Engineering Targeted Gene Delivery Systems for Primary Hereditary Skeletal Myopathies: Current Strategies and Future Perspectives
Abstract
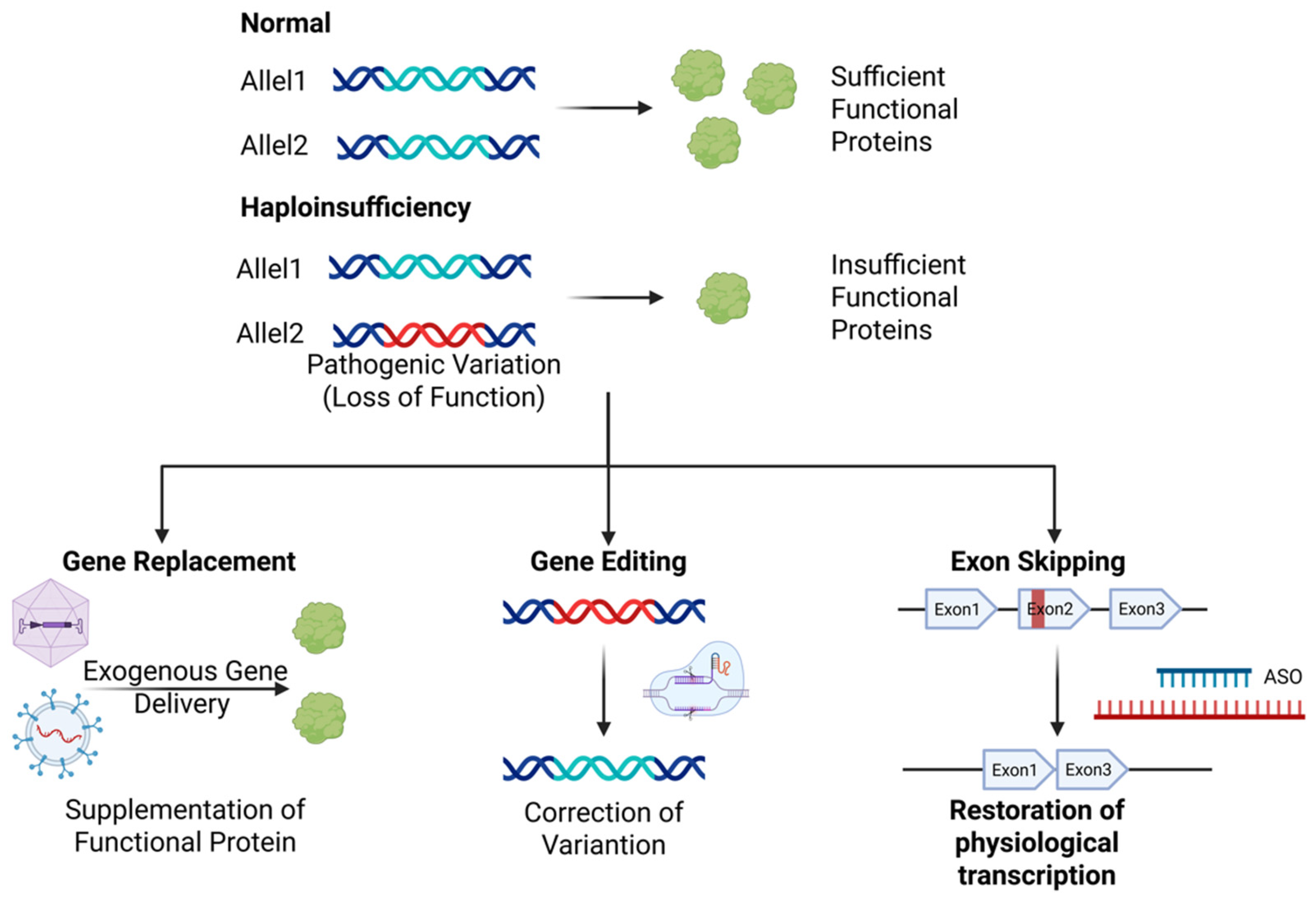
1. Introduction
2. Skeletal Muscle: An Ideal Target for Gene Therapy
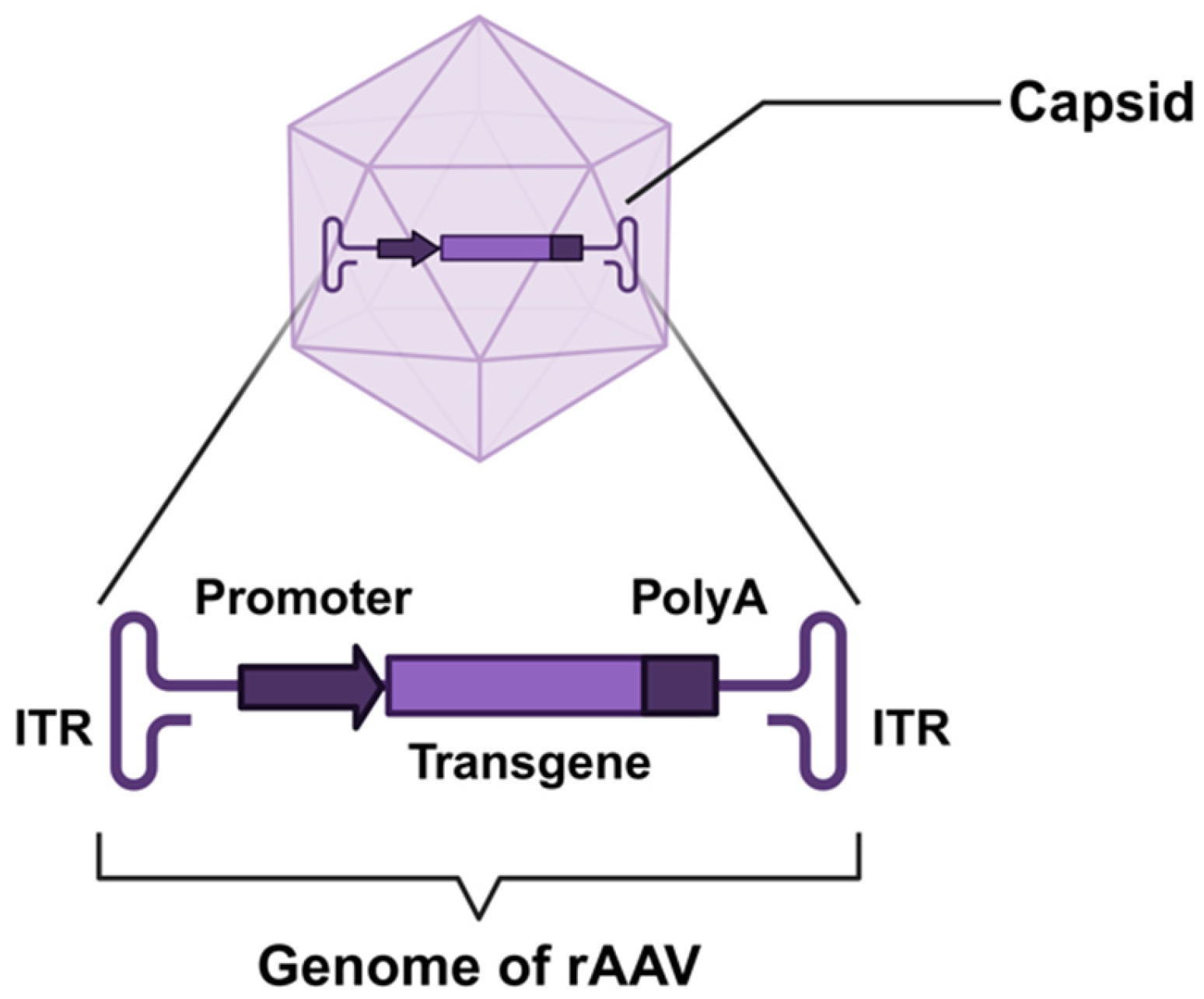
3. AAV Vector Development
3.1. Chemical Modification
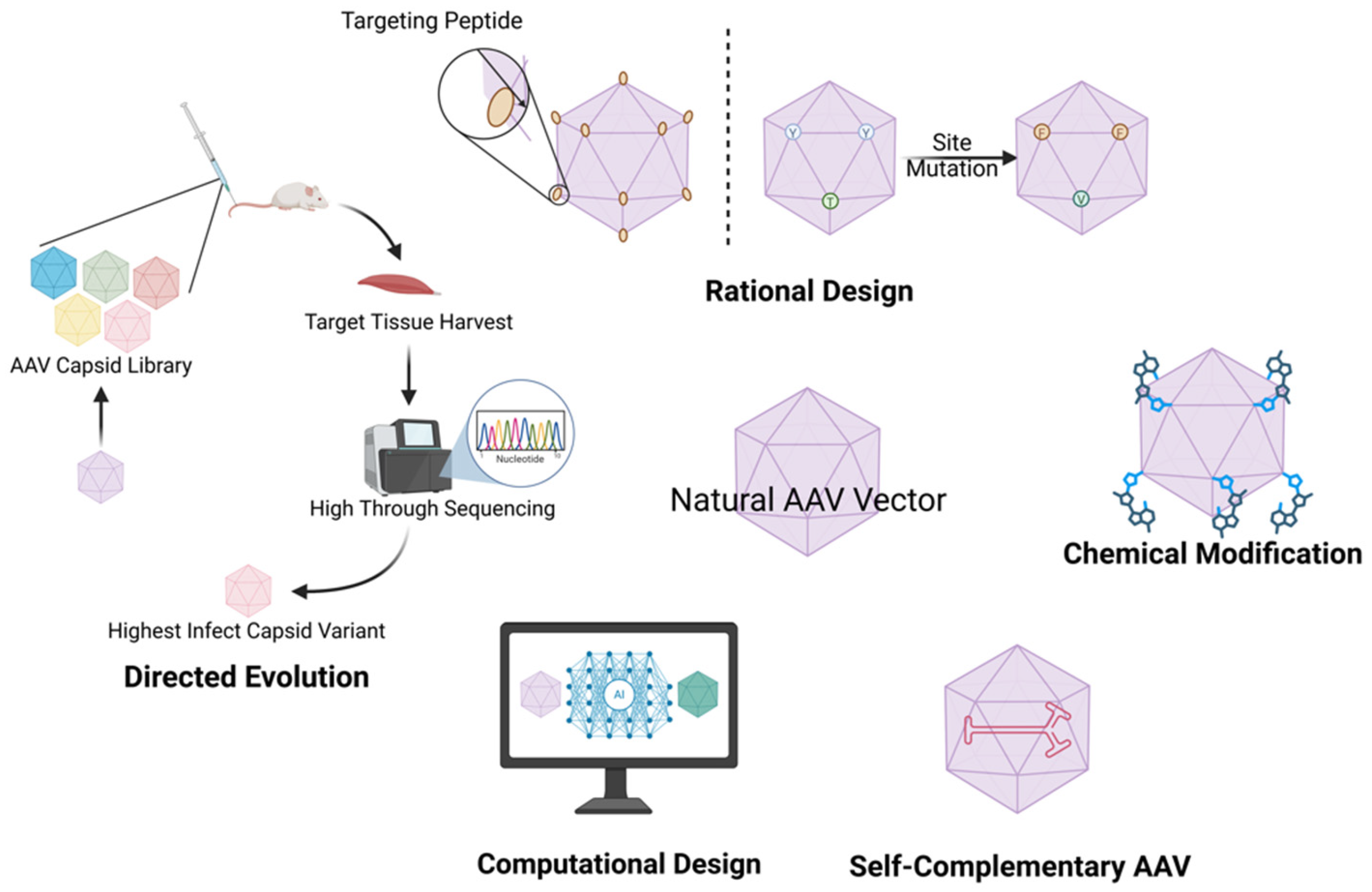
3.2. AAV Capsid Engineering
3.2.1. Rational Design
3.2.2. Directed Evolution
3.2.3. Computational Design
3.3. Self-Complementary AAV Vectors
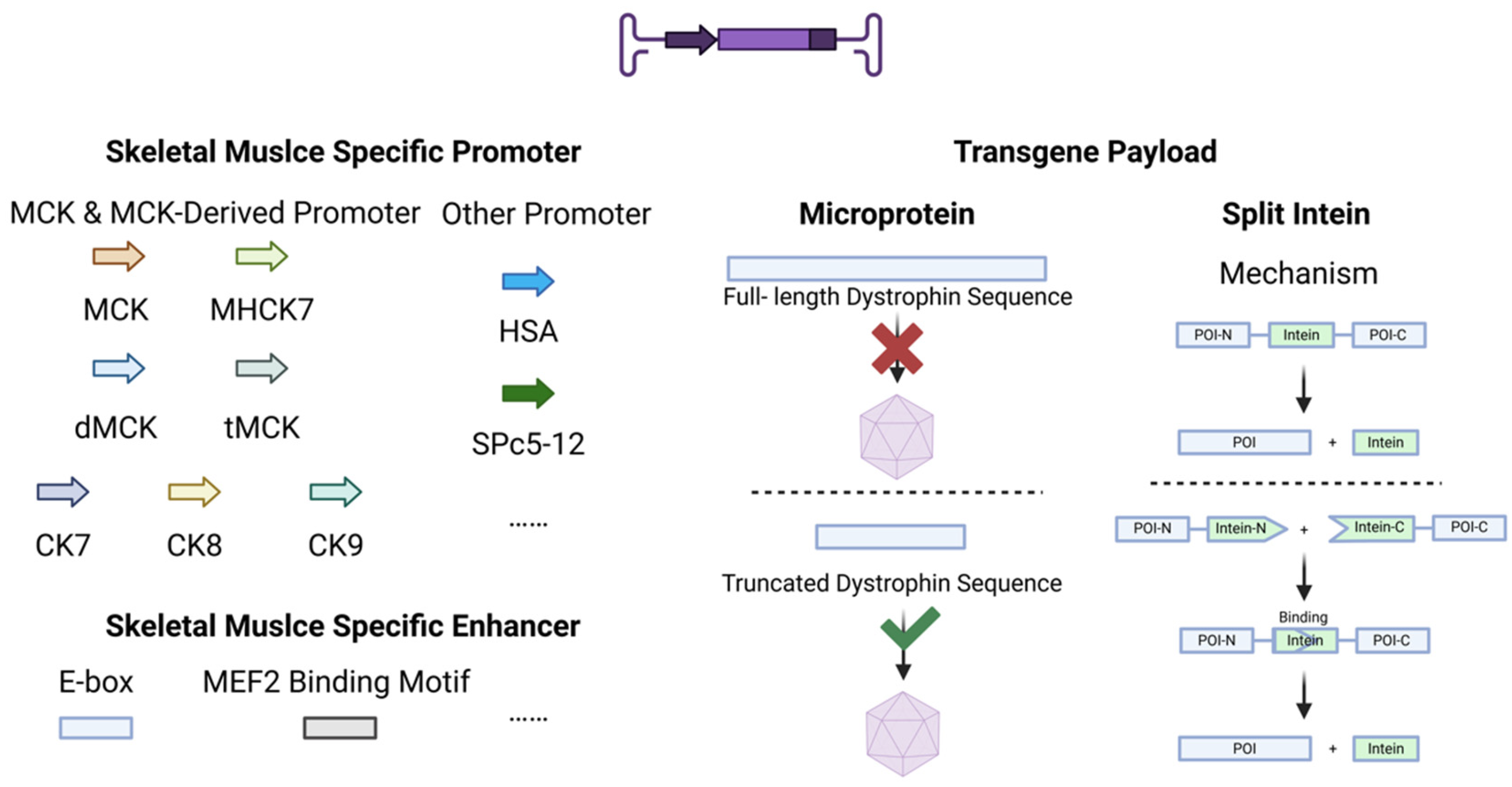
4. Genetic Cassette Engineering
4.1. Promoters
4.2. Enhancers
4.3. Transgene Payload
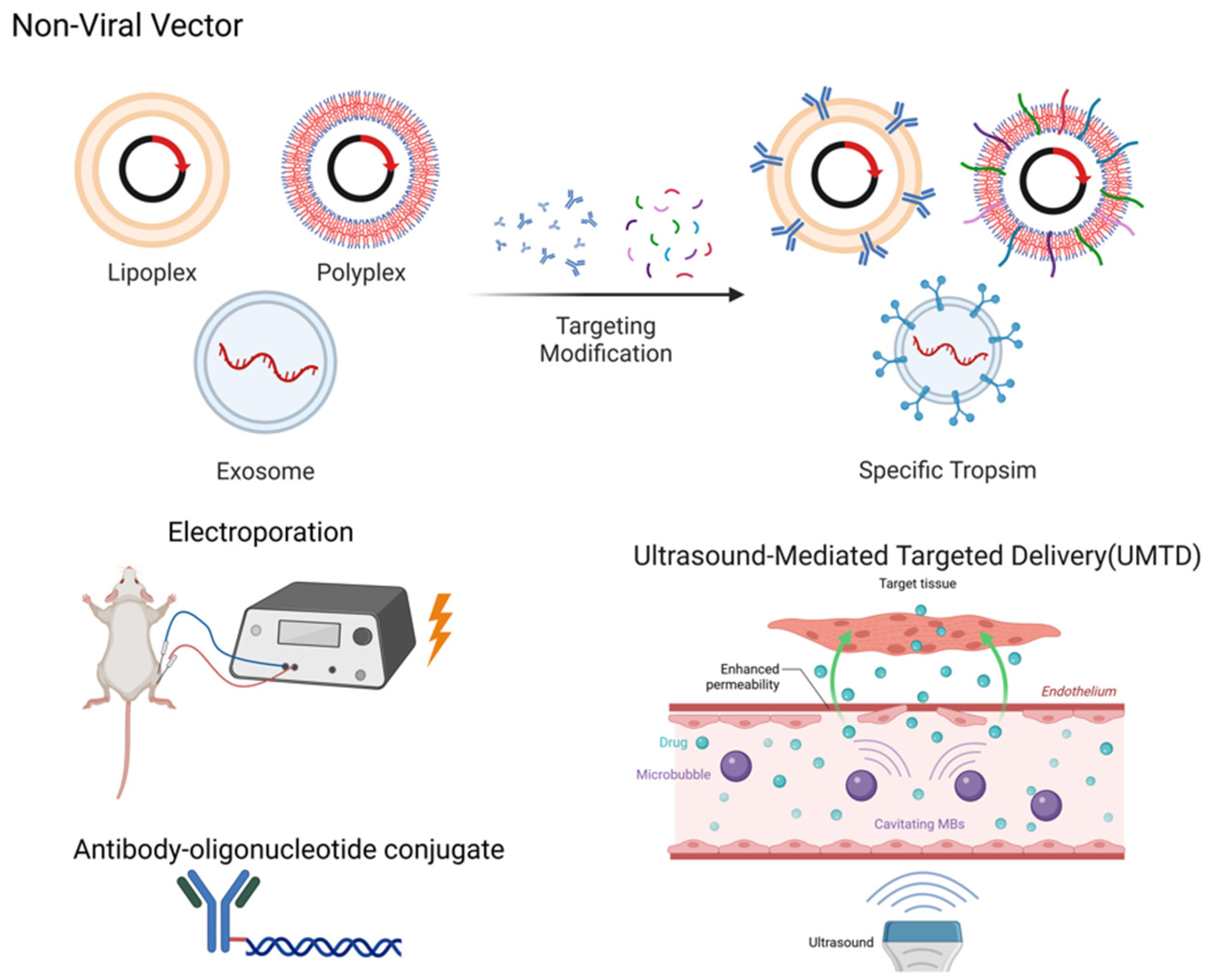
5. Non-Viral Vectors
5.1. Liposomal Vectors
5.2. Polymeric Vectors
5.3. Extracellular Vesicles/Exosomes
5.4. Physical Enhancement of Delivery
5.5. Antibody–Oligonucleotide Conjugate
6. Clinical Application
6.1. Gene Therapy Strategies
6.2. Clinical Implementation Scenarios
6.3. Cardiac Tropism in Gene Therapy
6.4. Comparison of AAV and Non-Viral Vectors
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yoshimoto, Y.; Oishi, Y. Mechanisms of skeletal muscle-tendon development and regeneration/healing as potential therapeutic targets. Pharmacol. Ther. 2023, 243, 108357. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Q.; Kim, Y.-R.; Gaddam, R.R.; Jacobs, J.S.; Bachschmid, M.M.; Younis, T.; Zhu, Z.; Zingman, L.; London, B.; et al. Deficiency of endothelial sirtuin1 in mice stimulates skeletal muscle insulin sensitivity by modifying the secretome. Nat. Commun. 2023, 14, 5595. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Ohashi, K.; Otaka, N.; Kawanishi, H.; Takikawa, T.; Fang, L.; Takahara, K.; Tatsumi, M.; Ishihama, S.; Takefuji, M.; et al. Myonectin protects against skeletal muscle dysfunction in male mice through activation of AMPK/PGC1α pathway. Nat. Commun. 2023, 14, 4675. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Bilan, P.J.; Klip, A. A Comprehensive View of Muscle Glucose Uptake: Regulation by Insulin, Contractile Activity and Exercise. Physiol. Rev. 2025, 105, 1867–1945. [Google Scholar] [CrossRef]
- Miranda-Cervantes, A.; Fritzen, A.M.; Raun, S.H.; Hodek, O.; Møller, L.L.V.; Johann, K.; Deisen, L.; Gregorevic, P.; Gudiksen, A.; Artati, A.; et al. Pantothenate kinase 4 controls skeletal muscle substrate metabolism. Nat. Commun. 2025, 16, 345. [Google Scholar] [CrossRef]
- Dumitras, A.G.; Piccoli, G.; Tellkamp, F.; Keufgens, L.; Baraldo, M.; Zorzato, S.; Cussonneau, L.; Nogara, L.; Krüger, M.; Blaauw, B. Neural stimulation suppresses mTORC1-mediated protein synthesis in skeletal muscle. Sci. Adv. 2025, 11, eadt4955. [Google Scholar] [CrossRef]
- Engel, A.G.; Shen, X.-M.; Selcen, D.; Sine, S.M. Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015, 14, 420–434. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef]
- Tihaya, M.S.; Mul, K.; Balog, J.; de Greef, J.C.; Tapscott, S.J.; Tawil, R.; Statland, J.M.; van der Maarel, S.M. Facioscapulohumeral muscular dystrophy: The road to targeted therapies. Nat. Rev. Neurol. 2023, 19, 91–108. [Google Scholar] [CrossRef]
- Bouchard, C.; Tremblay, J.P. Limb–girdle muscular dystrophies classification and therapies. J. Clin. Med. 2023, 12, 4769. [Google Scholar] [CrossRef]
- van der Ploeg, A.T.; Reuser, A.J.; Soltanzadeh, P. Myotonic dystrophies: A genetic overview. Genes 2008, 13, 367. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, M.; Chen, D.; Yang, J.; Zhang, Y.; Sun, J.; Yao, X.; Sun, H.; Gu, X.; Li, M.; et al. Congenital myopathies: Pathophysiological mechanisms and promising therapies. J. Transl. Med. 2024, 22, 815. [Google Scholar] [CrossRef] [PubMed]
- Sugie, K.; Nishino, I. History and perspective of LAMP-2 deficiency (danon disease). Biomolecules 2024, 14, 1272. [Google Scholar] [CrossRef] [PubMed]
- van der Ploeg, A.T.; Reuser, A.J.J. Pompe’s disease. Genes 2008, 372, 1342–1353. [Google Scholar] [CrossRef]
- Llavero, F.; Arrazola Sastre, A.; Luque Montoro, M.; Gálvez, P.; Lacerda, H.M.; Parada, L.A.; Zugaza, J.L.; Zhang, H.; Chang, M.; Chen, D.; et al. McArdle disease: New insights into its underlying molecular mechanisms. Int. J. Mol. Sci. 2019, 20, 5919. [Google Scholar] [CrossRef]
- Abrigo, J.; Simon, F.; Cabrera, D.; Vilos, C.; Cabello-Verrugio, C. Mitochondrial Dysfunction in Skeletal Muscle Pathologies. Curr. Protein Pept. Sci. 2019, 20, 536–546. [Google Scholar] [CrossRef]
- Azibani, F.; Muchir, A.; Vignier, N.; Bonne, G.; Bertrand, A.T.; Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Phillips, L.; Trivedi, J.R. Skeletal muscle channelopathies. Neurotherapeutics 2018, 15, 954–965. [Google Scholar] [CrossRef]
- Azibani, F.; Muchir, A.; Vignier, N.; Bonne, G.; Bertrand, A.T. Striated muscle laminopathies. Semin. Cell Dev. Biol. 2014, 29, 107–115. [Google Scholar] [CrossRef]
- Wang, D.; Stevens, G.; Flotte, T.R. Gene therapy then and now: A look back at changes in the field over the past 25 years. Mol. Ther. 2025, 33, 1889–1902. [Google Scholar] [CrossRef]
- Hoy, S.M. Delandistrogene Moxeparvovec: First Approval. Drugs 2023, 83, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Duan, D. Systemic AAV Micro-dystrophin Gene Therapy for Duchenne Muscular Dystrophy. Mol. Ther. 2018, 26, 2337–2356. [Google Scholar] [CrossRef] [PubMed]
- Komaki, H.; Nagata, T.; Saito, T.; Masuda, S.; Takeshita, E.; Sasaki, M.; Tachimori, H.; Nakamura, H.; Aoki, Y.; Takeda, S. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci. Transl. Med. 2018, 10, eaan0713. [Google Scholar] [CrossRef]
- Tasfaout, H.; Buono, S.; Guo, S.; Kretz, C.; Messaddeq, N.; Booten, S.; Greenlee, S.; Monia, B.P.; Cowling, B.S.; Laporte, J. Antisense oligonucleotide-mediated Dnm2 knockdown prevents and reverts myotubular myopathy in mice. Nat. Commun. 2017, 8, 15661. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Bengtsson, N.E.; Tasfaout, H.; Chamberlain, J.S. The road toward AAV-mediated gene therapy of Duchenne muscular dystrophy. Mol. Ther. 2025, 33, 2035–2051. [Google Scholar] [CrossRef]
- Costa Verdera, H.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef]
- Michele, D.E. Mechanisms of skeletal muscle repair and regeneration in health and disease. FEBS J. 2022, 289, 6460–6462. [Google Scholar] [CrossRef]
- Bachman, J.F.; Chakkalakal, J.V. Chapter one—Satellite cells in the growth and maintenance of muscle. In Current Topics in Developmental Biology; Rudnicki, M.A., Dilworth, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 158, pp. 1–14. [Google Scholar]
- Sun, C.; Swoboda, C.O.; Morales, F.M.; Calvo, C.; Petrany, M.J.; Parameswaran, S.; VonHandorf, A.; Weirauch, M.T.; Lepper, C.; Millay, D.P. Lineage tracing of nuclei in skeletal myofibers uncovers distinct transcripts and interplay between myonuclear populations. Nat. Commun. 2024, 15, 9372. [Google Scholar] [CrossRef]
- Meyer, N.L.; Chapman, M.S. Adeno-associated virus (AAV) cell entry: Structural insights. Trends Microbiol. 2022, 30, 432–451. [Google Scholar] [CrossRef]
- Wu, Z.; Asokan, A.; Samulski, R.J. Adeno-associated Virus Serotypes: Vector Toolkit for Human Gene Therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2006, 14, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Amaran, L.; Song, L.; Tretiakova, A.P.; Mikhail, S.A.; Samulski, R.J. AAV vector development, back to the future. Mol. Ther. 2025, 33, 1903–1936. [Google Scholar] [CrossRef] [PubMed]
- Pupo, A.; Fernández, A.; Low, S.H.; François, A.; Suárez-Amarán, L.; Samulski, R.J. AAV vectors: The Rubik’s cube of human gene therapy. Mol. Ther. 2022, 30, 3515–3541. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.; Glicksman, J.; Chatterjee, A. Chemical approaches to probe and engineer AAV vectors. Nanoscale 2024, 16, 13820–13833. [Google Scholar] [CrossRef]
- Mulcrone, P.L.; Lam, A.K.; Frabutt, D.; Zhang, J.; Chrzanowski, M.; Herzog, R.W.; Xiao, W. Chemical modification of AAV9 capsid with N-ethyl maleimide alters vector tissue tropism. Sci. Rep. 2023, 13, 8436. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, B.; Gu, W.; Luozhong, S.; Li, R.; Jiang, S. Mitigating the immunogenicity of AAV-mediated gene therapy with an immunosuppressive phosphoserine-containing zwitterionic peptide. J. Am. Chem. Soc. 2022, 144, 20507–20513. [Google Scholar] [CrossRef]
- Joo, K.-I.; Fang, Y.; Liu, Y.; Xiao, L.; Gu, Z.; Tai, A.; Lee, C.-L.; Tang, Y.; Wang, P. Enhanced Real-Time Monitoring of Adeno-Associated Virus Trafficking by Virus–Quantum Dot Conjugates. ACS Nano 2011, 5, 3523–3535. [Google Scholar] [CrossRef]
- Puzzo, F.; Zhang, C.; Powell Gray, B.; Zhang, F.; Sullenger, B.A.; Kay, M.A. Aptamer-programmable adeno-associated viral vectors as a novel platform for cell-specific gene transfer. Mol. Ther. Nucleic Acids 2023, 31, 383–397. [Google Scholar] [CrossRef]
- Horowitz, E.D.; Weinberg, M.S.; Asokan, A. Glycated AAV Vectors: Chemical Redirection of Viral Tissue Tropism. Bioconjug. Chem. 2011, 22, 529–532. [Google Scholar] [CrossRef]
- Pinto, M.S.-L.; Martí-Melero, L.; Fernandez-Alarcon, J.; Sitia, G.; Fornaguera, C.; Borrós, S.; Guerra-Rebollo, M. Polymer-based coating of adeno-associated viral particles as a new strategy to evade immune response for DMD treatment. J. Control. Release 2025, 384, 113896. [Google Scholar] [CrossRef]
- Milagros, S.; de Erenchun, P.R.-R.; Guembe, M.; Carte, B.; Méndez, M.; Uribarri, A.; Aldabe, R. The infectivity of AAV9 is influenced by the specific location and extent of chemically modified capsid residues. J. Biol. Eng. 2024, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Dhungel, B.P.; Winburn, I.; da Fonseca Pereira, C.; Huang, K.; Chhabra, A.; Rasko, J.E.J. Understanding AAV vector immunogenicity: From particle to patient. Theranostics 2024, 14, 1260–1288. [Google Scholar] [CrossRef] [PubMed]
- Vu Hong, A.; Suel, L.; Petat, E.; Dubois, A.; Le Brun, P.-R.; Guerchet, N.; Veron, P.; Poupiot, J.; Richard, I. An engineered AAV targeting integrin alpha V beta 6 presents improved myotropism across species. Nat. Commun. 2024, 15, 7965. [Google Scholar] [CrossRef]
- Zhong, L.; Li, B.; Mah, C.S.; Govindasamy, L.; Agbandje-McKenna, M.; Cooper, M.; Herzog, R.W.; Zolotukhin, I.; Warrington, K.H.; Weigel-Van Aken, K.A.; et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. USA 2008, 105, 7827–7832. [Google Scholar] [CrossRef] [PubMed]
- Shoti, J.; Qing, K.; Keeler, G.D.; Duan, D.; Byrne, B.J.; Srivastava, A. Development of capsid- and genome-modified optimized AAVrh74 vectors for muscle gene therapy. Mol. Ther. Methods Clin. Dev. 2023, 31, 101147. [Google Scholar] [CrossRef]
- Bönnemann, C.G. Designer AAV muscle up. Cell 2021, 184, 4845–4847. [Google Scholar] [CrossRef]
- Pulicherla, N.; Shen, S.; Yadav, S.; Debbink, K.; Govindasamy, L.; Agbandje-McKenna, M.; Asokan, A. Engineering Liver-detargeted AAV9 Vectors for Cardiac and Musculoskeletal Gene Transfer. Mol. Ther. 2011, 19, 1070–1078. [Google Scholar] [CrossRef]
- Weinmann, J.; Weis, S.; Sippel, J.; Tulalamba, W.; Remes, A.; El Andari, J.; Herrmann, A.-K.; Pham, Q.H.; Borowski, C.; Hille, S.; et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat. Commun. 2020, 11, 5432. [Google Scholar] [CrossRef]
- Tabebordbar, M.; Lagerborg, K.A.; Stanton, A.; King, E.M.; Ye, S.; Tellez, L.; Krunnfusz, A.; Tavakoli, S.; Widrick, J.J.; Messemer, K.A.; et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 2021, 184, 4919–4938.e22. [Google Scholar] [CrossRef]
- El Andari, J.; Renaud-Gabardos, E.; Tulalamba, W.; Weinmann, J.; Mangin, L.; Pham, Q.H.; Hille, S.; Bennett, A.; Attebi, E.; Bourges, E.; et al. Semirational bioengineering of AAV vectors with increased potency and specificity for systemic gene therapy of muscle disorders. Sci. Adv. 2022, 8, eabn4704. [Google Scholar] [CrossRef]
- Shay, T.F.; Jang, S.; Brittain, T.J.; Chen, X.; Walker, B.; Tebbutt, C.; Fan, Y.; Wolfe, D.A.; Arokiaraj, C.M.; Sullivan, E.E.; et al. Human cell surface-AAV interactomes identify LRP6 as blood-brain barrier transcytosis receptor and immune cytokine IL3 as AAV9 binder. Nat. Commun. 2024, 15, 7853. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, B.J.; Johnston, K.E.; Wu, Z.; Arnold, F.H. Advances in machine learning for directed evolution. Curr. Opin. Struct. Biol. 2021, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nisanov, A.M.; Rivera de Jesús, J.A.; Schaffer, D.V. Advances in AAV capsid engineering: Integrating rational design, directed evolution and machine learning. Mol. Ther. 2025, 33, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Dong, Y.; Qi, J.; Yu, W.; Chai, R. Artificial intelligence-based approaches for AAV vector engineering. Adv. Sci. 2025, 12, 2411062. [Google Scholar] [CrossRef]
- Han, Z.; Luo, N.; Wang, F.; Cai, Y.; Yang, X.; Feng, W.; Zhu, Z.; Wang, J.; Wu, Y.; Ye, C.; et al. Computer-Aided Directed Evolution Generates Novel AAV Variants with High Transduction Efficiency. Viruses 2023, 15, 848. [Google Scholar] [CrossRef]
- Zhu, D.; Brookes, D.H.; Busia, A.; Carneiro, A.; Fannjiang, C.; Popova, G.; Shin, D.; Donohue, K.C.; Lin, L.F.; Miller, Z.M.; et al. Optimal trade-off control in machine learning–based library design, with application to adeno-associated virus (AAV) for gene therapy. Sci. Adv. 2024, 10, eadj3786. [Google Scholar] [CrossRef]
- Eid, F.-E.; Chen, A.T.; Chan, K.Y.; Huang, Q.; Zheng, Q.; Tobey, I.G.; Pacouret, S.; Brauer, P.P.; Keyes, C.; Powell, M.; et al. Systematic multi-trait AAV capsid engineering for efficient gene delivery. Nat. Commun. 2024, 15, 6602. [Google Scholar] [CrossRef]
- Bryant, D.H.; Bashir, A.; Sinai, S.; Jain, N.K.; Ogden, P.J.; Riley, P.F.; Church, G.M.; Colwell, L.J.; Kelsic, E.D. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 2021, 39, 691–696. [Google Scholar] [CrossRef]
- McCarty, D.M. Self-complementary AAV Vectors; Advances and Applications. Mol. Ther. 2008, 16, 1648–1656. [Google Scholar] [CrossRef]
- McCarty, D.M.; Fu, H.; Monahan, P.E.; Toulson, C.E.; Naik, P.; Samulski, R.J. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003, 10, 2112–2118. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Min, Y.-L.; Sanchez-Ortiz, E.; Huang, J.; Mireault, A.A.; Shelton, J.M.; Kim, J.; Mammen, P.P.A.; Bassel-Duby, R.; et al. Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci. Adv. 2020, 6, eaay6812. [Google Scholar] [CrossRef] [PubMed]
- Rashnonejad, A.; Amini Chermahini, G.; Gündüz, C.; Onay, H.; Aykut, A.; Durmaz, B.; Baka, M.; Su, Q.; Gao, G.; Özkınay, F. Fetal Gene Therapy Using a Single Injection of Recombinant AAV9 Rescued SMA Phenotype in Mice. Mol. Ther. 2019, 27, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, E.R.; Griffin, D.A.; Heller, K.N.; Mendell, J.R.; Rodino-Klapac, L.R. Systemic AAV-Mediated β-Sarcoglycan Delivery Targeting Cardiac and Skeletal Muscle Ameliorates Histological and Functional Deficits in LGMD2E Mice. Mol. Ther. 2017, 25, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Zhang, P.; Wang, Y.; Liu, Y.; Ji, B.; Zhan, P.; Song, J. Investigation and Regulation of DNA Nanostructures on Activating cGAS-STING Signaling. Small Methods 2025, 9, 2401041. [Google Scholar] [CrossRef]
- Fougerousse, F.; Bartoli, M.; Poupiot, J.; Arandel, L.; Durand, M.; Guerchet, N.; Gicquel, E.; Danos, O.; Richard, I. Phenotypic Correction of α-Sarcoglycan Deficiency by Intra-arterial Injection of a Muscle-specific Serotype 1 rAAV Vector. Mol. Ther. 2007, 15, 53–61. [Google Scholar] [CrossRef]
- Wang, R.; Kumar, B.; Doud, E.H.; Mosley, A.L.; Alexander, M.S.; Kunkel, L.M.; Nakshatri, H. Skeletal muscle-specific overexpression of miR-486 limits mammary tumor-induced skeletal muscle functional limitations. Mol. Ther. Nucleic Acids 2022, 28, 231–248. [Google Scholar] [CrossRef]
- Xu, R.; Jia, Y.; Zygmunt, D.A.; Martin, P.T. rAAVrh74.MCK.GALGT2 Protects against Loss of Hemodynamic Function in the Aging mdx Mouse Heart. Mol. Ther. 2019, 27, 636–649. [Google Scholar] [CrossRef]
- Salva, M.Z.; Himeda, C.L.; Tai, P.W.; Nishiuchi, E.; Gregorevic, P.; Allen, J.M.; Finn, E.E.; Nguyen, Q.G.; Blankinship, M.J.; Meuse, L.; et al. Design of Tissue-specific Regulatory Cassettes for High-level rAAV-mediated Expression in Skeletal and Cardiac Muscle. Mol. Ther. 2007, 15, 320–329. [Google Scholar] [CrossRef]
- Willcocks, R.J.; Forbes, S.C.; Walter, G.A.; Sweeney, L.; Rodino-Klapac, L.R.; Mendell, J.R.; Vandenborne, K. Assessment of rAAVrh.74.MHCK7.micro-dystrophin Gene Therapy Using Magnetic Resonance Imaging in Children With Duchenne Muscular Dystrophy. JAMA Netw. Open 2021, 4, e2031851. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Fu, F.H.; Chen, C.; Zhu, X.; Zhou, L.; Jiang, X.; Xiao, X. Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther. 2008, 15, 1489–1499. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Crick, H.R.; Chaytow, H.; van der Hoorn, D.; Alhindi, A.; Jones, R.A.; Hector, R.D.; Cobb, S.R.; Gillingwater, T.H. Long-term muscle-specific overexpression of DOK7 in mice using AAV9-tMCK-DOK7. Mol. Ther. Nucleic Acids 2023, 33, 617–628. [Google Scholar] [CrossRef]
- Himeda, C.L.; Chen, X.; Hauschka, S.D. Design and testing of regulatory cassettes for optimal activity in skeletal and cardiac muscles. In Muscle Gene Therapy: Methods and Protocols; Duan, D., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 3–19. ISBN 978-1-61737-982-6. [Google Scholar]
- Ramos, J.N.; Hollinger, K.; Bengtsson, N.E.; Allen, J.M.; Hauschka, S.D.; Chamberlain, J.S. Development of Novel Micro-dystrophins with Enhanced Functionality. Mol. Ther. 2019, 27, 623–635. [Google Scholar] [CrossRef]
- Brennan, K.J.; Hardeman, E.C. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J. Biol. Chem. 1993, 268, 719–725. [Google Scholar] [CrossRef]
- Salyers, Z.R.; Coleman, M.; Le, D.; Ryan, T.E. AAV-mediated expression of PFKFB3 in myofibers, but not endothelial cells, improves ischemic muscle function in mice with critical limb ischemia. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H424–H436. [Google Scholar] [CrossRef]
- Li, X.; Eastman, E.M.; Schwartz, R.J.; Draghia-Akli, R. Synthetic muscle promoters: Activities exceeding naturally occurring regulatory sequences. Nat. Biotechnol. 1999, 17, 241–245. [Google Scholar] [CrossRef]
- Liu, M.; Cook, E.; Dai, Y.; Ehlert, E.; du Plessis, F.; Lubelski, J.; Sleczka, B.G.; Shipkova, P.; Li, Z.; Gamse, J.; et al. Systemic delivery of AAV5, AAV8, and AAV9 packaging a C5-12-microdystrophin-FLAG expression cassette in non-human primates. Mol. Ther. Methods Clin. Dev. 2025, 33, 101411. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Sandelin, A. Determinants of enhancer and promoter activities of regulatory elements. Nat. Rev. Genet. 2020, 21, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.D.; Nguyen, D.; Ramachandran, P.; Palidwor, G.A.; Porter, C.J.; Yin, H.; Perkins, T.J.; Rudnicki, M.A. Cis-regulatory determinants of MyoD function. Nucleic Acids Res. 2018, 46, 7221–7235. [Google Scholar] [CrossRef]
- Zia, A.; Imran, M.; Rashid, S. In Silico Exploration of Conformational Dynamics and Novel Inhibitors for Targeting MEF2-Associated Transcriptional Activity. J. Chem. Inf. Model. 2020, 60, 1892–1909. [Google Scholar] [CrossRef] [PubMed]
- Sarcar, S.; Tulalamba, W.; Rincon, M.Y.; Tipanee, J.; Pham, H.Q.; Evens, H.; Boon, D.; Samara-Kuko, E.; Keyaerts, M.; Loperfido, M.; et al. Next-generation muscle-directed gene therapy by in silico vector design. Nat. Commun. 2019, 10, 492. [Google Scholar] [CrossRef]
- Hart, C.C.; il Lee, Y.; Xie, J.; Gao, G.; Lin, B.L.; Hammers, D.W.; Sweeney, H.L. Potential limitations of microdystrophin gene therapy for Duchenne muscular dystrophy. JCI Insight 2024, 9, e165869. [Google Scholar] [CrossRef] [PubMed]
- Anastassov, S.; Filo, M.; Khammash, M. Inteins: A Swiss army knife for synthetic biology. Biotechnol. Adv. 2024, 73, 108349. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.J.; Sekar, G.; Gramespacher, J.A.; Cowburn, D.; Muir, T.W. An atypical mechanism of split intein molecular recognition and folding. J. Am. Chem. Soc. 2018, 140, 11791–11799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, C.; Xiao, W.; Herzog, R.W.; Han, R. Systemic delivery of full-length dystrophin in Duchenne muscular dystrophy mice. Nat. Commun. 2024, 15, 6141. [Google Scholar] [CrossRef]
- Tasfaout, H.; Halbert, C.L.; McMillen, T.S.; Allen, J.M.; Reyes, T.R.; Flint, G.V.; Grimm, D.; Hauschka, S.D.; Regnier, M.; Chamberlain, J.S. Split intein-mediated protein trans-splicing to express large dystrophins. Nature 2024, 632, 192–200. [Google Scholar] [CrossRef]
- Gapinske, M.; Winter, J.; Swami, D.; Gapinske, L.; Woods, W.S.; Shirguppe, S.; Miskalis, A.; Busza, A.; Joulani, D.; Kao, C.J.; et al. Targeting duchenne muscular dystrophy by skipping DMD exon 45 with base editors. Mol. Ther. Nucleic Acids 2023, 33, 572–586. [Google Scholar] [CrossRef]
- Chemello, F.; Chai, A.C.; Li, H.; Rodriguez-Caycedo, C.; Sanchez-Ortiz, E.; Atmanli, A.; Mireault, A.A.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 2021, 7, eabg4910. [Google Scholar] [CrossRef]
- Moretti, A.; Fonteyne, L.; Giesert, F.; Hoppmann, P.; Meier, A.B.; Bozoglu, T.; Baehr, A.; Schneider, C.M.; Sinnecker, D.; Klett, K.; et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat. Med. 2020, 26, 207–214. [Google Scholar] [CrossRef]
- Sameer Khan, M.; Gupta, G.; Alsayari, A.; Wahab, S.; Sahebkar, A.; Kesharwani, P. Advancements in liposomal formulations: A comprehensive exploration of industrial production techniques. Int. J. Pharm. 2024, 658, 124212. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Abri Aghdam, M.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef]
- Zaleski, M.H.; Omo-Lamai, S.; Nong, J.; Chase, L.S.; Myerson, J.W.; Glassman, P.M.; Lee, F.; Reyes-Esteves, S.; Wang, Z.; Patel, M.N.; et al. Nanocarriers’ repartitioning of drugs between blood subcompartments as a mechanism of improving pharmacokinetics, safety, and efficacy. J. Control. Release 2024, 374, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Hayashi, Y.; Kimura, Y.; Sashida, S.; Hamano, N.; Nirasawa, K.; Hamada, K.; Katagiri, F.; Kikkawa, Y.; Sakai, T.; et al. Alpha-dystroglycan binding peptide A2G80-modified stealth liposomes as a muscle-targeting carrier for duchenne muscular dystrophy. J. Control. Release 2021, 329, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Z.; Jiang, L.; Bojan, N.; Sha, Y.; Huang, B.; Ming, L.; Shen, J.; Pang, W. Specific muscle targeted delivery of miR-130a loaded lipid nanoparticles: A novel approach to inhibit lipid accumulation in skeletal muscle and obesity. J. Nanobiotechnol. 2025, 23, 159. [Google Scholar] [CrossRef]
- Kenjo, E.; Hozumi, H.; Makita, Y.; Iwabuchi, K.A.; Fujimoto, N.; Matsumoto, S.; Kimura, M.; Amano, Y.; Ifuku, M.; Naoe, Y.; et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun. 2021, 12, 7101. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Polymeric nanocarriers for therapeutic gene delivery. Asian J. Pharm. Sci. 2025, 20, 101015. [Google Scholar] [CrossRef]
- Cohen, S.A.; Bar-Am, O.; Fuoco, C.; Saar, G.; Gargioli, C.; Seliktar, D. In vivo restoration of dystrophin expression in mdx mice using intra-muscular and intra-arterial injections of hydrogel microsphere carriers of exon skipping antisense oligonucleotides. Cell Death Dis. 2022, 13, 779. [Google Scholar] [CrossRef]
- Chiper, M.; Tounsi, N.; Kole, R.; Kichler, A.; Zuber, G. Self-aggregating 1.8 kDa polyethylenimines with dissolution switch at endosomal acidic pH are delivery carriers for plasmid DNA, mRNA, siRNA and exon-skipping oligonucleotides. J. Control. Release 2017, 246, 60–70. [Google Scholar] [CrossRef]
- Hicks, M.R.; Liu, X.; Young, C.S.; Saleh, K.; Ji, Y.; Jiang, J.; Emami, M.R.; Mokhonova, E.; Spencer, M.J.; Meng, H.; et al. Nanoparticles systemically biodistribute to regenerating skeletal muscle in DMD. J. Nanobiotechnol. 2023, 21, 303. [Google Scholar] [CrossRef]
- Wan, R.; Liu, S.; Feng, X.; Luo, W.; Zhang, H.; Wu, Y.; Chen, S.; Shang, X. The Revolution of exosomes: From biological functions to therapeutic applications in skeletal muscle diseases. J. Orthop. Transl. 2024, 45, 132–139. [Google Scholar] [CrossRef]
- Dong, X.; Gao, X.; Dai, Y.; Ran, N.; Yin, H. Serum exosomes can restore cellular function in vitro and be used for diagnosis in dysferlinopathy. Theranostics 2018, 8, 1243–1255. [Google Scholar] [CrossRef]
- Gao, X.; Ran, N.; Dong, X.; Zuo, B.; Yang, R.; Zhou, Q.; Moulton, H.M.; Seow, Y.; Yin, H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018, 10, eaat0195. [Google Scholar] [CrossRef]
- Leng, L.; Dong, X.; Gao, X.; Ran, N.; Geng, M.; Zuo, B.; Wu, Y.; Li, W.; Yan, H.; Han, G.; et al. Exosome-mediated improvement in membrane integrity and muscle function in dystrophic mice. Mol. Ther. 2021, 29, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, Y.; Zhong, L.; Wang, B.; Qiu, S.; Song, J.; Lin, C.; Zou, F.; Wu, J.; Yu, H.; et al. Generalizable anchor aptamer strategy for loading nucleic acid therapeutics on exosomes. EMBO Mol. Med. 2024, 16, 1027–1045. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Shen, Y.; Jin, Y.; Weintraub, N.L.; Tang, Y. Identification of critical molecular pathways involved in exosome-mediated improvement of cardiac function in a mouse model of muscular dystrophy. Acta Pharmacol. Sin. 2021, 42, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef]
- Li, X.; La Salvia, S.; Liang, Y.; Adamiak, M.; Kohlbrenner, E.; Jeong, D.; Chepurko, E.; Ceholski, D.; Lopez-Gordo, E.; Yoon, S.; et al. Extracellular vesicle–encapsulated adeno-associated viruses for therapeutic gene delivery to the heart. Circulation 2023, 148, 405–425. [Google Scholar] [CrossRef]
- Hughes, D.C.; Hardee, J.P.; Waddell, D.S.; Goodman, C.A. CORP: Gene delivery into murine skeletal muscle using in vivo electroporation. J. Appl. Physiol. 2022, 133, 41–59. [Google Scholar] [CrossRef]
- Sokołowska, E.; Błachnio-Zabielska, A.U. A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2776. [Google Scholar] [CrossRef]
- Hughes, D.C.; Marcotte, G.R.; Baehr, L.M.; West, D.W.D.; Marshall, A.G.; Ebert, S.M.; Davidyan, A.; Adams, C.M.; Bodine, S.C.; Baar, K. Alterations in the muscle force transfer apparatus in aged rats during unloading and reloading: Impact of microRNA-31. J. Physiol. 2018, 596, 2883–2900. [Google Scholar] [CrossRef]
- Rizo-Roca, D.; Guimarães, D.S.P.S.F.; Pendergrast, L.A.; Di Leo, N.; Chibalin, A.V.; Maqdasy, S.; Rydén, M.; Näslund, E.; Zierath, J.R.; Krook, A. Decreased mitochondrial creatine kinase 2 impairs skeletal muscle mitochondrial function independently of insulin in type 2 diabetes. Sci. Transl. Med. 2024, 16, eado3022. [Google Scholar] [CrossRef]
- Fornelli, C.; Beltrà, M.; Zorzano, A.; Costelli, P.; Sebastian, D.; Penna, F. BNIP3 Downregulation Ameliorates Muscle Atrophy in Cancer Cachexia. Cancers 2024, 16, 4133. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Marcotte, G.R.; Baehr, L.M.; West, D.W.D.; Marshall, A.G.; Ebert, S.M.; Davidyan, A.; Adams, C.M.; Bodine, S.C.; Baar, K.; et al. Electroporation of Plasmid DNA into Mouse Skeletal Muscle. J. Vis. Exp. 2022, 182, e63916. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.; Graca, F.; Hunt, L.; Demontis, F. Electroporation of Small Interfering RNAs into Tibialis Anterior Muscles of Mice. BIO-Protocol 2022, 12, e4428. [Google Scholar] [CrossRef] [PubMed]
- Sales Conniff, A.; Tur, J.; Kohena, K.; Zhang, M.; Gibbons, J.; Heller, L. Transcriptomic analysis of the acute skeletal muscle effects after intramuscular DNA electroporation reveals inflammatory signaling. Vaccines 2022, 10, 2037. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Z.; Tan, H.; Hou, J.; Wen, X.; Yang, F.; Cheng, W. New Aspects of Ultrasound-Mediated Targeted Delivery and Therapy for Cancer. Int. J. Nanomed. 2020, 15, 401–418. [Google Scholar] [CrossRef]
- Xiang, X.; Leng, Q.; Tang, Y.; Wang, L.; Huang, J.; Zhang, Y.; Qiu, L. Ultrasound-targeted microbubble destruction delivery of insulin-like growth factor 1 cDNA and transforming growth factor beta short hairpin RNA enhances tendon regeneration and inhibits scar formation in vivo. Hum. Gene Ther. Clin. Dev. 2018, 29, 198–213. [Google Scholar] [CrossRef]
- Sekine, S.; Mayama, S.; Nishijima, N.; Kojima, T.; Endo-Takahashi, Y.; Ishii, Y.; Shiono, H.; Akiyama, S.; Sakurai, A.; Sashida, S.; et al. Development of a Gene and Nucleic Acid Delivery System for Skeletal Muscle Administration via Limb Perfusion Using Nanobubbles and Ultrasound. Pharmaceutics 2023, 15, 1665. [Google Scholar] [CrossRef]
- Chen, S.; Sun, S.; Moonen, D.; Lee, C.; Lee, A.Y.-F.; Schaffer, D.V.; He, L. CRISPR-READI: Efficient Generation of Knockin Mice by CRISPR RNP Electroporation and AAV Donor Infection. Cell Rep. 2019, 27, 3780–3789.e4. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Sun, X.; Sun, X.; Yang, G.; Shi, X. Exosome-mediated regulatory mechanisms in skeletal muscle: A narrative review. J. Zhejiang Univ.-Sci. B 2023, 24, 1–14. [Google Scholar] [CrossRef]
- Verma, N.; Tamang, R.; Mehata, A.K.; Setia, A.; Koch, B.; Muthu, M.S. Exosomes fused liposomes: Formulation, stability studies and theranostic evaluation for breast cancer applications. Int. J. Pharm. 2025, 682, 125907. [Google Scholar] [CrossRef]
- Sun, W.; Li, Z.; Zhou, X.; Yang, G.; Yuan, L. Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Deliv. 2019, 26, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Malecova, B.; Burke, R.S.; Cochran, M.; Hood, M.D.; Johns, R.; Kovach, P.R.; Doppalapudi, V.R.; Erdogan, G.; Arias, J.D.; Darimont, B.; et al. Targeted tissue delivery of RNA therapeutics using antibody–oligonucleotide conjugates (AOCs). Nucleic Acids Res. 2023, 51, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Sugo, T.; Terada, M.; Oikawa, T.; Miyata, K.; Nishimura, S.; Kenjo, E.; Ogasawara-Shimizu, M.; Makita, Y.; Imaichi, S.; Murata, S.; et al. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J. Control. Release 2016, 237, 1–13. [Google Scholar] [CrossRef]
- Liu, F.; Li, R.; Zhu, Z.; Yang, Y.; Lu, F. Current developments of gene therapy in human diseases. MedComm 2024, 5, e645. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Onasemnogene Abeparvovec: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 995–1005. [Google Scholar] [CrossRef]
- Song, Y.; Morales, L.; Malik, A.S.; Mead, A.F.; Greer, C.D.; Mitchell, M.A.; Petrov, M.T.; Su, L.T.; Choi, M.E.; Rosenblum, S.T.; et al. Non-immunogenic utrophin gene therapy for the treatment of muscular dystrophy animal models. Nat. Med. 2019, 25, 1505–1511. [Google Scholar] [CrossRef]
- Erkut, E.; Yokota, T. CRISPR Therapeutics for Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2022, 23, 1832. [Google Scholar] [CrossRef]
- Lin, J.; Jin, M.; Yang, D.; Li, Z.; Zhang, Y.; Xiao, Q.; Wang, Y.; Yu, Y.; Zhang, X.; Shao, Z.; et al. Adenine base editing-mediated exon skipping restores dystrophin in humanized Duchenne mouse model. Nat. Commun. 2024, 15, 5927. [Google Scholar] [CrossRef]
- Ryu, S.-M.; Koo, T.; Kim, K.; Lim, K.; Baek, G.; Kim, S.-T.; Kim, H.S.; Kim, D.; Lee, H.; Chung, E.; et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 2018, 36, 536–539. [Google Scholar] [CrossRef]
- Jin, M.; Lin, J.; Li, H.; Li, Z.; Yang, D.; Wang, Y.; Yu, Y.; Shao, Z.; Chen, L.; Wang, Z.; et al. Correction of human nonsense mutation via adenine base editing for Duchenne muscular dystrophy treatment in mouse. Mol. Ther. Nucleic Acids 2024, 35, 102165. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gough, V.; Bohning, J.D.; Liu, S.; Robinson-Hamm, J.N.; Daniels, H.; Majoros, W.H.; Devlin, G.; Asokan, A.; Gersbach, C.A. Full-length dystrophin restoration via targeted exon integration by AAV-CRISPR in a humanized mouse model of Duchenne muscular dystrophy. Mol. Ther. 2021, 29, 3243–3257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, D.; Ma, K.; Luo, H.; Mao, J.; Luo, J.; Shen, Q.; Xu, L.; Yang, S.; Ge, L.; et al. Lama1 upregulation prolongs the lifespan of the dyH/dyH mouse model of LAMA2-related congenital muscular dystrophy. J. Genet. Genom. 2024, 51, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.; Conner, H.; Groneman, M.; Blavo, C.; Parmar, M.S. Duchenne muscular dystrophy: Current treatment and emerging exon skipping and gene therapy approach. Eur. J. Pharmacol. 2023, 947, 175675. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Swayze, E.E. RNA targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef]
- Heo, Y.-A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Mendell, J.R.; Muntoni, F.; McDonald, C.M.; Mercuri, E.M.; Ciafaloni, E.; Komaki, H.; Leon-Astudillo, C.; Nascimento, A.; Proud, C.; Schara-Schmidt, U.; et al. AAV gene therapy for Duchenne muscular dystrophy: The EMBARK phase 3 randomized trial. Nat. Med. 2025, 31, 332–341. [Google Scholar] [CrossRef]
- Pascual-Morena, C.; Patiño-Cardona, S.; Martínez-García, I.; Fernández-Bravo-Rodrigo, J.; Garrido-Miguel, M. Efficacy of delandistrogene moxeparvovec on Duchenne muscular dystrophy: A systematic review and meta-analysis. Hum. Genet. 2025, 20, 1–13. [Google Scholar] [CrossRef]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, A.M.C.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Peña, L.D.M.; Shieh, P.B.; et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): An open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-F.; Chen, J.-A.; Jong, Y.-J. Treating neuromuscular diseases: Unveiling gene therapy breakthroughs and pioneering future applications. J. Biomed. Sci. 2025, 32, 30. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Catahay, J.A.; Macasaet, R.; Liu, J.; Velasco, J.V.; Peligro, P.J.; Vallo, J.; Goldrich, N.; Lahoti, L.; Zhou, J.; et al. Infusion reactions to adeno-associated virus (AAV)-based gene therapy: Mechanisms, diagnostics, treatment and review of the literature. J. Med. Virol. 2023, 95, e29305. [Google Scholar] [CrossRef] [PubMed]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 658399. [Google Scholar] [CrossRef]
- Duan, D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol. Ther. 2023, 31, 3123–3126. [Google Scholar] [CrossRef]
- Wilton-Clark, H.; Yokota, T. Safety concerns surrounding AAV and CRISPR therapies in neuromuscular treatment. Med 2023, 4, 855–856. [Google Scholar] [CrossRef]
- Wang, X.; Klann, P.J.; Wiedtke, E.; Sano, Y.; Fischer, N.; Schiller, L.; Elfert, A.; Güttsches, A.-K.; Weyen, U.; Grimm, D.; et al. Seroprevalence of binding and neutralizing antibodies against 18 adeno-associated virus types in patients with neuromuscular disorders. Front. Immunol. 2024, 15, 1450858. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- Torres-Masjoan, L.; Aguti, S.; Zhou, H.; Muntoni, F. Clinical applications of exon-skipping antisense oligonucleotides in neuromuscular diseases. Mol. Ther. 2025, 33, 2689–2704. [Google Scholar] [CrossRef]
- Goemans, N.; Mercuri, E.; Belousova, E.; Komaki, H.; Dubrovsky, A.; McDonald, C.M.; Kraus, J.E.; Lourbakos, A.; Lin, Z.; Campion, G.; et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul. Disord. 2018, 28, 4–15. [Google Scholar] [CrossRef]
- Servais, L.; Mercuri, E.; Straub, V.; Guglieri, M.; Seferian, A.M.; Scoto, M.; Leone, D.; Koenig, E.; Khan, N.; Dugar, A.; et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with duchenne muscular dystrophy amenable to exon 53 skipping: A first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther. 2022, 32, 29–39. [Google Scholar] [CrossRef]
- Datson, N.A.; Bijl, S.; Janson, A.; Testerink, J.; van den Eijnde, R.; Weij, R.; Puoliväli, J.; Lehtimäki, K.; Bragge, T.; Ahtoniemi, T.; et al. Using a state-of-the-art toolbox to evaluate molecular and functional readouts of antisense oligonucleotide-induced exon skipping in mdx mice. Nucleic Acid Ther. 2020, 30, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, F.; Domingos, J.; Manzur, A.Y.; Mayhew, A.; Guglieri, M.; Sajeev, G.; Signorovitch, J.; Ward, S.J. Categorising trajectories and individual item changes of the North Star Ambulatory Assessment in patients with Duchenne muscular dystrophy. PLoS ONE 2019, 14, e0221097. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, D.; Pang, L.; Liu, S. Extracellular vesicles for cancer therapy: Potential, progress, and clinical challenges. Front. Bioeng. Biotechnol. 2024, 12, 1476737. [Google Scholar] [CrossRef] [PubMed]
- Sani, F.; Shafiei, F.; Dehghani, F.; Mohammadi, Y.; Khorraminejad-Shirazi, M.; Anvari-Yazdi, A.F.; Moayedfard, Z.; Azarpira, N.; Sani, M. Unveiling exosomes: Cutting-edge isolation techniques and their therapeutic potential. J. Cell. Mol. Med. 2024, 28, e70139. [Google Scholar] [CrossRef]
- Musunuru, K.; Grandinette, S.A.; Wang, X.; Hudson, T.R.; Briseno, K.; Berry, A.M.; Hacker, J.L.; Hsu, A.; Silverstein, R.A.; Hille, L.T.; et al. Patient-specific in vivo gene editing to treat a rare genetic disease. N. Engl. J. Med. 2025, 392, 2235–2243. [Google Scholar] [CrossRef]
- Kamdar, F.; Garry, D.J. Dystrophin-deficient cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef]
- Garg, A.; Jansen, S.; Greenberg, L.; Zhang, R.; Lavine, K.J.; Greenberg, M.J. Dilated cardiomyopathy–associated skeletal muscle actin (ACTA1) mutation R256H disrupts actin structure and function and causes cardiomyocyte hypocontractility. Proc. Natl. Acad. Sci. USA 2024, 121, e2405020121. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Hsu, E.; Pinckard, K.M.; Stanford, K.I.; Han, R. Systemic AAV9.BVES delivery ameliorates muscular dystrophy in a mouse model of LGMDR25. Mol. Ther. 2023, 31, 398–408. [Google Scholar] [CrossRef]
- Switala, L.; Di, L.; Gao, H.; Asase, C.; Klos, M.; Rengasamy, P.; Fedyukina, D.; Maiseyeu, A. Engineered nanoparticles promote cardiac tropism of AAV vectors. J. Nanobiotechnol. 2024, 22, 223. [Google Scholar] [CrossRef]
- Tasfaout, H.; McMillen, T.S.; Reyes, T.R.; Halbert, C.L.; Tian, R.; Regnier, M.; Chamberlain, J.S. Expression of full-length dystrophin reverses muscular dystrophy defects in young and old mdx4cv mice. J. Clin. Investig. 2025, 135, e189075. [Google Scholar] [CrossRef]
- Muraine, L.; Bensalah, M.; Dhiab, J.; Cordova, G.; Arandel, L.; Marhic, A.; Chapart, M.; Vasseur, S.; Benkhelifa-Ziyyat, S.; Bigot, A.; et al. Transduction efficiency of adeno-associated virus serotypes after local injection in mouse and human skeletal muscle. Hum. Gene Ther. 2020, 31, 233–240. [Google Scholar] [CrossRef]
- Kessler, P.D.; Podsakoff, G.M.; Chen, X.; McQuiston, S.A.; Colosi, P.C.; Matelis, L.A.; Kurtzman, G.J.; Byrne, B.J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 1996, 93, 14082–14087. [Google Scholar] [CrossRef]
- Jackson, C.B.; Richard, A.S.; Ojha, A.; Conkright, K.A.; Trimarchi, J.M.; Bailey, C.C.; Alpert, M.D.; Kay, M.A.; Farzan, M.; Choe, H. AAV vectors engineered to target insulin receptor greatly enhance intramuscular gene delivery. Mol. Ther. Methods Clin. Dev. 2020, 19, 496–506. [Google Scholar] [CrossRef]
- Zaiss, A.; Muruve, D. Immune Responses to Adeno-Associated Virus Vectors. Curr. Gene Ther. 2005, 5, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.M.; Zhan, W.; Ram, S.; Fitzgerald, K.A.; Gao, G. The curious case of AAV immunology. Mol. Ther. 2025, 33, 1946–1965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Dalby, P.A. Challenges in scaling up AAV-based gene therapy manufacturing. Trends Biotechnol. 2023, 41, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Guyer, T.; Moore, A.C.; Stevens, M.M.; Guldberg, R.E.; Bursac, N. Translating musculoskeletal bioengineering into tissue regeneration therapies. Sci. Transl. Med. 2022, 14, eabn9074. [Google Scholar] [CrossRef]
| Therapy Type | Drug Name | Target Disease | Mechanism | Status |
|---|---|---|---|---|
| Gene Replacement | Zolgensma® (onasemnogene abeparvovec) | Spinal muscular atrophy (SMA) | AAV9-delivered SMN1 gene | Full approval (2019) |
| Elevidys® (delandistrogene moxeparvovec) | Duchenne muscular dystrophy (DMD) | AAVrh74-delivered microdystrophin | Traditional approval (2024) | |
| Exon Skipping | Eteplirsen | DMD (exon 51 amenable) | PMO-ASO skipping exon 51 | Accelerated approval (2016) |
| Golodirsen | DMD (exon 53 amenable) | PMO-ASO skipping exon 53 | Accelerated approval (2019) | |
| Viltolarsen | DMD (exon 53 amenable) | PMO-ASO skipping exon 53 | Accelerated approval (2020) | |
| Casimersen | DMD (exon 45 amenable) | PMO-ASO skipping exon 45 | Accelerated approval (2021) |
| AAV Vector | Non-Viral Vector | |
|---|---|---|
| Transduction efficiency | High | Low |
| Duration of transgene expression | Sustained expression | Transient expression |
| Immunogenicity | Prone to eliciting | Less likely to induce |
| Scalability of manufacturing | Higher manufacturing complexity | Streamlined production processes |
| Development complexity | Poses significant development challenges | Amenable to rapid development |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Hua, Y.; Zheng, Y.; Liu, X.; Li, Y. Engineering Targeted Gene Delivery Systems for Primary Hereditary Skeletal Myopathies: Current Strategies and Future Perspectives. Biomedicines 2025, 13, 1994. https://doi.org/10.3390/biomedicines13081994
Wu J, Hua Y, Zheng Y, Liu X, Li Y. Engineering Targeted Gene Delivery Systems for Primary Hereditary Skeletal Myopathies: Current Strategies and Future Perspectives. Biomedicines. 2025; 13(8):1994. https://doi.org/10.3390/biomedicines13081994
Chicago/Turabian StyleWu, Jiahao, Yimin Hua, Yanjiang Zheng, Xu Liu, and Yifei Li. 2025. "Engineering Targeted Gene Delivery Systems for Primary Hereditary Skeletal Myopathies: Current Strategies and Future Perspectives" Biomedicines 13, no. 8: 1994. https://doi.org/10.3390/biomedicines13081994
APA StyleWu, J., Hua, Y., Zheng, Y., Liu, X., & Li, Y. (2025). Engineering Targeted Gene Delivery Systems for Primary Hereditary Skeletal Myopathies: Current Strategies and Future Perspectives. Biomedicines, 13(8), 1994. https://doi.org/10.3390/biomedicines13081994






