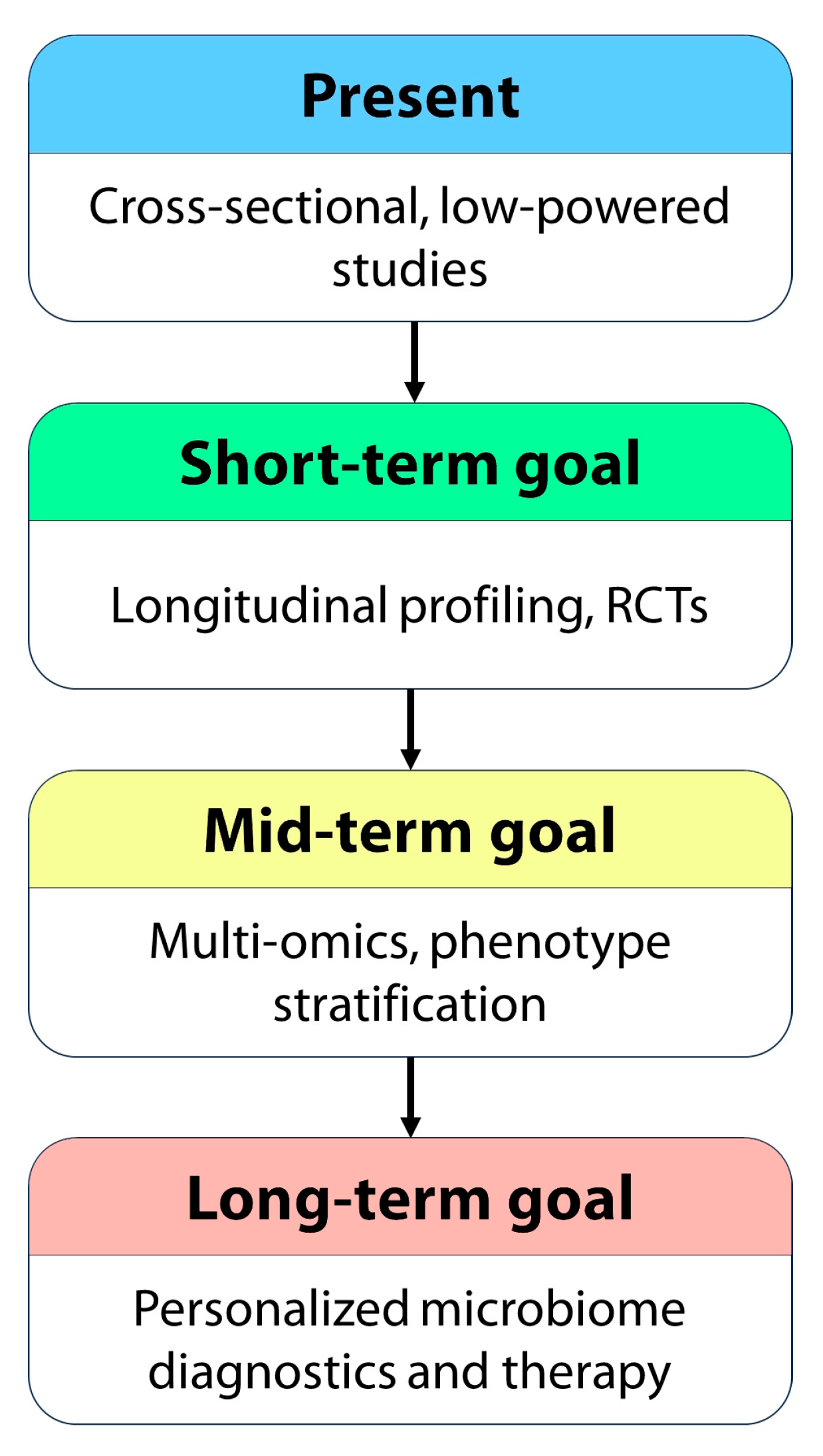
5. Challenges and Future Directions in Microbiome-Targeted Interventions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| CFU | Colony-forming unit |
| CIndU | Chronic inducible urticaria |
| CRP | C-reactive protein |
| CSU | Chronic spontaneous urticaria |
| FMT | Fecal microbiota transplantation |
| FOS | Fructo-oligosaccharides |
| GF | Germ-free |
| GOS | Galacto-oligosaccharides |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| LPS | Lipopolysaccharides |
| PAMPs | Pathogen-associated molecular patterns |
| RCT | Randomized controlled trial |
| SCFAs | Short-chain fatty acids |
| Th | T helper |
| TLRs | Toll-like receptors |
| TNF | Tumor necrosis factor |
| Treg | Regulatory T cell |
| UAS7 | Urticaria Activity Score over 7 days |
| UCT | Urticaria Control Test |
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Maurer, M.; Albuquerque, M.; Boursiquot, J.-N.; Dery, E.; Giménez-Arnau, A.; Godse, K.; Guitiérrez, G.; Kanani, A.; Lacuesta, G.; McCarthy, J.; et al. A Patient Charter for Chronic Urticaria. Adv. Ther. 2024, 41, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Giménez-Arnau, A.M.; Kulthanan, K.; Peter, J.; Metz, M.; Maurer, M. Urticaria. Nat. Rev. Dis. Prim. 2022, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Bonnekoh, H.; Metz, M.; Maurer, M. Chronic Spontaneous Urticaria: A Review. JAMA 2024, 332, 1464–1477. [Google Scholar] [CrossRef]
- Fricke, J.; Ávila, G.; Keller, T.; Weller, K.; Lau, S.; Maurer, M.; Zuberbier, T.; Keil, T. Prevalence of Chronic Urticaria in Children and Adults across the Globe: Systematic Review with Meta-Analysis. Allergy 2020, 75, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Gonçalo, M.; Gimenéz-Arnau, A.; Al-Ahmad, M.; Ben-Shoshan, M.; Bernstein, J.A.; Ensina, L.F.; Fomina, D.; Galvàn, C.A.; Godse, K.; Grattan, C.; et al. The Global Burden of Chronic Urticaria for the Patient and Society. Br. J. Dermatol. 2021, 184, 226–236. [Google Scholar] [CrossRef]
- Tomaszewska, K.; Słodka, A.; Tarkowski, B.; Zalewska-Janowska, A. Neuro–Immuno–Psychological Aspects of Chronic Urticaria. J. Clin. Med. 2023, 12, 3134. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Zhang, H.; Song, Y.; Wang, W.; Hu, Z.; Wang, S.; Huang, L.; Wang, Y.; Wu, S. Beyond the Itch: The Complex Interplay of Immune, Neurological, and Psychological Factors in Chronic Urticaria. J. Neuroinflamm. 2025, 22, 75. [Google Scholar] [CrossRef]
- Kaplan, A.; Lebwohl, M.; Giménez-Arnau, A.M.; Hide, M.; Armstrong, A.W.; Maurer, M. Chronic Spontaneous Urticaria: Focus on Pathophysiology to Unlock Treatment Advances. Allergy 2023, 78, 389–401. [Google Scholar] [CrossRef]
- He, L.; Yi, W.; Huang, X.; Long, H.; Lu, Q. Chronic Urticaria: Advances in Understanding of the Disease and Clinical Management. Clin. Rev. Allergy Immunol. 2021, 61, 424–448. [Google Scholar] [CrossRef]
- Mostmans, Y.; De Smedt, K.; Richert, B.; Elieh Ali Komi, D.; Maurer, M.; Michel, O. Markers for the Involvement of Endothelial Cells and the Coagulation System in Chronic Urticaria: A Systematic Review. Allergy 2021, 76, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Li, J.; Liu, R.; Zhu, L.; Peng, C. The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria. Front. Immunol. 2022, 13, 879754. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Fok, J.S.; Jiao, Q.; Kolkhir, P.; Pyatilova, P.; Romero, S.M.; Scheffel, J.; Siebenhaar, F.; Steinert, C.; Terhorst-Molawi, D.; et al. Total IgE as a Marker for Chronic Spontaneous Urticaria. Allergy. Asthma Immunol. Res. 2021, 13, 206–218. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Borro, M.; Ricciardi, L.; Gangemi, S. Prevalence of Autoimmune and Autoinflammatory Diseases in Chronic Urticaria: Pathogenetic, Diagnostic and Therapeutic Implications. Biomedicines 2023, 11, 410. [Google Scholar] [CrossRef]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.-K.; Maurer, M. Autoimmune Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef]
- Asero, R.; Ferrer, M.; Kocaturk, E.; Maurer, M. Chronic Spontaneous Urticaria: The Role and Relevance of Autoreactivity, Autoimmunity, and Autoallergy. J. Allergy Clin. Immunol. Pract. 2023, 11, 2302–2308. [Google Scholar] [CrossRef]
- Toubi, E.; Vadasz, Z. The Emerging Role of IL-17 in the Immune-Pathogenesis of Chronic Spontaneous Urticaria. ImmunoTargets Ther. 2020, 9, 217–223. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast Cells and Complement System: Ancient Interactions between Components of Innate Immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Aghdam, M.; van den Elzen, M.; van Os-Medendorp, H.; van Dijk, M.R.; Knol, E.F.; Knulst, A.C.; Röckmann, H.; Otten, H.G. Systemic and Local Evidence for Complement Involvement in Chronic Spontaneous Urticaria. Clin. Transl. Allergy 2021, 11, e12011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liang, B.; Li, R.; Li, J.; Lin, L.; Ma, S.; Wang, J. Activation of Coagulation, Anti-Coagulation, Fibrinolysis and the Complement System in Patients with Urticaria. Asian Pacific J. Allergy Immunol. 2013, 31, 43–50. [Google Scholar]
- Widhiati, S.; Purnomosari, D.; Wibawa, T.; Soebono, H. The Role of Gut Microbiome in Inflammatory Skin Disorders: A Systematic Review. Dermatol. Rep. 2021, 14, 9188. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S. Impact of Gut Microbiome on Skin Health: Gut-Skin Axis Observed through the Lenses of Therapeutics and Skin Diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef] [PubMed]
- Ryguła, I.; Pikiewicz, W.; Grabarek, B.O.; Wójcik, M.; Kaminiów, K. The Role of the Gut Microbiome and Microbial Dysbiosis in Common Skin Diseases. Int. J. Mol. Sci. 2024, 25, 1984. [Google Scholar] [CrossRef]
- Haidar, L.; Georgescu, M.; Drăghici, G.A.; Bănățean-Dunea, I.; Nica, D.V.; Șerb, A.-F. DNA Methylation Machinery in Gastropod Mollusks. Life 2024, 14, 537. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Celiberto, L.S.; Yang, H.; Sham, H.P.; Vallance, B.A. The Gut-Skin Axis: A Bi-Directional, Microbiota-Driven Relationship with Therapeutic Potential. Gut Microbes 2025, 17, 2473524. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Bah, Y.-R.; Law, J.W.-F.; Tan, L.T.-H.; He, Y.-W.; Wong, S.-H.; Thurairajasingam, S.; Chan, K.-G.; Lee, L.-H.; Letchumanan, V. Gut-Skin Axis: Unravelling the Connection between the Gut Microbiome and Psoriasis. Biomedicines 2022, 10, 1037. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What Defines a Healthy Gut Microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Effendi, R.M.R.A.; Anshory, M.; Kalim, H.; Dwiyana, R.F.; Suwarsa, O.; Pardo, L.M.; Nijsten, T.E.C.; Thio, H.B. Akkermansia muciniphila and Faecalibacterium prausnitzii in Immune-Related Diseases. Microorganisms 2022, 10, 2382. [Google Scholar] [CrossRef]
- Jan, T.; Negi, R.; Sharma, B.; Kumar, S.; Singh, S.; Rai, A.K.; Shreaz, S.; Rustagi, S.; Chaudhary, N.; Kaur, T.; et al. Next Generation Probiotics for Human Health: An Emerging Perspective. Heliyon 2024, 10, e35980. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Díez-Madueño, K.; de la Cueva Dobao, P.; Torres-Rojas, I.; Fernández-Gosende, M.; Hidalgo-Cantabrana, C.; Coto-Segura, P. Gut Dysbiosis and Adult Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2025, 14, 19. [Google Scholar] [CrossRef]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. An Altered Skin and Gut Microbiota Are Involved in the Modulation of Itch in Atopic Dermatitis. Cells 2022, 11, 3930. [Google Scholar] [CrossRef]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome—Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef]
- Sánchez-Pellicer, P.; Eguren-Michelena, C.; García-Gavín, J.; Llamas-Velasco, M.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Agüera-Santos, J.; Navarro-López, V. Rosacea, Microbiome and Probiotics: The Gut-Skin Axis. Front. Microbiol. 2024, 14, 1323644. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Chi, C.-C. Rosacea, Germs, and Bowels: A Review on Gastrointestinal Comorbidities and Gut–Skin Axis of Rosacea. Adv. Ther. 2021, 38, 1415–1424. [Google Scholar] [CrossRef]
- Sánchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Agüera-Santos, J.; Navarro-López, V. Acne, Microbiome, and Probiotics: The Gut–Skin Axis. Microorganisms 2022, 10, 1303. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Khan, N.A. The Increasing Importance of the Gut Microbiome in Acne Vulgaris. Folia Microbiol. 2022, 67, 825–835. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A.; et al. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2023, 16, 92. [Google Scholar] [CrossRef]
- Papa, V.; Li Pomi, F.; Di Gioacchino, M.; Mangifesta, R.; Borgia, F.; Gangemi, S. Mast Cells and Microbiome in Health and Disease. Front. Biosci. 2025, 30, 26283. [Google Scholar] [CrossRef]
- Solimando, A.G.; Desantis, V.; Ribatti, D. Mast Cells and Interleukins. Int. J. Mol. Sci. 2022, 23, 14004. [Google Scholar] [CrossRef]
- West, P.W.; Bahri, R.; Garcia-Rodriguez, K.M.; Sweetland, G.; Wileman, G.; Shah, R.; Montero, A.; Rapley, L.; Bulfone-Paus, S. Interleukin-33 Amplifies Human Mast Cell Activities Induced by Complement Anaphylatoxins. Front. Immunol. 2021, 11, 615236. [Google Scholar] [CrossRef]
- Pajulas, A.; Fu, Y.; Cheung, C.C.L.; Chu, M.; Cannon, A.; Alakhras, N.; Zhang, J.; Ulrich, B.J.; Nelson, A.S.; Zhou, B. Interleukin-9 Promotes Mast Cell Progenitor Proliferation and CCR2-Dependent Mast Cell Migration in Allergic Airway Inflammation. Mucosal Immunol. 2023, 16, 432–445. [Google Scholar] [CrossRef]
- Lauritano, D.; Mastrangelo, F.; D’Ovidio, C.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Frydas, I.; Kritas, S.K.; Trimarchi, M.; Carinci, F. Activation of Mast Cells by Neuropeptides: The Role of pro-Inflammatory and Anti-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 4811. [Google Scholar] [CrossRef]
- Lyons, D.O.; Pullen, N.A. Beyond IgE: Alternative Mast Cell Activation across Different Disease States. Int. J. Mol. Sci. 2020, 21, 1498. [Google Scholar] [CrossRef]
- Ney, L.-M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short Chain Fatty Acids: Key Regulators of the Local and Systemic Immune Response in Inflammatory Diseases and Infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Haidar, L.; Bănărescu, C.F.; Uța, C.; Moldovan, S.I.; Zimbru, E.-L.; Zimbru, R.-I.; Ciurariu, E.; Georgescu, M.; Panaitescu, C. Pollen–Food Allergy Syndrome: Allergens, Clinical Insights, Diagnostic and Therapeutic Challenges. Appl. Sci. 2025, 15, 66. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory Roles of Microbiota-Derived Short-Chain Fatty Acids in Bacterial Infections. Biomed. Pharmacother. 2021, 141, 111817. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, R.; Li, M.; Lu, M. Current Insights and Trends in Atopic Dermatitis and Microbiota Interactions: A Systematic Review and Bibliometric Analysis. Front. Microbiol. 2025, 16, 1613315. [Google Scholar] [CrossRef]
- Yu, L.; Deng, Y.-H.; Huang, Y.-H.; Ke, H.-J.; Guo, Y.; Wu, J.-L. Comparison of Gut Microbiota Between Infants with Atopic Dermatitis and Healthy Controls in Guangzhou, China. J. Asthma Allergy 2021, 14, 493–500. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the Gut Microbiota in Infancy and Its Impact on Health in Later Life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Sanchez-Lopez, M.F.; Barrero-Caicedo, P.A.; Olmos-Carval, H.M.; Torres-Medina, A.F.; Alzate-Granados, J.P. Relationship between Skin and Gut Microbiota Dysbiosis and Inflammatory Skin Diseases in Adult Patients: A Systematic Review. Microbe 2025, 7, 100342. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Wang, T. Gut Microbiota and Skin Pathologies: Mechanism of the Gut-Skin Axis in Atopic Dermatitis and Psoriasis. Int. Immunopharmacol. 2024, 141, 112658. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Serb, A.F.; Georgescu, M.; Onulov, R.; Novaconi, C.R.; Sisu, E.; Bolocan, A.; Sandu, R.E. Mass-Spectrometry-Based Research of Cosmetic Ingredients. Molecules 2024, 29, 1336. [Google Scholar] [CrossRef] [PubMed]
- Niers, L.; Martín, R.; Rijkers, G.; Sengers, F.; Timmerman, H.; Van Uden, N.; Smidt, H.; Kimpen, J.; Hoekstra, M. The Effects of Selected Probiotic Strains on the Development of Eczema (the PandA Study). Allergy 2009, 64, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus Rhamnosus and Lactobacillus Casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef]
- Dascălu, R.C.; Bărbulescu, A.L.; Stoica, L.E.; Dinescu; Ștefan, C.; Biță, C.E.; Popoviciu, H.V.; Ionescu, R.A.; Vreju, F.A. Review: A Contemporary, Multifaced Insight into Psoriasis Pathogenesis. J. Pers. Med. 2024, 14, 535. [Google Scholar] [CrossRef]
- Sikora, M.; Stec, A.; Chrabaszcz, M.; Knot, A.; Waskiel-Burnat, A.; Rakowska, A.; Olszewska, M.; Rudnicka, L. Gut Microbiome in Psoriasis: An Updated Review. Pathogens 2020, 9, 463. [Google Scholar] [CrossRef]
- Buhaș, M.C.; Gavrilaș, L.I.; Candrea, R.; Cătinean, A.; Mocan, A.; Miere, D.; Tătaru, A. Gut Microbiota in Psoriasis. Nutrients 2022, 14, 2970. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Dai, C.; Zeng, F. Cellular Mechanisms of Psoriasis Pathogenesis: A Systemic Review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2503–2515. [Google Scholar] [CrossRef]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Möller, S.; et al. Safety and Efficacy of Faecal Microbiota Transplantation for Active Peripheral Psoriatic Arthritis: An Exploratory Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, X.; Lu, Q.; Yao, X. Fecal Microbiota Transplantation for the Treatment of Chronic Inflammatory Skin Diseases. Heliyon 2024, 10, e37432. [Google Scholar] [CrossRef]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Guertler, A.; Hering, P.; Pacífico, C.; Gasche, N.; Sladek, B.; Irimi, M.; French, L.E.; Clanner-Engelshofen, B.M.; Reinholz, M. Characteristics of Gut Microbiota in Rosacea Patients—A Cross-Sectional, Controlled Pilot Study. Life 2024, 14, 585. [Google Scholar] [CrossRef]
- Manfredini, M.; Barbieri, M.; Milandri, M.; Longo, C. Probiotics and Diet in Rosacea: Current Evidence and Future Perspectives. Biomolecules 2025, 15, 411. [Google Scholar] [CrossRef]
- Yang, X. Relationship between Helicobacter Pylori and Rosacea: Review and Discussion. BMC Infect. Dis. 2018, 18, 318. [Google Scholar] [CrossRef]
- Zhu, W.; Hamblin, M.R.; Wen, X. Role of the Skin Microbiota and Intestinal Microbiome in Rosacea. Front. Microbiol. 2023, 14, 1108661. [Google Scholar] [CrossRef] [PubMed]
- Akaza, N.; Takasaki, K.; Nishiyama, E.; Usui, A.; Miura, S.; Yokoi, A.; Futamura, K.; Suzuki, K.; Yashiro, Y.; Yagami, A. The Microbiome in Comedonal Contents of Inflammatory Acne Vulgaris is Composed of an Overgrowth of Cutibacterium spp. and Other Cutaneous Microorganisms. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2003–2012. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes (Propionibacterium Acnes) and Acne Vulgaris: A Brief Look at the Latest Updates. J. Eur. Acad. Dermatology Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Peña-Durán, E.; García-Galindo, J.J.; López-Murillo, L.D.; Huerta-Huerta, A.; Balleza-Alejandri, L.R.; Beltrán-Ramírez, A.; Anaya-Ambriz, E.J.; Suárez-Rico, D.O. Microbiota and Inflammatory Markers: A Review of Their Interplay, Clinical Implications, and Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 1773. [Google Scholar] [CrossRef] [PubMed]
- Ryguła, I.; Pikiewicz, W.; Kaminiów, K. Impact of Diet and Nutrition in Patients with Acne Vulgaris. Nutrients 2024, 16, 1476. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.H.; Manaf, Z.A.; Azizan, N.Z. High Glycemic Load Diet, Milk and Ice Cream Consumption Are Related to Acne Vulgaris in Malaysian Young Adults: A Case Control Study. BMC Dermatol. 2012, 12, 13. [Google Scholar] [CrossRef]
- Meixiong, J.; Ricco, C.; Vasavda, C.; Ho, B.K. Diet and Acne: A Systematic Review. JAAD Int. 2022, 7, 95–112. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Puxeddu, I.; Pistone, F.; Pisani, F.; Levi-Schaffer, F. Mast Cell Signaling and Its Role in Urticaria. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2024, 133, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Podder, I.; Pesqué, D.; Carrón, N.; González Torres, P.I.; Pujol, R.M.; Giménez-Arnau, A.M. Gut Microbial Alteration in Chronic Spontaneous Urticaria Unresponsive to Second Generation Antihistamines and Its Correlation with Disease Characteristics—A Cross-Sectional Case-Control Study. Clin. Transl. Allergy 2025, 15, e70027. [Google Scholar] [CrossRef]
- Krišto, M.; Lugović-Mihić, L.; Muñoz, M.; Rupnik, M.; Mahnic, A.; Ozretić, P.; Jaganjac, M.; Ćesić, D.; Kuna, M. Gut Microbiome Composition in Patients with Chronic Urticaria: A Review of Current Evidence and Data. Life 2023, 13, 152. [Google Scholar] [CrossRef]
- Grzanka, A.; Machura, E.; Misiolek, M.; Polaniak, R.; Kasperski, J.; Kasperska-Zajac, A. Systemic Inflammatory Response and Calcification Markers in Patients with Long Lasting Moderate-Severe Chronic Spontaneous Urticaria. Eur. J. Dermatol. 2015, 25, 26–28. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Chu, Z.; Shi, L.; Geng, S.; Guo, K. Gut Microbiome Alterations and Functional Prediction in Chronic Spontaneous Urticaria Patients. J. Microbiol. Biotechnol. 2021, 31, 747–755. [Google Scholar] [CrossRef]
- Cai, R.; Zhou, C.; Tang, R.; Meng, Y.; Zeng, J.; Li, Y.; Wen, X. Current Insights on Gut Microbiome and Chronic Urticaria: Progress in the Pathogenesis and Opportunities for Novel Therapeutic Approaches. Gut Microbes 2024, 16, 2382774. [Google Scholar] [CrossRef]
- Bosveld, C.J.; Guth, C.; Limjunyawong, N.; Pundir, P. Emerging Role of the Mast Cell–Microbiota Crosstalk in Cutaneous Homeostasis and Immunity. Cells 2023, 12, 2624. [Google Scholar] [CrossRef]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodríguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal Mucosal Mast Cells: Key Modulators of Barrier Function and Homeostasis. Cells 2019, 8, 135. [Google Scholar] [CrossRef]
- Wang, D.; Guo, S.; He, H.; Gong, L.; Cui, H. Gut Microbiome and Serum Metabolome Analyses Identify Unsaturated Fatty Acids and Butanoate Metabolism Induced by Gut Microbiota in Patients With Chronic Spontaneous Urticaria. Front. Cell. Infect. Microbiol. 2020, 10, 24. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut Microbiome Stability and Resilience: Elucidating the Response to Perturbations in Order to Modulate Gut Health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Wang, X.; Yi, W.; He, L.; Luo, S.; Wang, J.; Jiang, L.; Long, H.; Zhao, M.; Lu, Q. Abnormalities in Gut Microbiota and Metabolism in Patients With Chronic Spontaneous Urticaria. Front. Immunol. 2021, 12, 691304. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Y.; Guo, Y.; Sun, J.; Shen, W.; Yuan, M.; Zhang, S.; He, P.; Jiao, X. Altered Gut Microbiota Diversity and Composition in Chronic Urticaria. Dis. Markers 2019, 2019, 6417471. [Google Scholar] [CrossRef]
- Nabizadeh, E.; Jazani, N.H.; Bagheri, M.; Shahabi, S. Association of Altered Gut Microbiota Composition with Chronic Urticaria. Ann. Allergy, Asthma Immunol. 2017, 119, 48–53. [Google Scholar] [CrossRef]
- Kim, C.H. Control of Lymphocyte Functions by Gut Microbiota-Derived Short-Chain Fatty Acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Shahabi, S.; Bagheri, M.; Nabizadeh, E.; Jazani, N.H. The Protective Effect of Lactobacillus and Bifidobacterium as the Gut Microbiota Members against Chronic Urticaria. Int. Immunopharmacol. 2018, 59, 168–173. [Google Scholar] [CrossRef]
- De Zuani, M.; Dal Secco, C.; Tonon, S.; Arzese, A.; Pucillo, C.E.M.; Frossi, B. LPS Guides Distinct Patterns of Training and Tolerance in Mast Cells. Front. Immunol. 2022, 13, 835348. [Google Scholar] [CrossRef]
- Song, Y.; Dan, K.; Yao, Z.; Yang, X.; Chen, B.; Hao, F. Altered Gut Microbiota in H1-Antihistamine-Resistant Chronic Spontaneous Urticaria Associates with Systemic Inflammation. Front. Cell. Infect. Microbiol. 2022, 12, 831489. [Google Scholar] [CrossRef]
- Zhu, L.; Jian, X.; Zhou, B.; Liu, R.; Muñoz, M.; Sun, W.; Xie, L.; Chen, X.; Peng, C.; Maurer, M. Gut Microbiota Facilitate Chronic Spontaneous Urticaria. Nat. Commun. 2024, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, L.; Wang, K.; Qin, Y.; Jin, J.; Wang, D.; Yan, H.; You, C. Changes of Gut Microbiome in Adolescent Patients with Chronic Spontaneous Urticaria after Omalizumab Treatment. Clin. Cosmet. Investig. Dermatol. 2023, 16, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, J.; Cao, H.; Lu, Z.; Shen, H.; Ji, J.; Jiao, Q. Causal Effect Between Gut Microbiota, Gut Bacterial Pathway, and Chronic Spontaneous Urticaria: A Large-Scale Bidirectional Mendelian Randomization Analysis. J. Investig. Allergol. Clin. Immunol. 2025, 35, 1. [Google Scholar] [CrossRef]
- Liu, R.; Peng, C.; Jing, D.; Xiao, Y.; Zhu, W.; Zhao, S.; Zhang, J.; Chen, X.; Li, J. Biomarkers of Gut Microbiota in Chronic Spontaneous Urticaria and Symptomatic Dermographism. Front. Cell. Infect. Microbiol. 2021, 11, 703126. [Google Scholar] [CrossRef]
- Luo, Z.; Jin, Z.; Tao, X.; Wang, T.; Wei, P.; Zhu, C.; Wang, Z. Combined Microbiome and Metabolome Analysis of Gut Microbiota and Metabolite Interactions in Chronic Spontaneous Urticaria. Front. Cell. Infect. Microbiol. 2022, 12, 1094737. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Hermanova, P.; Srutkova, D.; Golias, J.; Hudcovic, T.; Zwicker, C.; Sinkora, M.; Akgün, J.; Wiedermann, U.; Tuckova, L.; et al. Germ-Free Mice Exhibit Mast Cells With Impaired Functionality and Gut Homing and Do Not Develop Food Allergy. Front. Immunol. 2019, 10, 205. [Google Scholar] [CrossRef]
- Hornikova, T.; Jelinkova, A.; Jiraskova Zakostelska, Z.; Thon, T.; Coufal, S.; Polouckova, A.; Kopelentova, E.; Kverka, M.; Makovicky, P.; Tlaskalova-Hogenova, H.; et al. Genetic Background and Microbiome Drive Susceptibility to Epicutaneous Sensitization and Food Allergy in Adjuvant-Free Mouse Model. Front. Immunol. 2024, 15, 1509691. [Google Scholar] [CrossRef]
- Rodriguez, B.; Prioult, G.; Bibiloni, R.; Nicolis, I.; Mercenier, A.; Butel, M.-J.; Waligora-Dupriet, A.-J. Germ-Free Status and Altered Caecal Subdominant Microbiota Are Associated with a High Susceptibility to Cow’s Milk Allergy in Mice. FEMS Microbiol. Ecol. 2011, 76, 133–144. [Google Scholar] [CrossRef]
- Kim, A.-R.; Jeon, S.-G.; Kim, H.-R.; Hong, H.; Yoon, Y.W.; Lee, B.-M.; Yoon, C.H.; Choi, S.J.; Jang, M.H.; Yang, B.-G. Preventive and Therapeutic Effects of Lactiplantibacillus plantarum HD02 and MD159 through Mast Cell Degranulation Inhibition in Mouse Models of Atopic Dermatitis. Nutrients 2024, 16, 3021. [Google Scholar] [CrossRef]
- Xie, A.; Chen, A.; Chen, Y.; Luo, Z.; Jiang, S.; Chen, D.; Yu, R. Lactobacillus for the Treatment and Prevention of Atopic Dermatitis: Clinical and Experimental Evidence. Front. Cell. Infect. Microbiol. 2023, 13, 1137275. [Google Scholar] [CrossRef]
- Kim, W.-K.; Jang, Y.J.; Han, D.H.; Jeon, K.; Lee, C.; Han, H.S.; Ko, G. Lactobacillus Paracasei KBL382 Administration Attenuates Atopic Dermatitis by Modulating Immune Response and Gut Microbiota. Gut Microbes 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Gryaznova, M.; Burakova, I.; Smirnova, Y.; Morozova, P.; Chirkin, E.; Gureev, A.; Mikhaylov, E.; Korneeva, O.; Syromyatnikov, M. Effect of Probiotic Bacteria on the Gut Microbiome of Mice with Lipopolysaccharide-Induced Inflammation. Microorganisms 2024, 12, 1341. [Google Scholar] [CrossRef]
- Li, Y.; Jia, D.; Wang, J.; Li, H.; Yin, X.; Liu, J.; Wang, J.; Guan, G.; Luo, J.; Yin, H.; et al. Probiotics Isolated From Animals in Northwest China Improve the Intestinal Performance of Mice. Front. Vet. Sci. 2021, 8, 750895. [Google Scholar] [CrossRef]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like Receptor 9 Signaling Mediates the Anti-Inflammatory Effects of Probiotics in Murine Experimental Colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar] [CrossRef]
- Shi, C.-Z.; Chen, H.-Q.; Liang, Y.; Xia, Y.; Yang, Y.-Z.; Yang, J.; Zhang, J.-D.; Wang, S.-H.; Liu, J.; Qin, H.-L. Combined Probiotic Bacteria Promotes Intestinal Epithelial Barrier Function in Interleukin-10-Gene-Deficient Mice. World J. Gastroenterol. 2014, 20, 4636–4647. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, L.; Wang, J.; Hao, M.; Che, H. Antibiotic-Induced Gut Microbiota Dysbiosis Damages the Intestinal Barrier, Increasing Food Allergy in Adult Mice. Nutrients 2021, 13, 3315. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.-H.; Hong, S.-J. Antibiotics-Induced Dysbiosis of Intestinal Microbiota Aggravates Atopic Dermatitis in Mice by Altered Short-Chain Fatty Acids. Allergy. Asthma Immunol. Res. 2019, 12, 137–148. [Google Scholar] [CrossRef]
- Reynolds, L.A.; Finlay, B.B. A Case for Antibiotic Perturbation of the Microbiota Leading to Allergy Development. Expert Rev. Clin. Immunol. 2013, 9, 1019–1030. [Google Scholar] [CrossRef]
- Zhang, X.; Borbet, T.C.; Fallegger, A.; Wipperman, M.F.; Blaser, M.J.; Müller, A. An Antibiotic-Impacted Microbiota Compromises the Development of Colonic Regulatory T Cells and Predisposes to Dysregulated Immune Responses. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Nettis, E.; Di Leo, E.; Pastore, A.; Distaso, M.; Zaza, I.; Vacca, M.; Macchia, L.; Vacca, A. Probiotics and Refractory Chronic Spontaneous Urticaria. Eur. Ann. Allergy Clin. Immunol. 2016, 48, 182–187. [Google Scholar]
- Dabaghzadeh, A.; Ghaffari, J.; Moradi, S.; Sayadian Separghan, D. Probiotics on Chronic Urticaria: A Randomized Clinical Trial Study. Casp. J. Intern. Med. 2023, 14, 192–198. [Google Scholar] [CrossRef]
- Bi, X.-D.; Lu, B.-Z.; Pan, X.-X.; Liu, S.; Wang, J.-Y. Adjunct Therapy with Probiotics for Chronic Urticaria in Children: Randomised Placebo-Controlled Trial. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2021, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Atefi, N.; Fallahpour, M.; Sharifi, S.; Ghassemi, M.; Roohaninasab, M.; Goodarzi, A. Probiotic as an Adjuvant Therapy in Chronic Urticaria: A Blinded Randomized Controlled Clinical Trial. Eur. Ann. Allergy Clin. Immunol. 2022, 54, 123–130. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Yu, H.; Bai, Y.-P.; Yue, L.-F.; Wang, H.-M.; Li, L.-L. Effect and Safety of Probiotics for Treating Urticaria: A Systematic Review and Meta-Analysis. J. Cosmet. Dermatol. 2023, 22, 2663–2670. [Google Scholar] [CrossRef]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 1, 3673. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory Effects of Inulin and Its Intestinal Metabolites. Front. Immunol. 2023, 14, 1224092. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; Cheng, J.; Wang, J.; Liu, Q. Prebiotic Oligosaccharides in Skin Health: Benefits, Mechanisms, and Cosmetic Applications. Antioxidants 2025, 14, 754. [Google Scholar] [CrossRef]
- Lee, L.Y.G.N.; Leow, S.Y.; Wen, H.; Soh, J.Y.; Chiang, W.C.; Zhong, Y.; Tham, E.H.; Loh, W.; Delsing, D.J.; Lee, B.W.; et al. An Evaluation of the Mechanisms of Galacto-Oligosaccharide (GOS)-Induced IgE Cross-Linking on Basophils in GOS Allergy. Front. Allergy 2022, 3, 840454. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Lekkala, L.; Yadav, D.; Jain, S.; Yadav, H. Microbiome and Postbiotics in Skin Health. Biomedicines 2025, 13, 791. [Google Scholar] [CrossRef]
- Zdybel, K.; Śliwka, A.; Polak-Berecka, M.; Polak, P.; Waśko, A. Postbiotics Formulation and Therapeutic Effect in Inflammation: A Systematic Review. Nutrients 2025, 17, 2187. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W.; et al. Unlocking the Power of Postbiotics: A Revolutionary Approach to Nutrition for Humans and Animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Yuan, Q.-F.; Qin, Z.-C.; Xu, Y.-D.; Li, L.; Xu, J.-T.; He, X.-X.; Xie, W.-R.; Wu, L.-H. Faecal Microbiota Transplantation for Treatment of Chronic Urticaria with Recurrent Abdominal Pain and Food Allergy. Singapore Med. J. 2023. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Stolfi, C.; Pacifico, T.; Monteleone, G.; Laudisi, F. Impact of Western Diet and Ultra-Processed Food on the Intestinal Mucus Barrier. Biomedicines 2023, 11, 2015. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Tsigalou, C.; Tsolou, A.; Stavropoulou, E.; Konstantinidis, T.; Zafiriou, E.; Dardiotis, E.; Tsirogianni, A.; Bogdanos, D. Unraveling the Intricate Dance of the Mediterranean Diet and Gut Microbiota in Autoimmune Resilience. Front. Nutr. 2024, 11, 1383040. [Google Scholar] [CrossRef]
- Kimble, R.; Gouinguenet, P.; Ashor, A.; Stewart, C.; Deighton, K.; Matu, J.; Griffiths, A.; Malcomson, F.C.; Joel, A.; Houghton, D.; et al. Effects of a Mediterranean Diet on the Gut Microbiota and Microbial Metabolites: A Systematic Review of Randomized Controlled Trials and Observational Studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 8698–8719. [Google Scholar] [CrossRef]
- Deleu, S.; Becherucci, G.; Godny, L.; Mentella, M.C.; Petito, V.; Scaldaferri, F. The Key Nutrients in the Mediterranean Diet and Their Effects in Inflammatory Bowel Disease: A Narrative Review. Nutrients 2024, 16, 4201. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- Ayvaz, H.H.; Kuyumcu, A. Effect of the Mediterranean Diet in Patients with Chronic Spontaneous Urticaria. Rev. Assoc. Med. Bras. 2021, 67, 675–680. [Google Scholar] [CrossRef]
- Jaros, J.; Shi, V.Y.; Katta, R. Diet and Chronic Urticaria: Dietary Modification as a Treatment Strategy. Dermatol. Pract. Concept. 2020, 10, e2020004. [Google Scholar] [CrossRef]
- Podder, I.; Jaiswal, S.; Das, A. Dietary Strategies for Chronic Spontaneous Urticaria: An Evidence-Based Review. Int. J. Dermatol. 2023, 62, 143–153. [Google Scholar] [CrossRef]
- Wagner, N.; Dirk, D.; Peveling-Oberhag, A.; Reese, I.; Rady-Pizarro, U.; Mitzel, H.; Staubach, P. A Popular Myth—Low-Histamine Diet Improves Chronic Spontaneous Urticaria—Fact or Fiction? J. Eur. Acad. Dermatol. Venereol. 2017, 31, 650–655. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Melde, A.; Magerl, M.; Zuberbier, T.; Church, M.K.; Maurer, M. Histamine Intolerance in Patients with Chronic Spontaneous Urticaria. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Cornillier, H.; Giraudeau, B.; Samimi, M.; Munck, S.; Hacard, F.; Jonville-Bera, A.-P.; Jegou, M.-H.; d’Acremont, G.; Pham, B.-N.; Chosidow, O.; et al. Effect of Diet in Chronic Spontaneous Urticaria: A Systematic Review. Acta Derm. Venereol. 2019, 99, 127–132. [Google Scholar] [CrossRef]
- Tripathy, A.; Swain, N.; Padhan, P.; Raghav, S.K.; Gupta, B. Lactobacillus Rhamnosus Reduces CD8+ T Cell Mediated Inflammation in Patients with Rheumatoid Arthritis. Immunobiology 2023, 228, 152415. [Google Scholar] [CrossRef]
- Guo, H.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. The Potential Therapeutic Role of Lactobacillaceae Rhamnosus for Treatment of Inflammatory Bowel Disease. Foods 2023, 12, 692. [Google Scholar] [CrossRef]
- Bornholdt, J.; Broholm, C.; Chen, Y.; Rago, A.; Sloth, S.; Hendel, J.; Melsæther, C.; Müller, C.V.; Juul Nielsen, M.; Strickertsson, J.; et al. Personalized B Cell Response to the Lactobacillus Rhamnosus GG Probiotic in Healthy Human Subjects: A Randomized Trial. Gut Microbes 2020, 12, 1854639. [Google Scholar] [CrossRef] [PubMed]
| Phylum | Representative Genera | Notable Species/Examples | Functions/Notes |
|---|---|---|---|
| Firmicutes | Faecalibacterium | F. prausnitzii | Anti-inflammatory, SCFA (butyrate) producer |
| Clostridium | C. leptum, C. coccoides | SCFA production, immune modulation | |
| Lactobacillus | L. rhamnosus, L. acidophilus | Probiotic, lactic acid production | |
| Ruminococcus | R. bromii, R. flavefaciens | Resistant starch degradation | |
| Blautia | B. obeum, B. wexlerae | SCFA producer, potential metabolic benefits | |
| Eubacterium | E. rectale | Butyrate production, colonic health | |
| Bacteroidetes | Bacteroides | B. fragilis, B. thetaiotaomicron, B. vulgatus | Carbohydrate metabolism, immune homeostasis |
| Prevotella | P. copri | Fiber fermentation, controversial inflammatory links | |
| Alistipes | A. putredinis | Potentially protective, involved in amino acid metabolism | |
| Actinobacteria | Bifidobacterium | B. longum, B. breve, B. adolescentis | Early colonizer, probiotic, carbohydrate metabolism |
| Collinsella | C. aerofaciens | Role in lipid metabolism | |
| Proteobacteria | Escherichia | E. coli (commensal strains) | Vitamin K production, immune interaction (can become pathogenic if dysregulated) |
| Klebsiella | K. pneumoniae (commensal strains) | May act as pathobiont if overgrown | |
| Enterobacter | E. cloacae | Often transient, opportunistic potential | |
| Verrucomicrobia | Akkermansia | A. muciniphila | Mucus layer degradation, metabolic health benefits |
| Fusobacteria | Fusobacterium | F. nucleatum (low abundance) | Normally low in abundance; associated with disease in overgrowth |
| Synergistetes | Synergistes | S. jonesii | Present in low abundance; limited known function |
| Tenericutes | Mycoplasma | M. hominis (rarely detected) | Occasional, typically not dominant in healthy microbiome |
| Feature | Atopic Dermatitis (AD) [62,63,87] | Psoriasis [70,71] | Rosacea [75,77] | Acne Vulgaris [42,82] |
|---|---|---|---|---|
| Microbial diversity | Decreased | Decreased | Decreased | Decreased |
| Key changes in composition | ↓ Bifidobacterium, ↓ Lactobacillus, ↑ Clostridium clusters (pro-inflammatory) | ↓ SCFA producers (Faecalibacterium prausnitzii—key anti-inflammatory bacterium), ↓ Actinobacteria, ↑ Escherichia coli, ↑ Ruminococcus gnavus | ↑ Helicobacter pylori, ↑ Firmicutes/Bacteroidetes ratio | ↑ Firmicutes/Bacteroidetes ratio Shift toward pro-inflammatory taxa |
| Gut barrier function | Increased permeability (“leaky gut”) | Increased permeability (“leaky gut”) | Increased permeability (“leaky gut”) | Possible increased permeability (less studied) |
| Associated immune changes | Th2 skewing, elevated IgE, systemic inflammation | Th17 polarization, systemic inflammation | Systemic inflammation | Systemic inflammation, possible hyperandrogenism links |
| Clinical/Dietary modulators | Probiotics show potential benefits (strain-specific) Breastfeeding protective | Preliminary data on FMT; SCFA-focused dietary interventions under investigation | Probiotics under investigation; dietary modifications (e.g., low-histamine, gluten-free) anecdotally helpful but limited evidence | High-glycemic-load diets linked to gut dysbiosis; probiotics under early investigation |
| Evidence level | Moderate (RCTs in prevention; smaller studies in treatment) | Moderate (observational studies; pilot interventions) | Preliminary (small studies; emerging field) | Preliminary (small studies; emerging field) |
| Feature/Genera Category | Observation in Urticaria Patients (Compared to Healthy Controls) | Key Genera/Phyla Examples | Important Notes & Implications |
|---|---|---|---|
| Overall diversity | Alpha diversity: usually decreased or no significant difference [90,108]. | - | Alpha diversity measures richness and evenness within a sample. A decrease suggests a less diverse and potentially less resilient microbial community. Some studies report no significant difference, highlighting the need for larger and more standardized cohorts. |
| Beta diversity: usually significantly different [63,90,93]. | - | Beta diversity measures the differences in microbial composition between groups (e.g., CSU vs. healthy controls). A significant difference indicates distinct microbial communities in urticaria patients. | |
| Genera (Decreased) | Beneficial/Commensal bacteria: often decreased [93,102]. | Lactobacillus spp. Bifidobacterium spp. Faecalibacterium prausnitzii Roseburia spp. Bacteroides spp. (though some studies vary) Lachnospiraceae family (many SCFA producers) Prevotella spp. (often varied) | These genera are known for producing beneficial metabolites like SCFAs (e.g., butyrate), which are crucial for gut barrier integrity, immune regulation, and anti-inflammatory effects. Their reduction can contribute to increased gut permeability and systemic inflammation. |
| Genera (Increased) | Opportunistic pathogens/Pro-inflammatory bacteria: often increased [93,105]. | Proteobacteria phylum, Enterobacteriaceae family (Escherichia coli and Klebsiella spp.) Peptostreptococcaceae family (Clostridioides difficile and other anaerobes) | An increase in these taxa is often associated with dysbiosis and a pro-inflammatory gut environment. Proteobacteria is often considered a hallmark of dysbiosis and may contribute to increased gut permeability and LPS production. |
| Phyla level | Firmicutes and Bacteroidetes: often decreased in relative abundance, or altered ratios [89,90]. | Firmicutes, Bacteroidetes | These are the two most dominant phyla. While general trends suggest a decrease in ““beneficial” Firmicutes members and some Bacteroidetes, the exact alterations can vary between studies. The Firmicutes-to-Bacteroidetes ratio is often examined, but findings are not always consistent. |
| Proteobacteria: often increased [90,92,93]. | Proteobacteria | An enrichment of Proteobacteria is frequently observed and is often considered a key indicator of gut dysbiosis in various inflammatory conditions. | |
| Functional alteration | Reduced SCFA production: linked to decreased beneficial bacteria [93,105]. | - | Dysbiosis can lead to a reduction in SCFA-producing bacteria, which are vital for gut health and immune modulation, potentially exacerbating inflammation in CSU. |
| Altered amino acid and bile acid metabolism: due to shifts in microbial communities [109]. | - | Changes in the gut microbiota can significantly impact host metabolism, including the processing of amino acids (e.g., tryptophan pathways) and bile acids, which can have systemic immune effects. |
| Strategy | Current Evidence | Limitations | Potential Role |
|---|---|---|---|
| Probiotics | Preliminary | Strain specificity, RCT gaps | Adjunct to antihistamines and omalizumab |
| Prebiotics | Theoretical | No CSU trials yet | Microbial support |
| Fecal microbiota transplant (FMT) | Conceptual | Ethical/regulatory concerns | Future therapy for refractory CSU |
| Anti-inflammatory diet | Indirect | Low adherence in some patients | Lifestyle adjunct |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidar, L.; Bănărescu, C.F.; Uța, C.; Zimbru, E.-L.; Zimbru, R.-I.; Tîrziu, A.; Pătrașcu, R.; Șerb, A.-F.; Georgescu, M.; Nistor, D.; et al. Beyond the Skin: Exploring the Gut–Skin Axis in Chronic Spontaneous Urticaria and Other Inflammatory Skin Diseases. Biomedicines 2025, 13, 2014. https://doi.org/10.3390/biomedicines13082014
Haidar L, Bănărescu CF, Uța C, Zimbru E-L, Zimbru R-I, Tîrziu A, Pătrașcu R, Șerb A-F, Georgescu M, Nistor D, et al. Beyond the Skin: Exploring the Gut–Skin Axis in Chronic Spontaneous Urticaria and Other Inflammatory Skin Diseases. Biomedicines. 2025; 13(8):2014. https://doi.org/10.3390/biomedicines13082014
Chicago/Turabian StyleHaidar, Laura, Camelia Felicia Bănărescu, Cristina Uța, Elena-Larisa Zimbru, Răzvan-Ionuț Zimbru, Alexandru Tîrziu, Raul Pătrașcu, Alina-Florina Șerb, Marius Georgescu, Daciana Nistor, and et al. 2025. "Beyond the Skin: Exploring the Gut–Skin Axis in Chronic Spontaneous Urticaria and Other Inflammatory Skin Diseases" Biomedicines 13, no. 8: 2014. https://doi.org/10.3390/biomedicines13082014
APA StyleHaidar, L., Bănărescu, C. F., Uța, C., Zimbru, E.-L., Zimbru, R.-I., Tîrziu, A., Pătrașcu, R., Șerb, A.-F., Georgescu, M., Nistor, D., & Panaitescu, C. (2025). Beyond the Skin: Exploring the Gut–Skin Axis in Chronic Spontaneous Urticaria and Other Inflammatory Skin Diseases. Biomedicines, 13(8), 2014. https://doi.org/10.3390/biomedicines13082014





.png)


