Therapeutic Opportunities in Melanoma Through PRAME Expression
Abstract
1. Introduction
2. Background
2.1. Tumor-Associated Antigens (TAAs)
2.2. Cancer-Testis Antigens (CTAs)
2.3. Preferentially Expressed Antigen in Melanoma (PRAME)
3. PRAME Expression and Its Clinical Implications
| Neoplasm Type | Cohort Size | Expression Rate | Relevance | Reference |
|---|---|---|---|---|
| AML | >2000 AML patients (children and young adults) | 30% (pediatric leukemias) | - The practicality and therapeutic impact of using next-generation PRAME-specific mTCRCAR T cells | [41] |
| 50 (40 AL patients and 10 healthy individuals) | PRAME mRNA: 80% in AL and 20% in controls | - Potentially useful as a marker for tracking MRD - Its presence has been associated with unfavorable outcomes in AML patients | [42] | |
| 204 AML patients, 22 healthy controls | 4.01% (AML patients) | - Increasing PRAME levels over time predicted clinical relapses, highlighting its value as a dynamic MRD monitoring biomarker | [43] | |
| Breast cancer | 220 cases | 24.1% | - PRAME is more frequently expressed in HER2+ and triple-negative breast cancers and may serve as an immunotherapy target - However, it is not an independent prognostic factor | [45] |
| Ovarian cancer | 119 cases of EOC, 17 healthy ovaries | ~60% of primary EOCs | - PRAME is often expressed in EOC and HGSC, supporting its potential as an immunotherapy target, possibly enhanced by epigenetic agents like decitabine | [46] |
| Neuroblastoma | 94 primary neuroblastoma patients | 93% in primary neuroblastoma (and 100% in advanced disease) | - PRAME expression significantly influences neuroblastoma outcomes, making it a promising target for immunotherapy | [49] |
| Sarcoma | 93 myxoid liposarcoma samples, 46 dedifferentiated liposarcoma samples, 32 well-differentiated liposarcoma samples, and 14 pleomorphic liposarcomas samples | 90% in myxoid liposarcomas, 43% in dedifferentiated liposarcomas, 9% in well-differentiated liposarcomas, and 50% in pleomorphic liposarcomas | - High PRAME levels in sarcomas are linked to aggressive features like larger tumors, necrosis, and higher grade - These associations point to PRAME’s potential role as a prognostic biomarker in specific sarcoma subtypes | [51] |
| NSCLC | 377 specimens | 49.9% | - PRAME expression in NSCLC correlates with factors like smoking status and tumor histology - Its prognostic value is uncertain, as no clear link to OS has been found | [53] |
| NMSCs | 42 BCCs | 62% | - PRAME’s limited specificity as an IHC marker for NMSCs reduces its utility in diagnostic surgical pathology | [36] |
| 27 MCCs | 30% | - IHC offers a reliable and cost-effective way to identify PRAME-positive cancers for potential immunotherapy | [36] | |
| Melanoma | 155 primary melanomas | 83.2% | - PRAME IHC may aid in confirming a suspected melanoma diagnosis - It could also help assess surgical margins in known PRAME-positive melanomas - PRAME expression in benign skin lesions poses diagnostic challenges, requiring further study | [22] |
| Metastatic melanoma | 100 metastatic melanomas | 92% | - Widespread PRAME expression in metastatic melanoma supports its potential as an immunotherapy target | [22] |
| Mucosal melanoma | 29 mucosal melanoma cases | 83.3% | - High PRAME expression in mucosal melanomas suggests it could be a promising target for future therapies | [56] |
| Acral melanoma | 10 acral melanomas | 100% in acral melanoma | - Strong PRAME expression is a highly sensitive and specific diagnostic marker for acral melanomas, outperforming p16 IHC | [57] |
| Ocular melanoma | 389 UM patients | 84–100% | - PRAME independently predicts metastasis risk in uveal melanoma, improving prognostic testing and precision care | [58] |
| 40 (30 invasive conjunctival melanoma samples and 10 in situ conjunctival melanoma samples) | 57% in invasive melanoma samples and 70% in situ melanoma samples | - Diffuse 4+ PRAME staining is highly specific for malignant conjunctival melanocytic lesions, aiding in melanoma diagnosis | [59] |
4. PRAME as a Diagnostic and Prognostic Biomarker in Melanocytic Tumors
5. Advancing Melanoma Diagnosis with PRAME Immunohistochemistry
6. PRAME in Spitzoid Melanocytic Lesions
7. Immunotherapy and the Emerging Role of PRAME
7.1. PRAME as a Target in the Development of T-Cell Immunotherapies
7.2. New Cancer Vaccines Based on PRAME as a Tumor Antigen
8. Solving Key Barriers in PRAME-Directed Immunotherapy
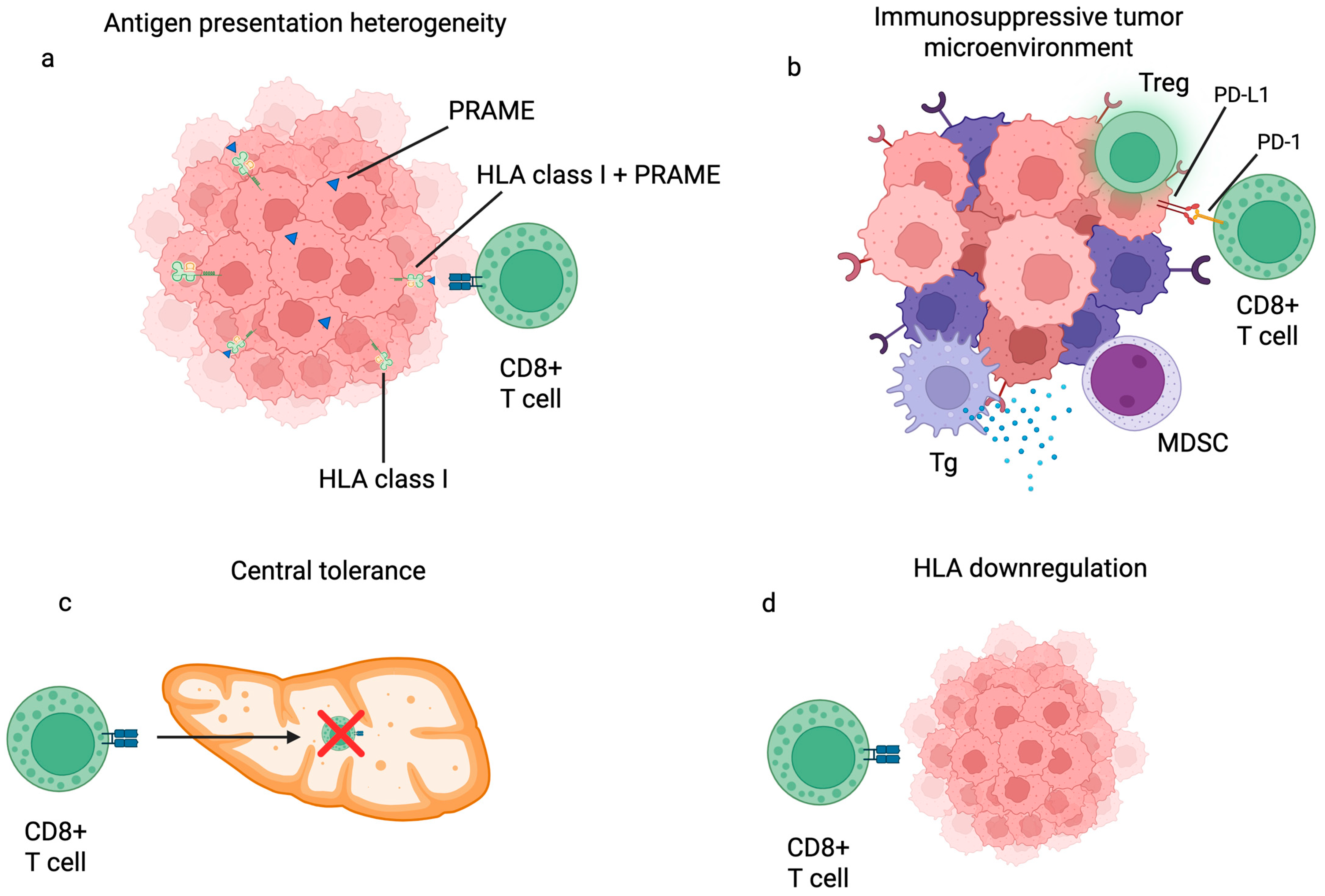
9. Conclusions
Funding
Conflicts of Interest
References
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; De Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495. [Google Scholar] [CrossRef]
- NIH. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Melanoma of the Skin. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 2 August 2025).
- Lam, G.T.; Prabhakaran, S.; Sorvina, A.; Martini, C.; Ung, B.S.-Y.; Karageorgos, L.; Hickey, S.M.; Lazniewska, J.; Johnson, I.R.D.; Williams, D.B.; et al. Pitfalls in Cutaneous Melanoma Diagnosis and the Need for New Reliable Markers. Mol. Diagn. Ther. 2023, 27, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ohsie, S.J.; Sarantopoulos, G.P.; Cochran, A.J.; Binder, S.W. Immunohistochemical Characteristics of Melanoma. J. Cutan. Pathol. 2008, 35, 433–444. [Google Scholar] [CrossRef]
- Eisenstein, A.; Gonzalez, E.C.; Raghunathan, R.; Xu, X.; Wu, M.; McLean, E.O.; McGee, J.; Ryu, B.; Alani, R.M. Emerging Biomarkers in Cutaneous Melanoma. Mol. Diagn. Ther. 2018, 22, 203–218. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Britton-Rivet, C.; Collins, L.; Carreira, R.J.; Moureau, S.; Benlahrech, A.; Stanhope, S.; Harper, S.; Liddy, N.; Mahon, T.M.; et al. High-Affinity T Cell Receptor ImmTAC® Bispecific Efficiently Redirects T Cells to Kill Tumor Cells Expressing the Cancer–Testis Antigen PRAME. Immunother. Adv. 2024, 4, ltae008. [Google Scholar] [CrossRef]
- Criscitiello, C. Tumor-Associated Antigens in Breast Cancer. Breast Care 2012, 7, 262–266. [Google Scholar] [CrossRef]
- Liu, C.-C.; Yang, H.; Zhang, R.; Zhao, J.-J.; Hao, D.-J. Tumour-Associated Antigens and Their Anti-Cancer Applications. Eur. J. Cancer Care 2017, 26, e12446. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Guddati, A.K. Carcinoembryonic Antigen, Carbohydrate Antigen 19-9, Cancer Antigen 125, Prostate-Specific Antigen and Other Cancer Markers: A Primer on Commonly Used Cancer Markers. World J. Oncol. 2023, 14, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Nasr, D.; Kumar, P.A.; Zerdan, M.B.; Ghelani, G.; Dutta, D.; Graziano, S.; Lim, S.H. Radioimmunoconjugates in the Age of Modern Immuno-Oncology. Life Sci. 2022, 310, 121126. [Google Scholar] [CrossRef]
- Parakh, S.; Lee, S.T.; Gan, H.K.; Scott, A.M. Radiolabeled Antibodies for Cancer Imaging and Therapy. Cancers 2022, 14, 1454. [Google Scholar] [CrossRef]
- Effer, B.; Perez, I.; Ulloa, D.; Mayer, C.; Muñoz, F.; Bustos, D.; Rojas, C.; Manterola, C.; Vergara-Gómez, L.; Dappolonnio, C.; et al. Therapeutic Targets of Monoclonal Antibodies Used in the Treatment of Cancer: Current and Emerging. Biomedicines 2023, 11, 2086. [Google Scholar] [CrossRef]
- Nin, D.S.; Deng, L.-W. Biology of Cancer-Testis Antigens and Their Therapeutic Implications in Cancer. Cells 2023, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, W.; Xia, T.; Liu, Y.; Bi, Z.; Guo, L.; Xie, W.; Xiang, Y.; Xu, Z.; Yu, Z.; et al. Diagnostic Value of Tumor-Associated Autoantibodies Panel in Combination with Traditional Tumor Markers for Lung Cancer. Front. Oncol. 2023, 13, 1022331. [Google Scholar] [CrossRef]
- Xie, K.; Fu, C.; Wang, S.; Xu, H.; Liu, S.; Shao, Y.; Gong, Z.; Wu, X.; Xu, B.; Han, J.; et al. Cancer-Testis Antigens in Ovarian Cancer: Implication for Biomarkers and Therapeutic Targets. J. Ovarian Res. 2019, 12, 1. [Google Scholar] [CrossRef]
- Gjerstorff, M.F.; Andersen, M.H.; Ditzel, H.J. Oncogenic Cancer/Testis Antigens: Prime Candidates for Immunotherapy. Oncotarget 2015, 6, 15772–15787. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, Z.; Li, M.; Wang, D.; Guo, R.; Fang, X.; Chen, F. Cancer Testis Antigen Subfamilies: Attractive Targets for Therapeutic Vaccine (Review). Int. J. Oncol. 2023, 62, 71. [Google Scholar] [CrossRef]
- Ai, H.; Yang, H.; Li, L.; Ma, J.; Liu, K.; Li, Z. Cancer/Testis Antigens: Promising Immunotherapy Targets for Digestive Tract Cancers. Front. Immunol. 2023, 14, 1190883. [Google Scholar] [CrossRef]
- Yang, P.; Meng, M.; Zhou, Q. Oncogenic Cancer/Testis Antigens Are a Hallmarker of Cancer and a Sensible Target for Cancer Immunotherapy. Biochim. Biophys. Acta BBA Rev. Cancer 2021, 1876, 188558. [Google Scholar] [CrossRef]
- Ikeda, H.; Lethé, B.; Lehmann, F.; Van Baren, N.; Baurain, J.-F.; De Smet, C.; Chambost, H.; Vitale, M.; Moretta, A.; Boon, T.; et al. Characterization of an Antigen That Is Recognized on a Melanoma Showing Partial HLA Loss by CTL Expressing an NK Inhibitory Receptor. Immunity 1997, 6, 199–208. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME Expression in Melanocytic Tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Al-Khadairi, G.; Decock, J. Cancer Testis Antigens and Immunotherapy: Where Do We Stand in the Targeting of PRAME? Cancers 2019, 11, 984. [Google Scholar] [CrossRef]
- Bose, M. Preferentially Expressed Antigen in Melanoma Is a Multifaceted Cancer Testis Antigen with Diverse Roles as a Biomarker and Therapeutic Target. Int. J. Transl. Med. 2023, 3, 334–359. [Google Scholar] [CrossRef]
- Dwivedi, R.; Mehrotra, D.; Chandra, S.; Pandey, R. A Systematic Review of Potential Immunotherapies TargetingPRAME in Retinoid Resistant Oral Potentially Malignant Disordersand Oral Cancer. Curr. Mol. Med. 2022, 22, 735–746. [Google Scholar] [CrossRef]
- Cassalia, F.; Danese, A.; Tudurachi, I.; Federico, S.; Zambello, A.; Guidotti, A.; Franceschin, L.; Bolzon, A.; Naldi, L.; Belloni Fortina, A. PRAME Updated: Diagnostic, Prognostic, and Therapeutic Role in Skin Cancer. Int. J. Mol. Sci. 2024, 25, 1582. [Google Scholar] [CrossRef]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef]
- Kurtenbach, S.; Sanchez, M.I.; Kuznetsoff, J.; Rodriguez, D.A.; Weich, N.; Dollar, J.J.; Cruz, A.; Kurtenbach, S.; Field, M.G.; Durante, M.A.; et al. PRAME Induces Genomic Instability in Uveal Melanoma. Oncogene 2024, 43, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Al-Khadairi, G.; Naik, A.; Thomas, R.; Al-Sulaiti, B.; Rizly, S.; Decock, J. PRAME Promotes Epithelial-to-Mesenchymal Transition in Triple Negative Breast Cancer. J. Transl. Med. 2019, 17, 9. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, M.; Zhou, S.; Chen, H.; Song, G.; Wu, Y.; Zhu, X. PRAME Promotes Cervical Cancer Proliferation and Migration via Wnt/β-Catenin Pathway Regulation. Cancers 2023, 15, 1801. [Google Scholar] [CrossRef]
- Li, J.; Zou, X.; Li, C.; Zhong, J.; Chen, Y.; Zhang, X.; Qi, F.; Li, M.; Cai, Z.; Tang, A. Expression of Novel Cancer/Testis Antigen TMEM31 Increases during Metastatic Melanoma Progression. Oncol. Lett. 2017, 13, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Blount, S.L.; Liu, X.; McBride, J.D. The Utilization of PRAME in the Diagnosis, Prognosis, and Treatment of Melanoma. Cells 2024, 13, 1740. [Google Scholar] [CrossRef]
- Naik, A.; Thomas, R.; Al-Khadairi, G.; Bacha, R.; Hendrickx, W.; Decock, J. Cancer Testis Antigen PRAME: An Anti-cancer Target with Immunomodulatory Potential. J. Cell. Mol. Med. 2021, 25, 10376–10388. [Google Scholar] [CrossRef]
- Xu, B.; Jungbluth, A.A.; Frosina, D.; Alzumaili, B.; Aleynick, N.; Slodkowska, E.; Higgins, K.; Ho, A.; Morris, L.; Ghossein, R.; et al. The Immune Microenvironment and Expression of PD-L1, PD-1, PRAME and MHC I in Salivary Duct Carcinoma. Histopathology 2019, 75, 672–682. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Alexis, J. PRAME Staining of Adnexal Lesions and Common Skin Cancer Types: Biomarker with Potential Diagnostic Utility. Dermatopathology 2024, 11, 364–373. [Google Scholar] [CrossRef]
- Kaczorowski, M.; Chłopek, M.; Kruczak, A.; Ryś, J.; Lasota, J.; Miettinen, M. PRAME Expression in Cancer. A Systematic Immunohistochemical Study of >5800 Epithelial and Nonepithelial Tumors. Am. J. Surg. Pathol. 2022, 46, 1467–1476. [Google Scholar] [CrossRef]
- Hedrich, V.; Breitenecker, K.; Ortmayr, G.; Pupp, F.; Huber, H.; Chen, D.; Sahoo, S.; Jolly, M.K.; Mikulits, W. PRAME Is a Novel Target of Tumor-Intrinsic Gas6/Axl Activation and Promotes Cancer Cell Invasion in Hepatocellular Carcinoma. Cancers 2023, 15, 2415. [Google Scholar] [CrossRef]
- Gelmi, M.C.; Gezgin, G.; Van Der Velden, P.A.; Luyten, G.P.M.; Luk, S.J.; Heemskerk, M.H.M.; Jager, M.J. PRAME Expression: A Target for Cancer Immunotherapy and a Prognostic Factor in Uveal Melanoma. Investig. Opthalmology Vis. Sci. 2023, 64, 36. [Google Scholar] [CrossRef]
- Kaczorowski, M.; Lasota, J.; Dudek, K.; Małkiewicz, B.; Miettinen, M.; Hałoń, A. Expression of Immunotherapy Target PRAME in Cancer Correlates with Histone H3 Acetylation and Is Unrelated to Expression of Methylating (DMNT3A/3B) and Demethylating (TET1) Enzymes. J. Clin. Med. 2024, 13, 1554. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, M.; Ye, J.; Ma, H. Targeting PRAME for Acute Myeloid Leukemia Therapy. Front. Immunol. 2024, 15, 1378277. [Google Scholar] [CrossRef]
- Kirkey, D.C.; Loeb, A.M.; Castro, S.; McKay, C.N.; Perkins, L.; Pardo, L.; Leonti, A.R.; Tang, T.T.; Loken, M.R.; Brodersen, L.E.; et al. Therapeutic Targeting of PRAME with mTCRCAR T Cells in Acute Myeloid Leukemia. Blood Adv. 2023, 7, 1178–1189. [Google Scholar] [CrossRef]
- Alyaqubi, K.J.; Al-Kraity, W.R.H.; Alfatlawi, J.H.; Taher, T.M.J. Assessment of mRNA Levels of Tumor Antigen (PRAME) and Clinical Outcomes in Newly Diagnosed Cases of Acute Leukemia. Al-Rafidain J. Med. Sci. 2025, 8, 195–200. [Google Scholar] [CrossRef]
- Qin, Y.; Zhu, H.; Jiang, B.; Li, J.; Lu, X.; Li, L.; Ruan, G.; Liu, Y.; Chen, S.; Huang, X. Expression Patterns of WT1 and PRAME in Acute Myeloid Leukemia Patients and Their Usefulness for Monitoring Minimal Residual Disease. Leuk. Res. 2009, 33, 384–390. [Google Scholar] [CrossRef]
- Asato, M.A.; Moraes Neto, F.A.; Moraes, M.P.D.T.; Ocanha-Xavier, J.P.; Takita, L.C.; Fung, M.A.; Marques, M.E.A.; Xavier-Júnior, J.C.C. The Utility of PRAME and Ki-67 as Prognostic Markers for Cutaneous Melanoma. Am. J. Dermatopathol. 2025, 47, 9–16. [Google Scholar] [CrossRef]
- Korša, L.; Abramović, M.; Kovačević, L.; Milošević, M.; Podolski, P.; Prutki, M.; Marušić, Z. PRAME Expression and Its Prognostic Significance in Invasive Breast Carcinoma. Pathol. Res. Pract. 2024, 254, 155096. [Google Scholar] [CrossRef]
- Zhang, W.; Barger, C.J.; Eng, K.H.; Klinkebiel, D.; Link, P.A.; Omilian, A.; Bshara, W.; Odunsi, K.; Karpf, A.R. PRAME Expression and Promoter Hypomethylation in Epithelial Ovarian Cancer. Oncotarget 2016, 7, 45352–45369. [Google Scholar] [CrossRef]
- Van Amerongen, R.A.; Tuit, S.; Wouters, A.K.; Van De Meent, M.; Siekman, S.L.; Meeuwsen, M.H.; Wachsmann, T.L.A.; Remst, D.F.G.; Hagedoorn, R.S.; Van Der Steen, D.M.; et al. PRAME and CTCFL-Reactive TCRs for the Treatment of Ovarian Cancer. Front. Immunol. 2023, 14, 1121973. [Google Scholar] [CrossRef]
- Epping, M.T.; Hart, A.A.M.; Glas, A.M.; Krijgsman, O.; Bernards, R. PRAME Expression and Clinical Outcome of Breast Cancer. Br. J. Cancer 2008, 99, 398–403. [Google Scholar] [CrossRef]
- Oberthuer, A.; Hero, B.; Spitz, R.; Berthold, F.; Fischer, M. The Tumor-Associated Antigen PRAME Is Universally Expressed in High-Stage Neuroblastoma and Associated with Poor Outcome. Clin. Cancer Res. 2004, 10, 4307–4313. [Google Scholar] [CrossRef]
- Touioui, S.; Desandes, E.; Jannot, L.; Mansuy, L.; Clabaut, D.; Peuchmaur, M.; Rioux-leclercq, N.; Khneisser, P.; Thiebaut, P.-A.; Gallo, M.; et al. Expression Evaluated by Digital Image Analysis Techniques of PRAME More than MCM6 Is Associated with Poor Prognosis in Neuroblastoma: A Pilot Study with 84 Cases. Hum. Pathol. 2025, 155, 105718. [Google Scholar] [CrossRef]
- Iura, K.; Kohashi, K.; Hotokebuchi, Y.; Ishii, T.; Maekawa, A.; Yamada, Y.; Yamamoto, H.; Iwamoto, Y.; Oda, Y. Cancer-testis Antigens PRAME and NY-ESO-1 Correlate with Tumour Grade and Poor Prognosis in Myxoid Liposarcoma. J. Pathol. Clin. Res. 2015, 1, 144–159. [Google Scholar] [CrossRef]
- Pan, S.; Su, K.; Spiessens, B.; Kusuma, N.; Delahaye, N.F.; Gruselle, O.; Myo, A.; De Creus, A.; Louahed, J.; Chang, G.; et al. Gene Expression of MAGE-A3 and PRAME Tumor Antigens and EGFR Mutational Status in Taiwanese Non–Small Cell Lung Cancer Patients. Asia Pac. J. Clin. Oncol. 2017, 13, e212–e223. [Google Scholar] [CrossRef]
- Thongprasert, S.; Yang, P.-C.; Lee, J.S.; Soo, R.; Gruselle, O.; Myo, A.; Louahed, J.; Lehmann, F.F.; Brichard, V.G.; Coche, T. The Prevalence of Expression of MAGE-A3 and PRAME Tumor Antigens in East and South East Asian Non-Small Cell Lung Cancer Patients. Lung Cancer 2016, 101, 137–144. [Google Scholar] [CrossRef]
- Elsensohn, A.; Hanson, J.; Ferringer, T. Preferentially Expressed Antigen in Melanoma Expression in Nonmelanoma Skin Cancers and Melanocytes in Surrounding Skin. J. Cutan. Pathol. 2021, 48, 1150–1155. [Google Scholar] [CrossRef]
- Miller, E.; Biesemier, A.; Coomes, D.M.; Raghavan, S.S. PRAME Expression in Merkel Cell Carcinoma. Am. J. Surg. Pathol. 2024, 48, 1270–1276. [Google Scholar] [CrossRef]
- Toyama, A.; Siegel, L.; Nelson, A.C.; Najmuddin, M.; Bu, L.; LaRue, R.; Henzler, C.; Caicedo-Granados, E.; Giubellino, A.; Li, F. Analyses of Molecular and Histopathologic Features and Expression of PRAME by Immunohistochemistry in Mucosal Melanomas. Mod. Pathol. 2019, 32, 1727–1733. [Google Scholar] [CrossRef]
- McBride, J.D.; McAfee, J.L.; Piliang, M.; Bergfeld, W.F.; Fernandez, A.P.; Ronen, S.; Billings, S.D.; Ko, J.S. Preferentially Expressed Antigen in Melanoma and P16 Expression in Acral Melanocytic Neoplasms. J. Cutan. Pathol. 2022, 49, 220–230. [Google Scholar] [CrossRef]
- Field, M.G.; Decatur, C.L.; Kurtenbach, S.; Gezgin, G.; Van Der Velden, P.A.; Jager, M.J.; Kozak, K.N.; Harbour, J.W. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin. Cancer Res. 2016, 22, 1234–1242. [Google Scholar] [CrossRef]
- Šekoranja, D.; Hawlina, G.; Pižem, J. PRAME Expression in Melanocytic Lesions of the Conjunctiva. Histopathology 2021, 79, 989–996. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Skin Tumours, 5th ed.; WHO Classification of Tumours Series; Beta Version Ahead of Print; International Agency for Research on Cancer: Lyon, France, 2023; Volume 12. Available online: https://Tumourclassification.Iarc.Who.Int/Chapters/64 (accessed on 2 August 2025).
- Da Silveira, H.G.; Junior, H.F.; De Souza Campos, L.D.; Stall, J.; Blasius, R.; Kricheski, C.; Silva, B.L.; De França, P.H.C.; De Paula Alves Coelho, K.M. PRAME Immunohistochemistry Distinguishes Nodal Nevi from Metastatic Melanoma. Surg. Exp. Pathol. 2024, 7, 28. [Google Scholar] [CrossRef]
- Gezgin, G.; Luk, S.J.; Cao, J.; Dogrusöz, M.; Van Der Steen, D.M.; Hagedoorn, R.S.; Krijgsman, D.; Van Der Velden, P.A.; Field, M.G.; Luyten, G.P.M.; et al. PRAME as a Potential Target for Immunotherapy in Metastatic Uveal Melanoma. JAMA Ophthalmol. 2017, 135, 541. [Google Scholar] [CrossRef]
- Vizcaino, M.A.; Dalvin, L.A.; Salomao, D.R. Correlation of PRAME Immunohistochemistry with PRAME Status on Gene Expression Profiling in Enucleated Uveal Melanoma. Can. J. Ophthalmol. 2024, 59, e279–e282. [Google Scholar] [CrossRef]
- Perez-Perez, M.; García De Sola-Llamas, C.; Mariscal, G.; Macías-García, L. Prognostic Value of PRAME Expression in Uveal Melanoma: A Meta-Analysis. J. Clin. Pathol. 2025, 78, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Zito Marino, F.; Toni, G.; Pagliuca, F.; Russo, D.; Signoriello, G.; Moscarella, E.; Brancaccio, G.; Argenziano, G.; Franco, R.; et al. Diagnostic Performance of Melanocytic Markers for Immunocytochemical Evaluation of Lymph-Node Melanoma Metastases on Cytological Samples. J. Clin. Pathol. 2022, 75, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Johnson, G.; Wang, J.; Cohen, C. SOX10: A Useful Marker for Identifying Metastatic Melanoma in Sentinel Lymph Nodes. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 109–112. [Google Scholar] [CrossRef]
- Saleem, A.; Narala, S.; Raghavan, S.S. Immunohistochemistry in Melanocytic Lesions: Updates with a Practical Review for Pathologists. Semin. Diagn. Pathol. 2022, 39, 239–247. [Google Scholar] [CrossRef]
- Mohamed, A.; Gonzalez, R.S.; Lawson, D.; Wang, J.; Cohen, C. SOX10 Expression in Malignant Melanoma, Carcinoma, and Normal Tissues. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 506–510. [Google Scholar] [CrossRef]
- Nonaka, D.; Chiriboga, L.; Rubin, B.P. Sox10: A Pan-Schwannian and Melanocytic Marker. Am. J. Surg. Pathol. 2008, 32, 1291–1298. [Google Scholar] [CrossRef]
- Pop, A.M.; Monea, M.; Olah, P.; Moraru, R.; Cotoi, O.S. The Importance of Immunohistochemistry in the Evaluation of Tumor Depth of Primary Cutaneous Melanoma. Diagnostics 2023, 13, 1020. [Google Scholar] [CrossRef]
- Rasic, D.; Korsgaard, N.; Marcussen, N.; Precht Jensen, E.M. Diagnostic Utility of Combining PRAME and HMB-45 Stains in Primary Melanocytic Tumors. Ann. Diagn. Pathol. 2023, 67, 152211. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Park, Y.; Moon, S.; Ahn, S.; Lee, K.; Park, H.J.; Lee, H.S.; Choe, G.; Lee, K.S. Clinicopathological and Prognostic Significance of Programmed Death Ligand 1 Expression in Korean Melanoma Patients. J. Cancer 2019, 10, 3070–3078. [Google Scholar] [CrossRef]
- Mandalà, M.; Merelli, B.; Massi, D. PD-L1 in Melanoma: Facts and Myths. Melanoma Manag. 2016, 3, 187–194. [Google Scholar] [CrossRef]
- Grossman, J.E.; Vasudevan, D.; Joyce, C.E.; Hildago, M. Is PD-L1 a Consistent Biomarker for Anti-PD-1 Therapy? The Model of Balstilimab in a Virally-Driven Tumor. Oncogene 2021, 40, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Sorroche, B.P.; Teixeira, R.D.J.; Pereira, C.A.D.; Santana, I.V.V.; Vujanovic, L.; Vazquez, V.D.L.; Arantes, L.M.R.B. PD-L1 Tumor Expression as a Predictive Biomarker of Immune Checkpoint Inhibitors’ Response and Survival in Advanced Melanoma Patients in Brazil. Diagnostics 2023, 13, 1041. [Google Scholar] [CrossRef]
- Kline, N.; Menge, T.D.; Hrycaj, S.M.; Andea, A.A.; Patel, R.M.; Harms, P.W.; Chan, M.P.; Bresler, S.C. PRAME Expression in Challenging Dermal Melanocytic Neoplasms and Soft Tissue Tumors with Melanocytic Differentiation. Am. J. Dermatopathol. 2022, 44, 404–410. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Oh, K.S.; Alexis, J. Potential Diagnostic Utility of PRAME and P16 Immunohistochemistry in Melanocytic Nevi and Malignant Melanoma. J. Cutan. Pathol. 2023, 50, 763–772. [Google Scholar] [CrossRef]
- Cazzato, G.; Cascardi, E.; Colagrande, A.; Belsito, V.; Lospalluti, L.; Foti, C.; Arezzo, F.; Dellino, M.; Casatta, N.; Lupo, C.; et al. PRAME Immunoexpression in 275 Cutaneous Melanocytic Lesions: A Double Institutional Experience. Diagnostics 2022, 12, 2197. [Google Scholar] [CrossRef]
- Ronchi, A.; Zito Marino, F.; Moscarella, E.; Brancaccio, G.; Argenziano, G.; Troiani, T.; Napolitano, S.; Franco, R.; Cozzolino, I. PRAME Immunocytochemistry for the Diagnosis of Melanoma Metastases in Cytological Samples. Diagnostics 2022, 12, 646. [Google Scholar] [CrossRef]
- Ricci, C.; Altavilla, M.V.; Corti, B.; Pasquini, E.; Presutti, L.; Baietti, A.M.; Amorosa, L.; Balbi, T.; Baldovini, C.; Ambrosi, F.; et al. PRAME Expression in Mucosal Melanoma of the Head and Neck Region. Am. J. Surg. Pathol. 2023, 47, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Santandrea, G.; Valli, R.; Zanetti, E.; Ragazzi, M.; Pampena, R.; Longo, C.; Lai, M.; Piana, S.; Cesinaro, A.M. Comparative Analysis of PRAME Expression in 127 Acral and Nail Melanocytic Lesions. Am. J. Surg. Pathol. 2022, 46, 579–590. [Google Scholar] [CrossRef]
- Hu, J.; Cai, X.; Lv, J.-J.; Wan, X.-C.; Zeng, X.-Y.; Feng, M.-L.; Dai, B.; Kong, Y.-Y. Preferentially Expressed Antigen in Melanoma Immunohistochemistry as an Adjunct for Differential Diagnosis in Acral Lentiginous Melanoma and Acral Nevi. Hum. Pathol. 2022, 120, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.S.; Lau, S.K.; Scapa, J.V.; Cassarino, D.S. PRAME Immunohistochemistry of Spitzoid Neoplasms. J. Cutan. Pathol. 2022, 49, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Gassenmaier, M.; Hahn, M.; Metzler, G.; Bauer, J.; Yazdi, A.S.; Keim, U.; Garbe, C.; Wagner, N.B.; Forchhammer, S. Diffuse PRAME Expression Is Highly Specific for Thin Melanomas in the Distinction from Severely Dysplastic Nevi but Does Not Distinguish Metastasizing from Non-Metastasizing Thin Melanomas. Cancers 2021, 13, 3864. [Google Scholar] [CrossRef]
- Rothrock, A.T.; Torres-Cabala, C.A.; Milton, D.R.; Cho, W.C.; Nagarajan, P.; Vanderbeck, K.; Curry, J.L.; Ivan, D.; Prieto, V.G.; Aung, P.P. Diagnostic Utility of PRAME Expression by Immunohistochemistry in Subungual and Non-Subungual Acral Melanocytic Lesions. J. Cutan. Pathol. 2022, 49, 859–867. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.A.; Busam, K.J. Immunohistochemistry for PRAME in Dermatopathology. Am. J. Dermatopathol. 2023, 45, 733–747. [Google Scholar] [CrossRef]
- Parra, O.; Linos, K.; Li, Z.; Yan, S. PRAME Expression in Melanocytic Lesions of the Nail. J. Cutan. Pathol. 2022, 49, 610–617. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jung, C.J.; Na, H.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Park, C.-S.; Lim, Y.; Won, C.H. Cyclin D1 and PRAME Expression in Distinguishing Melanoma in Situ from Benign Melanocytic Proliferation of the Nail Unit. Diagn. Pathol. 2022, 17, 41. [Google Scholar] [CrossRef]
- Daruish, M.; Karunaratne, S.; Duffy-Gadd, P.; Hansford, S.; Taibjee, S. Utility of PRAME Immunohistochemistry in the Detection of Subtle Melanoma Microsatellites. Am. J. Dermatopathol. 2024, 46, 668–671. [Google Scholar] [CrossRef]
- Mudhar, H.S.; Milman, T.; Stevenson, S.; Watson, M.; Kim, J.; Magan, T.; Salvi, S.M.; Harley, U.; Lally, S.E.; Shields, C.L. PRAME Expression by Immunohistochemistry and Reverse Transcription Quantitative PCR in Conjunctival Melanocytic Lesions—A Comprehensive Clinicopathologic Study of 202 Cases and Correlation of Cytogenetics with PRAME Expression in Challenging Conjunctival Melanocytic Lesions. Hum. Pathol. 2023, 134, 1–18. [Google Scholar] [CrossRef]
- Cassalia, F.; Danese, A.; Cocchi, E.; Danese, E.; Ambrogio, F.; Cazzato, G.; Mazza, M.; Zambello, A.; Belloni Fortina, A.; Melandri, D. Misdiagnosis and Clinical Insights into Acral Amelanotic Melanoma—A Systematic Review. J. Pers. Med. 2024, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Soon, S.L.; Solomon, A.R.; Papadopoulos, D.; Murray, D.R.; McAlpine, B.; Washington, C.V. Acral Lentiginous Melanoma Mimicking Benign Disease: The Emory Experience. J. Am. Acad. Dermatol. 2003, 48, 183–188. [Google Scholar] [CrossRef]
- Matteucci, P.; Pinder, R.; Magdum, A.; Stanley, P. Accuracy in Skin Lesion Diagnosis and the Exclusion of Malignancy. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1460–1465. [Google Scholar] [CrossRef]
- Tsao, H.; Olazagasti, J.M.; Cordoro, K.M.; Brewer, J.D.; Taylor, S.C.; Bordeaux, J.S.; Chren, M.-M.; Sober, A.J.; Tegeler, C.; Bhushan, R.; et al. Early Detection of Melanoma: Reviewing the ABCDEs. J. Am. Acad. Dermatol. 2015, 72, 717–723. [Google Scholar] [CrossRef]
- Alheejawi, S.; Berendt, R.; Jha, N.; Maity, S.P.; Mandal, M. Detection of Malignant Melanoma in H&E-Stained Images Using Deep Learning Techniques. Tissue Cell 2021, 73, 101659. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Zuo, K.; Zhang, Y.; Li, F.; Yin, M.; Lu, K. Interpretable Classification from Skin Cancer Histology Slides Using Deep Learning: A Retrospective Multicenter Study. arXiv 2019, arXiv:1904.06156. [Google Scholar] [CrossRef]
- Harvey, N.T.; Wood, B.A. A Practical Approach to the Diagnosis of Melanocytic Lesions. Arch. Pathol. Lab. Med. 2019, 143, 789–810. [Google Scholar] [CrossRef]
- Prieto, V.G.; Shea, C.R. Use of Immunohistochemistry in Melanocytic Lesions. J. Cutan. Pathol. 2008, 35 (Suppl. S2), 1–10. [Google Scholar] [CrossRef]
- Drabeni, M.; Lopez-Vilaró, L.; Barranco, C.; Trevisan, G.; Gallardo, F.; Pujol, R.M. Differences in Tumor Thickness Between Hematoxylin and Eosin and Melan-A Immunohistochemically Stained Primary Cutaneous Melanomas. Am. J. Dermatopathol. 2013, 35, 56–63. [Google Scholar] [CrossRef]
- Gerami, P.; Jewell, S.S.; Morrison, L.E.; Blondin, B.; Schulz, J.; Ruffalo, T.; Matushek, P.; Legator, M.; Jacobson, K.; Dalton, S.R.; et al. Fluorescence In Situ Hybridization (FISH) as an Ancillary Diagnostic Tool in the Diagnosis of Melanoma. Am. J. Surg. Pathol. 2009, 33, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Hedayat, A.A.; Linos, K.; Jung, H.-S.; Tafe, L.J.; Yan, S.; LeBlanc, R.E.; Lefferts, J.A. Evaluating Melanocytic Lesions with Single Nucleotide Polymorphism (SNP) Chromosomal Microarray. Exp. Mol. Pathol. 2017, 103, 279–287. [Google Scholar] [CrossRef]
- Rawson, R.V.; Shteinman, E.R.; Ansar, S.; Vergara, I.A.; Thompson, J.F.; Long, G.V.; Scolyer, R.A.; Wilmott, J.S. Diagnostic Utility of PRAME, P53 and 5-hmC Immunostaining for Distinguishing Melanomas from Naevi, Neurofibromas, Scars and Other Histological Mimics. Pathology 2022, 54, 863–873. [Google Scholar] [CrossRef]
- Wakefield, C.; O’Keefe, L.; Heffron, C.C.B.B. Refining the Application of PRAME—A Useful Marker in High CSD and Acral Melanoma Subtypes. Virchows Arch. 2023, 483, 847–854. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Qiu, Y.; Ke, L.; Chen, H.; Chen, G. PRAME Is a Useful Marker for the Differential Diagnosis of Melanocytic Tumours and Histological Mimics. Histopathology 2023, 82, 285–295. [Google Scholar] [CrossRef]
- Warbasse, E.; Mehregan, D.; Utz, S.; Stansfield, R.B.; Abrams, J. PRAME Immunohistochemistry Compared to Traditional FISH Testing in Spitzoid Neoplasms and Other Difficult to Diagnose Melanocytic Neoplasms. Front. Med. 2023, 10, 1265827. [Google Scholar] [CrossRef]
- Harvey, N.T.; Peverall, J.; Acott, N.; Mesbah Ardakani, N.; Leecy, T.N.; Iacobelli, J.; McCallum, D.; Van Vliet, C.; Wood, B.A. Correlation of FISH and PRAME Immunohistochemistry in Ambiguous Superficial Cutaneous Melanocytic Proliferations. Am. J. Dermatopathol. 2021, 43, 913–920. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, M.Y.; Won, C.H.; Chang, S.E.; Lee, M.W.; Lee, W.J. Performance of PRAME Immunohistochemistry Compared with That of c-Kit, c-Myc, or Cyclin D1 for the Diagnosis of Acral Melanocytic Tumors. Pathol. Int. 2023, 73, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Jungbluth, A.A.; Busam, K.J. Comparison of Immunohistochemistry for PRAME with Cytogenetic Test Results in the Evaluation of Challenging Melanocytic Tumors. Am. J. Surg. Pathol. 2020, 44, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Parra, O.; Ma, W.; Li, Z.; Coffing, B.N.; Linos, K.; LeBlanc, R.E.; Momtahen, S.; Sriharan, A.; Cloutier, J.M.; Wells, W.A.; et al. PRAME Expression in Cutaneous Melanoma Does Not Correlate with Disease-specific Survival. J. Cutan. Pathol. 2023, 50, 903–912. [Google Scholar] [CrossRef]
- Durzynska, M.; Dos Santos, F.L.C.; Matuszczyk, A.; Derezinska-Wolek, E.; Michalek, I.M. Prognostic Implications of PRAME Expression and Clinicopathological Factors in Sinonasal Mucosal Melanoma: A Single-Center Cohort Study of 30 Cases. Anticancer Res. 2023, 43, 4551–4557. [Google Scholar] [CrossRef] [PubMed]
- Field, M.G.; Durante, M.A.; Decatur, C.L.; Tarlan, B.; Oelschlager, K.M.; Stone, J.F.; Kuznetsov, J.; Bowcock, A.M.; Kurtenbach, S.; Harbour, J.W. Epigenetic Reprogramming and Aberrant Expression of PRAME Are Associated with Increased Metastatic Risk in Class 1 and Class 2 Uveal Melanomas. Oncotarget 2016, 7, 59209–59219. [Google Scholar] [CrossRef]
- Googe, P.B.; Flanigan, K.L.; Miedema, J.R. Preferentially Expressed Antigen in Melanoma Immunostaining in a Series of Melanocytic Neoplasms. Am. J. Dermatopathol. 2021, 43, 794–800. [Google Scholar] [CrossRef]
- Raghavan, S.S.; Wang, J.Y.; Kwok, S.; Rieger, K.E.; Novoa, R.A.; Brown, R.A. PRAME Expression in Melanocytic Proliferations with Intermediate Histopathologic or Spitzoid Features. J. Cutan. Pathol. 2020, 47, 1123–1131. [Google Scholar] [CrossRef]
- Gerami, P.; Benton, S.; Zhao, J.; Zhang, B.; Lampley, N.; Roth, A.; Boutko, A.; Olivares, S.; Busam, K.J. PRAME Expression Correlates with Genomic Aberration and Malignant Diagnosis of Spitzoid Melanocytic Neoplasms. Am. J. Dermatopathol. 2022, 44, 575–580. [Google Scholar] [CrossRef]
- McAfee, J.L.; Scarborough, R.; Jia, X.S.; Azzato, E.M.; Astbury, C.; Ronen, S.; Andea, A.A.; Billings, S.D.; Ko, J.S. Combined Utility of P16 and BRAF V600E in the Evaluation of Spitzoid Tumors: Superiority to PRAME and Correlation with FISH. J. Cutan. Pathol. 2023, 50, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Marot-Lassauzaie, V.; Beneyto-Calabuig, S.; Obermayer, B.; Velten, L.; Beule, D.; Haghverdi, L. Identifying Cancer Cells from Calling Single-Nucleotide Variants in scRNA-Seq Data. Bioinformatics 2024, 40, btae512. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Floros, T.; Tarhini, A.A. Anticancer Cytokines: Biology and Clinical Effects of Interferon-A2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin. Oncol. 2015, 42, 539–548. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Sznol, M.; Melero, I. Revisiting Anti-CTLA-4 Antibodies in Combination with PD-1 Blockade for Cancer Immunotherapy. Ann. Oncol. 2021, 32, 295–297. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Nguyen, P.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Esandrio, J.; Xu, H.; Ogurtsova, A.; Bleich, K.B.; Cornish, T.C.; et al. PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin. Cancer Res. 2017, 23, 4938–4944. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Freeman, G.J.; McDermott, D.F. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin. Ther. 2015, 37, 764–782. [Google Scholar] [CrossRef]
- Lodde, G.C.; Zhao, F.; Herbst, R.; Terheyden, P.; Utikal, J.; Pföhler, C.; Ulrich, J.; Kreuter, A.; Mohr, P.; Gutzmer, R.; et al. Early versus Late Response to PD-1-Based Immunotherapy in Metastatic Melanoma. Eur. J. Cancer 2024, 210, 114295. [Google Scholar] [CrossRef]
- Comito, F.; Pagani, R.; Grilli, G.; Sperandi, F.; Ardizzoni, A.; Melotti, B. Emerging Novel Therapeutic Approaches for Treatment of Advanced Cutaneous Melanoma. Cancers 2022, 14, 271. [Google Scholar] [CrossRef]
- Li, T.; Niu, M.; Zhou, J.; Wu, K.; Yi, M. The Enhanced Antitumor Activity of Bispecific Antibody Targeting PD-1/PD-L1 Signaling. Cell Commun. Signal. 2024, 22, 179. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.F.; Wang, Y.; Liu, B.; Molina, J.R. Comparative Analysis of Predictive Biomarkers for PD-1/PD-L1 Inhibitors in Cancers: Developments and Challenges. Cancers 2021, 14, 109. [Google Scholar] [CrossRef]
- Mehta, A.; Motavaf, M.; Nebo, I.; Luyten, S.; Osei-Opare, K.D.; Gru, A.A. Advancements in Melanoma Treatment: A Review of PD-1 Inhibitors, T-VEC, mRNA Vaccines, and Tumor-Infiltrating Lymphocyte Therapy in an Evolving Landscape of Immunotherapy. J. Clin. Med. 2025, 14, 1200. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination Strategies with PD-1/PD-L1 Blockade: Current Advances and Future Directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Maul, L.V.; Ramelyte, E.; Dummer, R.; Mangana, J. Management of Metastatic Melanoma with Combinations Including PD-1 Inhibitors. Expert Opin. Biol. Ther. 2025, 25, 487–498. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Li, J.; Adhikari, R.; Fu, L. PD-1/PD-L1 Based Combinational Cancer Therapy: Icing on the Cake. Front. Pharmacol. 2020, 11, 722. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Yao, Q.; Du, N.; Fu, X.; Lou, Y.; Wang, M.; Mao, F.; Mao, D.; Khadaroo, P.A.; et al. The Efficacy and Safety of Combined Immune Checkpoint Inhibitors (Nivolumab plus Ipilimumab): A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2020, 18, 150. [Google Scholar] [CrossRef]
- He, R.; Zhao, X.; Liu, J.; Zhou, Y.; Zhang, X.; Cheng, F. PD-1 and CTLA-4 Inhibitors in Combination vs. Alone for the Treatment of Advanced Melanoma: A Systematic Review and Meta-Analysis. Medicine 2022, 101, e30561. [Google Scholar] [CrossRef]
- Pinto, E.; Lione, L.; Compagnone, M.; Paccagnella, M.; Salvatori, E.; Greco, M.; Frezza, V.; Marra, E.; Aurisicchio, L.; Roscilli, G.; et al. From Ex Vivo to in Vivo Chimeric Antigen T Cells Manufacturing: New Horizons for CAR T-Cell Based Therapy. J. Transl. Med. 2025, 23, 10. [Google Scholar] [CrossRef]
- Chong, C.; Coukos, G.; Bassani-Sternberg, M. Identification of Tumor Antigens with Immunopeptidomics. Nat. Biotechnol. 2022, 40, 175–188. [Google Scholar] [CrossRef]
- Yang, P.; Qiao, Y.; Meng, M.; Zhou, Q. Cancer/Testis Antigens as Biomarker and Target for the Diagnosis, Prognosis, and Therapy of Lung Cancer. Front. Oncol. 2022, 12, 864159. [Google Scholar] [CrossRef]
- Norberg, S.M.; Hinrichs, C.S. Engineered T Cell Therapy for Viral and Non-Viral Epithelial Cancers. Cancer Cell 2023, 41, 58–69. [Google Scholar] [CrossRef]
- Kazemi, M.H.; Sadri, M.; Najafi, A.; Rahimi, A.; Baghernejadan, Z.; Khorramdelazad, H.; Falak, R. Tumor-Infiltrating Lymphocytes for Treatment of Solid Tumors: It Takes Two to Tango? Front. Immunol. 2022, 13, 1018962. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Packard, B.S.; Aebersold, P.M.; Solomon, D.; Topalian, S.L.; Toy, S.T.; Simon, P.; Lotze, M.T.; Yang, J.C.; Seipp, C.A.; et al. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef]
- Dafni, U.; Michielin, O.; Lluesma, S.M.; Tsourti, Z.; Polydoropoulou, V.; Karlis, D.; Besser, M.J.; Haanen, J.; Svane, I.-M.; Ohashi, P.S.; et al. Efficacy of Adoptive Therapy with Tumor-Infiltrating Lymphocytes and Recombinant Interleukin-2 in Advanced Cutaneous Melanoma: A Systematic Review and Meta-Analysis. Ann. Oncol. 2019, 30, 1902–1913. [Google Scholar] [CrossRef]
- Kessler, J.H.; Beekman, N.J.; Bres-Vloemans, S.A.; Verdijk, P.; Van Veelen, P.A.; Kloosterman-Joosten, A.M.; Vissers, D.C.J.; Ten Bosch, G.J.A.; Kester, M.G.D.; Sijts, A.; et al. Efficient Identification of Novel Hla-A*0201–Presented Cytotoxic T Lymphocyte Epitopes in the Widely Expressed Tumor Antigen Prame by Proteasome-Mediated Digestion Analysis. J. Exp. Med. 2001, 193, 73–88. [Google Scholar] [CrossRef]
- Griffioen, M.; Kessler, J.H.; Borghi, M.; Van Soest, R.A.; Van Der Minne, C.E.; Nouta, J.; Van Der Burg, S.H.; Medema, J.P.; Schrier, P.I.; Falkenburg, J.H.F.; et al. Detection and Functional Analysis of CD8+ T Cells Specific for PRAME: A Target for T-Cell Therapy. Clin. Cancer Res. 2006, 12, 3130–3136. [Google Scholar] [CrossRef]
- Greiner, J.; Schmitt, M.; Li, L.; Giannopoulos, K.; Bosch, K.; Schmitt, A.; Dohner, K.; Schlenk, R.F.; Pollack, J.R.; Dohner, H.; et al. Expression of Tumor-Associated Antigens in Acute Myeloid Leukemia: Implications for Specific Immunotherapeutic Approaches. Blood 2006, 108, 4109–4117. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Dunmall, L.C.; Wang, Y.Y.; Fan, Z.; Cheng, Z.; Wang, Y. The Dilemmas and Possible Solutions for CAR-T Cell Therapy Application in Solid Tumors. Cancer Lett. 2024, 591, 216871. [Google Scholar] [CrossRef]
- Montalvo, M.J.; Bandey, I.N.; Rezvan, A.; Wu, K.-L.; Saeedi, A.; Kulkarni, R.; Li, Y.; An, X.; Sefat, K.M.S.R.; Varadarajan, N. Decoding the Mechanisms of Chimeric Antigen Receptor (CAR) T Cell-Mediated Killing of Tumors: Insights from Granzyme and Fas Inhibition. Cell Death Dis. 2024, 15, 109. [Google Scholar] [CrossRef]
- Keane, J.T.; Posey, A.D. Chimeric Antigen Receptors Expand the Repertoire of Antigenic Macromolecules for Cellular Immunity. Cells 2021, 10, 3356. [Google Scholar] [CrossRef]
- Chang, A.Y.; Dao, T.; Gejman, R.S.; Jarvis, C.A.; Scott, A.; Dubrovsky, L.; Mathias, M.D.; Korontsvit, T.; Zakhaleva, V.; Curcio, M.; et al. A Therapeutic T Cell Receptor Mimic Antibody Targets Tumor-Associated PRAME Peptide/HLA-I Antigens. J. Clin. Investig. 2017, 127, 2705–2718. [Google Scholar] [CrossRef]
- Bellicum Pharmaceuticals. Safety & Activity of Controllable PRAME-TCR Therapy in Previously Treated AML/MDS or Metastatic Uveal Melanoma. ClinicalTrials.Gov. Available online: https://Clinicaltrials.Gov/Study/NCT02743611#more-Information (accessed on 16 July 2025).
- Wermke, M.; Tsimberidou, A.-M.; Mohamed, A.; Mayer-Mokler, A.; Satelli, A.; Reinhardt, C.; Araujo, D.; Maurer, D.; Blumenschein, G.J.; Singh, H.; et al. 959 Safety and Anti-Tumor Activity of TCR-Engineered Autologous, PRAME-Directed T Cells across Multiple Advanced Solid Cancers at Low Doses—Clinical Update on the ACTengine® IMA203 Trial. In Late-Breaking Abstracts; BMJ Publishing Group Ltd.: London, UK, 2021; p. A1009. [Google Scholar] [CrossRef]
- Weber, J.S.; Vogelzang, N.J.; Ernstoff, M.S.; Goodman, O.B.; Cranmer, L.D.; Marshall, J.L.; Miles, S.; Rosario, D.; Diamond, D.C.; Qiu, Z.; et al. A Phase 1 Study of a Vaccine Targeting Preferentially Expressed Antigen in Melanoma and Prostate-Specific Membrane Antigen in Patients with Advanced Solid Tumors. J. Immunother. 2011, 34, 556–567. [Google Scholar] [CrossRef]
- Immunocore Reports Updated Phase 1 Data of Brenetafusp (IMC-F106C), an ImmTAC Bispecific Targeting PRAME, in Immune Checkpoint Pre-Treated Cutaneous Melanoma Patients at ASCO 2024. Available online: https://www.immunocore.com/investors/news/press-releases/detail/92/immunocore-reports-updated-phase-1-data-of-brenetafusp-imc-f106c-an-immtac-bispecific-targeting-prame-in-immune-checkpoint-pre-treated-cutaneous-melanoma-patients-at-asco-2024 (accessed on 5 August 2025).
- Immunocore Ltd. IMC-F106C Regimen Versus Nivolumab Regimens in Previously Untreated Advanced Melanoma (PRISM-MEL-301) (PRISM-MEL-301). ClinicalTrials.Gov. Available online: https://Clinicaltrials.Gov/Study/NCT06112314?term=NCT06112314&rank=1 (accessed on 16 July 2025).
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised Neoantigen Therapy mRNA-4157 (V940) plus Pembrolizumab versus Pembrolizumab Monotherapy in Resected Melanoma (KEYNOTE-942): A Randomised, Phase 2b Study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic Cancer Vaccines: Advancements, Challenges and Prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef]
- Lei, W.; Zhou, K.; Lei, Y.; Li, Q.; Zhu, H. Cancer Vaccines: Platforms and Current Progress. Mol. Biomed. 2025, 6, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Tian, X.; Wei, X. Cancer Vaccines: Current Status and Future Directions. J. Hematol. Oncol. 2025, 18, 18. [Google Scholar] [CrossRef]
- Biri-Kovács, B.; Bánóczi, Z.; Tummalapally, A.; Szabó, I. Peptide Vaccines in Melanoma: Chemical Approaches towards Improved Immunotherapeutic Efficacy. Pharmaceutics 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Tagliamonte, M. Peptide-Based Vaccine for Cancer Therapies. Front. Immunol. 2023, 14, 1210044. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA Vaccines for Cancer Immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Zielinski, M.; Linder, A.; Dahabreh, J.; Gonzalez, E.E.; Malinowski, W.; Lopez-Brea, M.; Vanakesa, T.; Jassem, J.; Kalofonos, H.; et al. Adjuvant MAGE-A3 Immunotherapy in Resected Non–Small-Cell Lung Cancer: Phase II Randomized Study Results. J. Clin. Oncol. 2013, 31, 2396–2403. [Google Scholar] [CrossRef]
- Dreno, B.; Thompson, J.F.; Smithers, B.M.; Santinami, M.; Jouary, T.; Gutzmer, R.; Levchenko, E.; Rutkowski, P.; Grob, J.-J.; Korovin, S.; et al. MAGE-A3 Immunotherapeutic as Adjuvant Therapy for Patients with Resected, MAGE-A3-Positive, Stage III Melanoma (DERMA): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2018, 19, 916–929. [Google Scholar] [CrossRef]
- Cho, H.; Binder, J.; Weeratna, R.; Dermyer, M.; Dai, S.; Boccia, A.; Li, W.; Li, S.; Jooss, K.; Merson, J.; et al. Preclinical Development of a Vaccine-Based Immunotherapy Regimen (VBIR) That Induces Potent and Durable T Cell Responses to Tumor-Associated Self-Antigens. Cancer Immunol. Immunother. 2023, 72, 287–300. [Google Scholar] [CrossRef]
- León-Letelier, R.A.; Bonifaz, L.C.; Fuentes-Pananá, E.M. OMIC Signatures to Understand Cancer Immunosurveillance and Immunoediting: Melanoma and Immune Cells Interplay in Immunotherapy. J. Leukoc. Biol. 2019, 105, 915–933. [Google Scholar] [CrossRef]
- Czajka-Francuz, P.; Prendes, M.J.; Mankan, A.; Quintana, Á.; Pabla, S.; Ramkissoon, S.; Jensen, T.J.; Peiró, S.; Severson, E.A.; Achyut, B.R.; et al. Mechanisms of Immune Modulation in the Tumor Microenvironment and Implications for Targeted Therapy. Front. Oncol. 2023, 13, 1200646. [Google Scholar] [CrossRef]
- Shende, S.; Rathored, J.; Budhbaware, T. Role of Metabolic Transformation in Cancer Immunotherapy Resistance: Molecular Mechanisms and Therapeutic Implications. Discov. Oncol. 2025, 16, 453. [Google Scholar] [CrossRef]
- Cabezón-Gutiérrez, L.; Palka-Kotlowska, M.; Custodio-Cabello, S.; Chacón-Ovejero, B.; Pacheco-Barcia, V. Metabolic Mechanisms of Immunotherapy Resistance. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002297. [Google Scholar] [CrossRef]
- Lezcano, C.; Pulitzer, M.; Moy, A.P.; Hollmann, T.J.; Jungbluth, A.A.; Busam, K.J. Immunohistochemistry for PRAME in the Distinction of Nodal Nevi from Metastatic Melanoma. Am. J. Surg. Pathol. 2020, 44, 503–508. [Google Scholar] [CrossRef]
- Brocks, D.; Schmidt, C.R.; Daskalakis, M.; Jang, H.S.; Shah, N.M.; Li, D.; Li, J.; Zhang, B.; Hou, Y.; Laudato, S.; et al. DNMT and HDAC Inhibitors Induce Cryptic Transcription Start Sites Encoded in Long Terminal Repeats. Nat. Genet. 2017, 49, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, M.; Brocks, D.; Sheng, Y.-H.; Islam, M.S.; Ressnerova, A.; Assenov, Y.; Milde, T.; Oehme, I.; Witt, O.; Goyal, A.; et al. Reactivation of Endogenous Retroviral Elements via Treatment with DNMT- and HDAC-Inhibitors. Cell Cycle 2018, 17, 811–822. [Google Scholar] [CrossRef]
- Enevoldsen, J.; Brogård, M.B.; Lade-Keller, J.; Christensen, K.B.; Georgsen, J.B.; Nielsen, P.S.; Steiniche, T. Digital Quantification of PRAME for Distinguishing Melanoma from Nevi Compared to Manual Assessment. Pathol. Res. Pract. 2024, 262, 155543. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, S.; Aebischer, V.; Lenders, D.; Seitz, C.M.; Schroeder, C.; Liebmann, A.; Abele, M.; Wild, H.; Bien, E.; Krawczyk, M.; et al. Characterization of PRAME Immunohistochemistry Reveals Lower Expression in Pediatric Melanoma Compared to Adult Melanoma. Pigment Cell Melanoma Res. 2024, 37, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Elkord, E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Basak, U.; Sarkar, T.; Mukherjee, S.; Chakraborty, S.; Dutta, A.; Dutta, S.; Nayak, D.; Kaushik, S.; Das, T.; Sa, G. Tumor-Associated Macrophages: An Effective Player of the Tumor Microenvironment. Front. Immunol. 2023, 14, 1295257. [Google Scholar] [CrossRef]
- He, Z.-N.; Zhang, C.-Y.; Zhao, Y.-W.; He, S.-L.; Li, Y.; Shi, B.-L.; Hu, J.-Q.; Qi, R.-Z.; Hua, B.-J. Regulation of T Cells by Myeloid-Derived Suppressor Cells: Emerging Immunosuppressor in Lung Cancer. Discov. Oncol. 2023, 14, 185. [Google Scholar] [CrossRef]
- Sui, H.; Dongye, S.; Liu, X.; Xu, X.; Wang, L.; Jin, C.Q.; Yao, M.; Gong, Z.; Jiang, D.; Zhang, K.; et al. Immunotherapy of Targeting MDSCs in Tumor Microenvironment. Front. Immunol. 2022, 13, 990463. [Google Scholar] [CrossRef]
- Sharma, P.; Kranz, D.M. T Cell Receptors for Gene Transfer in Adoptive T Cell Therapy. Crit. Rev. Immunol. 2019, 39, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Balko, J.M. Mechanisms of MHC-I Downregulation and Role in Immunotherapy Response. Front. Immunol. 2022, 13, 844866. [Google Scholar] [CrossRef] [PubMed]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Ritter, C.; Fan, K.; Paschen, A.; Reker Hardrup, S.; Ferrone, S.; Nghiem, P.; Ugurel, S.; Schrama, D.; Becker, J.C. Epigenetic Priming Restores the HLA Class-I Antigen Processing Machinery Expression in Merkel Cell Carcinoma. Sci. Rep. 2017, 7, 2290. [Google Scholar] [CrossRef]
- Rodems, T.S.; Heninger, E.; Stahlfeld, C.N.; Gilsdorf, C.S.; Carlson, K.N.; Kircher, M.R.; Singh, A.; Krueger, T.E.G.; Beebe, D.J.; Jarrard, D.F.; et al. Reversible Epigenetic Alterations Regulate Class I HLA Loss in Prostate Cancer. Commun. Biol. 2022, 5, 897. [Google Scholar] [CrossRef]
- Limagne, E.; Nuttin, L.; Thibaudin, M.; Jacquin, E.; Aucagne, R.; Bon, M.; Revy, S.; Barnestein, R.; Ballot, E.; Truntzer, C.; et al. MEK Inhibition Overcomes Chemoimmunotherapy Resistance by Inducing CXCL10 in Cancer Cells. Cancer Cell 2022, 40, 136–152.e12. [Google Scholar] [CrossRef]
- Stopfer, L.E.; Rettko, N.J.; Leddy, O.; Mesfin, J.M.; Brown, E.; Winski, S.; Bryson, B.; Wells, J.A.; White, F.M. MEK Inhibition Enhances Presentation of Targetable MHC-I Tumor Antigens in Mutant Melanomas. Proc. Natl. Acad. Sci. USA 2022, 119, e2208900119. [Google Scholar] [CrossRef]
| Marker | Specificity | Sensitivity | Additional Notes | Reference Numbers |
|---|---|---|---|---|
| S100 | Low | High | Stains both benign and malignant melanocytes | [4,65,66,67,68,69] |
| SOX10 | Low | High | Limited specificity similar to S100 | [4,65,66,67,68,69] |
| Melan-A | Low | High | Difficulties in differentiating benign from malignant lesions | [70] |
| HMB-45 | Moderate | Moderate | Slightly improved specificity compared to others | [67,71] |
| PD-L1 | Variable | Variable | Predictive value inconsistent; depends on tumor microenvironment | [72,73,74,75] |
| PRAME | High (86.4% negative in benign nevi) | 92% in primary and metastatic melanoma; 85.4% in metastases | Higher specificity than traditional markers | [22,66,76,77,78,79] |
| Trial Name | Main Observations | Clinical Phase | Reference Number |
|---|---|---|---|
| Safety & Activity of Controllable PRAME-TCR Therapy in Previously Treated AML/MDS or Metastatic Uveal Melanoma | TCR-engineered autologous T-cell therapy targeting PRAME in metastatic uveal melanoma; features a rimiducid-controlled safety switch. | Phase II | [148] |
| ACTengine® IMA203/IMA203CD8 as Monotherapy or in Combination With Nivolumab in Recurrent and/or Refractory Solid Tumors (ACTengine) | Phase I trial showed a manageable safety profile, 52.5% overall response rate, 29% confirmed response in PRAME-positive tumors; supports further development. | Phase I | [149] |
| Safety and Immune Response to a Multi-component Immune Based Therapy (MKC1106-PP) for Patients With Advanced Cancer. | Triggered CD8+ T-cell expansion in 15 of 24 prostate cancer patients; some showed disease stability over 6 months (this included one out of ten patients with metastatic melanoma). | Phase I | [150] |
| Safety and Efficacy of IMC-F106C as a Single Agent and in Combination With Checkpoint Inhibitors | Bispecific ImmTAC molecule targeting PRAME and CD3; showed 58% disease control rate and median PFS of 4.2 months in heavily pre-treated cutaneous melanoma; 42% of PRAME-positive patients showed molecular responses via ctDNA; adverse events were manageable (e.g., mild cytokine release syndrome, rash) | Phase I | [151] |
| IMC-F106C Regimen Versus Nivolumab Regimens in Previously Untreated Advanced Melanoma (PRISM-MEL-301) (PRISM-MEL-301) | Phase III randomized global study evaluating IMC-F106C (PRAME × CD3) in combination with nivolumab in previously untreated HLA-A*02:01+ advanced melanoma patients. Aims to improve PFS, OS, and response rates versus standard nivolumab regimens. Trial includes patients with cutaneous and select non-cutaneous melanoma subtypes. No results published yet. | Phase III | [152] |
| An Efficacy Study of Adjuvant Treatment With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Participants With High-Risk Melanoma (KEYNOTE-942) | Improved recurrence-free survival in high-risk melanoma patients; well tolerated; received FDA Breakthrough Therapy Designation. | Phase IIb | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokos, M.; Prkačin, I.; Gaćina, K.; Brkić, A.; Pondeljak, N.; Šitum, M. Therapeutic Opportunities in Melanoma Through PRAME Expression. Biomedicines 2025, 13, 1988. https://doi.org/10.3390/biomedicines13081988
Mokos M, Prkačin I, Gaćina K, Brkić A, Pondeljak N, Šitum M. Therapeutic Opportunities in Melanoma Through PRAME Expression. Biomedicines. 2025; 13(8):1988. https://doi.org/10.3390/biomedicines13081988
Chicago/Turabian StyleMokos, Mislav, Ivana Prkačin, Klara Gaćina, Ana Brkić, Nives Pondeljak, and Mirna Šitum. 2025. "Therapeutic Opportunities in Melanoma Through PRAME Expression" Biomedicines 13, no. 8: 1988. https://doi.org/10.3390/biomedicines13081988
APA StyleMokos, M., Prkačin, I., Gaćina, K., Brkić, A., Pondeljak, N., & Šitum, M. (2025). Therapeutic Opportunities in Melanoma Through PRAME Expression. Biomedicines, 13(8), 1988. https://doi.org/10.3390/biomedicines13081988






