1. Introduction
Citrate is a key inhibitor of calcium-containing kidney stone formation. It binds urinary calcium, forming soluble complexes and reducing supersaturation of calcium oxalate and calcium phosphate [
1,
2]. Hypocitraturia, defined as low urinary citrate excretion, is a common metabolic abnormality reported in 20–60% of patients with recurrent nephrolithiasis [
3,
4]. This condition may result from metabolic acidosis, gastrointestinal disorders, medications, or genetic predisposition [
5,
6].
The reabsorption of filtered citrate primarily occurs in the renal proximal tubule and is mediated by sodium-dependent dicarboxylate cotransporter 1 (NaDC1), a membrane transport protein encoded by the
SLC13A2 gene [
7,
8]. NaDC1 plays a central role in determining urinary citrate concentration, and its activity is regulated by systemic acid-base status. Functional alterations in NaDC1 can significantly affect urinary citrate levels and thereby influence stone risk [
9,
10].
Genetic variation within the
SLC13A2 gene has been proposed as a contributing factor to hypocitraturia. Several single-nucleotide polymorphisms (SNPs) have been identified, with varying effects on transporter function and expression [
11]. Among them, the rs11568476 polymorphism, also known as the V477M variant, has been shown to reduce NaDC1 transport activity by approximately 90% in vitro, without affecting its expression [
12]. Despite its functional relevance, the clinical implications of this SNP remain poorly understood, and few studies have examined its association with hypocitraturia or stone formation in human populations.
Most existing genetic studies have focused on alternative
SLC13A2 variants such as rs11567842 (I550V), which has been associated with hypocitraturia in East Asian cohorts [
11,
13]. However, there is a lack of data regarding the prevalence and significance of rs11568476 in other ethnic groups. Notably, its distribution in Turkish individuals remains unknown.
Türkiye is part of the so-called “stone belt,” with a high prevalence of urolithiasis. While several clinical and metabolic studies have been conducted in the country, genetic investigations into the etiopathogenesis of nephrolithiasis are limited. Understanding population-specific genetic susceptibility may provide insight into disease mechanisms and inform targeted prevention strategies [
14,
15].
The aim of this study is to determine the frequency of the rs11568476 polymorphism in the SLC13A2 gene among Turkish patients with calcium-containing kidney stones and to assess its association with hypocitraturia. To our knowledge, this is the first study to evaluate this SNP in a Turkish cohort.
2. Materials and Methods
2.1. Study Design and Ethical Approval
This prospective cross-sectional study was conducted at the Department of Urology, Düzce University Faculty of Medicine, Türkiye, between March 2015 and June 2015. The study protocol was approved by the Clinical Research Ethics Committee of Düzce University (approval No. 2015/135, dated 3 March 2015), and written informed consent was obtained from all participants. This study complied with the ethical principles out-lined in the Declaration of Helsinki and institutional guidelines.
2.2. Patient Selection
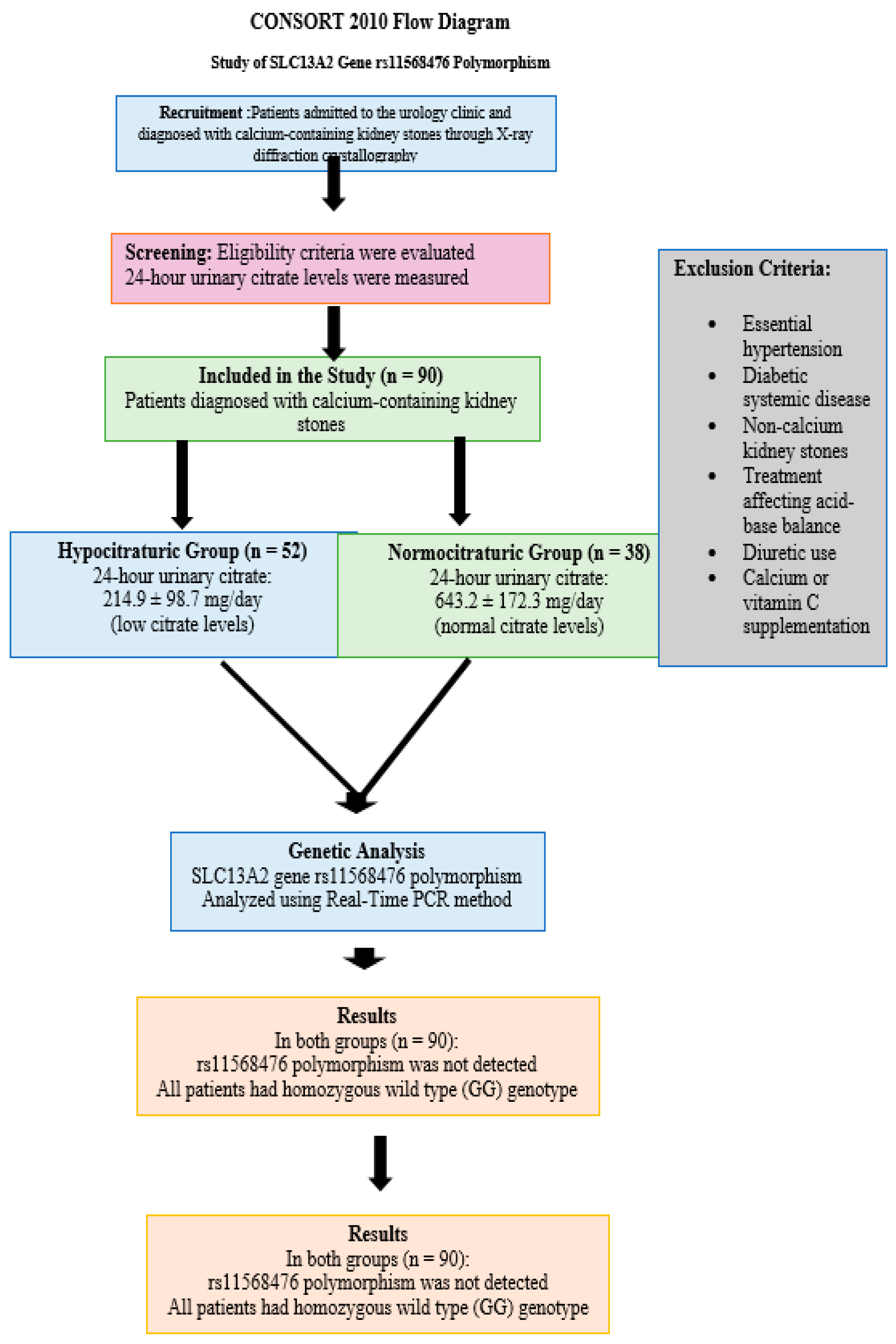
Patients presenting to the urology outpatient clinic with a diagnosis of calcium-containing kidney stones, confirmed via X-ray diffraction crystallography of stone samples, were evaluated for inclusion. Exclusion criteria were as follows: presence of any systemic disease other than well-controlled essential hypertension, history of non-calcium-based kidney stones, use of diuretics, calcium or vitamin C supplementation, and receipt of any pharmacologic treatment known to alter systemic acid-base balance (e.g., bicarbonate therapy or alkali treatment). Additionally, patients with secondary hypertension or those under antihypertensive therapies affecting renal citrate handling were excluded. Patient selection and enrollment are illustrated in the CONSORT 2010 flow diagram (
Figure 1).
2.3. Data Collection and Dietary Control
Detailed clinical and demographic data were obtained through structured interviews and medical record reviews. Information collected included age, sex, height, weight, previous history of nephrolithiasis, history of surgical or medical treatments, systemic or metabolic disorders, and family history of kidney stones. Anthropometric measurements were performed using standardized clinical protocols. In order to minimize dietary variation that could influence urinary citrate levels, all patients were provided with written and verbal dietary instructions. These included restrictions on the intake of red meat, high-sodium foods, chocolate, spinach, leafy vegetables, tea, and coffee during the 48 h preceding the 24 h urine collection. Dietary adherence was not clinically supervised but was verified through direct patient interviews.
2.4. Biochemical Analysis
Venous blood samples were collected from all patients to measure serum creatinine, sodium, potassium, calcium, and uric acid concentrations. These analyses were performed using reagents from Biochemical Enterprise™, Milan, Italy (catalog no: CI8820), based on standardized enzymatic and colorimetric methods. Venous blood samples were collected from all patients to measure serum creatinine, sodium, potassium, calcium, and uric acid concentrations. These analyses were performed using an automated clinical chemistry analyzer (Architect c8000, Abbott Diagnostics™, Santa Clara, CA, USA), based on standardized enzymatic and colorimetric methods. Urine samples were collected over a 24 h period in acid-washed containers, and hydrochloric acid was added at the time of collection to reduce the pH below 3.0, ensuring citrate and oxalate stability. The following parameters were measured in the 24 h urine samples: citrate, oxalate, uric acid, calcium, and magnesium. All measurements were performed using photometric methods with commercial kits (Ben Biochemical Enterprise™, Milan, Italy; catalog no: CI8820). Based on 24 h urinary citrate excretion, patients were divided into two groups: normocitraturic (≥320 mg/1.73 m2/24 h) and hypocitraturic (<320 mg/1.73 m2/24 h).
2.5. Genetic Analysis
Genomic DNA was extracted from peripheral blood samples collected in Na-EDTA tubes using the High Pure PCR Template Preparation Kit (Roche Diagnostics™, Mannheim, Germany) following the manufacturer’s instructions. The rs11568476 polymorphism (V477M variant) of the SLC13A2 gene was analyzed using a LightCycler 480 II Real-Time PCR system (Roche Diagnostics™) with LightSNIP-specific primers and HybProbe technology. Each 15 µL reaction mixture contained 10.4 µL PCR-grade water, 1 µL LightSNIP reagent mix, 2 µL FastStart DNA Master HybProbe (Roche Diagnostics GmbH, Mannheim, Germany), 1.6 µL 25 mM MgCl2, and 5 µL of DNA template. FastStart DNA Master HybProbe, 1.6 µL 25 mM MgCl2, and 5 µL of DNA template. Following centrifugation, PCR amplification and melting curve analysis were performed. Genotypes were classified based on melting temperature (Tm) peaks as wild-type homozygous (GG), heterozygous (AG), or homozygous mutant (AA).
2.6. Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA). A priori power analysis indicated that at least 30 patients per group were required to detect statistically significant differences with 80% power and a significance level (α) of 0.05. Continuous variables were assessed for normal distribution using the Kolmogorov–Smirnov test and were expressed as mean ± standard deviation. Categorical variables were reported as frequencies and percentages. Group comparisons were performed using Student’s t-test for continuous variables and Pearson’s chi-square test for categorical variables. As all patients were found to carry the homozygous wild-type genotype (GG), Hardy–Weinberg equilibrium could not be assessed, and no genotype–phenotype association analysis could be performed.
3. Results
A total of 90 patients diagnosed with calcium-containing kidney stones were included and stratified into two groups based on their 24 h urinary citrate excretion: the hypocitraturic group (
n = 52) and the normocitraturic group (
n = 38).
Table 1 summarizes the demographic and clinical characteristics of the study population.
There were no statistically significant differences between the two groups regarding age, gender distribution, height, or body weight (all p > 0.05). However, family history of urinary stone disease was significantly more prevalent in the normocitraturic group compared to the hypocitraturic group (60.5% vs. 36.5%, p = 0.024). This finding is somewhat counterintuitive and may suggest sample-specific or hereditary factors unrelated to urinary citrate levels.
Biochemical parameters revealed no significant differences in serum creatinine or calcium concentrations between the groups. Notably, serum uric acid levels were significantly elevated in the hypocitraturic group (5.08 ± 1.58 mg/dL vs. 4.12 ± 1.14 mg/dL, p = 0.002), suggesting a potential alternative metabolic contribution to stone formation in these patients. Urine specific gravity and pH values were comparable between groups (both p > 0.05).
Twenty-four-hour urine analysis results are presented in
Table 2. As expected, urinary citrate excretion was markedly lower in the hypocitraturic group (155.3 ± 86.8 vs. 750.2 ± 238.1 mg/1.73 m
2/24 h,
p < 0.0001), confirming successful stratification. No significant differences were observed in 24 h urine volume, oxalate, uric acid, calcium, or magnesium excretion (all
p > 0.05). Although urinary oxalate levels appeared higher in the normocitraturic group, the difference did not reach statistical significance (
p = 0.097).
Genetic analysis of the rs11568476 region of the SLC13A2 gene was successfully performed in all 90 patients using Real-Time PCR. Interestingly, none of the patients in either group carried the polymorphic variant. All participants (100%) were found to have the homozygous wild-type genotype (GG). As a result, no heterozygous (AG) or mutant homozygous (AA) individuals were identified. Due to the complete absence of allelic variation, genotype-based association analyses and Hardy–Weinberg equilibrium assessments could not be performed.
4. Discussion
This study investigated the presence of the rs11568476 (V477M) polymorphism in the
SLC13A2 gene in Turkish patients with calcium-containing nephrolithiasis, particularly focusing on its relationship with hypocitraturia, a known and common metabolic risk factor for kidney stone formation. Our main finding was the complete absence of the rs11568476 variant in all patients, regardless of urinary citrate status, suggesting this SNP may be rare or absent in the Turkish population [
2,
16].
Citrate is a central inhibitor of calcium stone formation, exerting its anti-lithogenic effect by complexing with urinary calcium, thus reducing ionized calcium availability and preventing crystal nucleation and aggregation [
17]. The significance of hypocitraturia, defined as a reduction in urinary citrate excretion below reference values, has been well established as a recurrent and independent risk factor for calcium-based stone formation. Its etiology is multifactorial, involving dietary acid load, renal tubular defects, and genetic variants affecting citrate transporters, particularly
SLC13A2, which encodes the sodium-dependent dicarboxylate cotransporter (NaDC1) in the proximal renal tubule [
7,
18].
The rs11568476 polymorphism has previously been associated with significantly reduced NaDC1 transport activity in functional studies, leading to the hypothesis that it may contribute to impaired urinary citrate excretion and, therefore, a predisposition to stone formation [
19]. However, our study found no carriers of this polymorphism among the 90 patients analyzed, all of whom exhibited a homozygous GG (wild-type) genotype. While this may initially seem surprising, such absence likely reflects ethnic or population-specific differences in allele frequency. Prior reports of this variant have been limited and geographically restricted, with no established prevalence in Turkish or broader Middle Eastern cohorts [
12].
This population-specific genomic profile is important for interpreting genetic risk and underscores the need for ethnically diverse genetic epidemiology studies. It is possible that
SLC13A2 polymorphisms other than rs11568476—such as rs11567842 (I550V)—may be more relevant in this context, as previously demonstrated in Thai and Japanese cohorts. These variants may exhibit distinct effects on transporter function and citrate handling, highlighting the polygenic and complex nature of hypocitraturia pathogenesis [
20].
Another significant aspect of our findings was the lack of correlation between hypocitraturia and the studied polymorphism, supporting the view that this condition is not attributable to a single-gene defect in most cases [
21]. Our study also revealed that serum uric acid levels were significantly higher in the hypocitraturic group, suggesting the presence of broader metabolic derangements. Hyperuricemia may reflect underlying acid-base disturbances or increased purine metabolism, both of which could influence citrate excretion through renal tubular pathways [
22].
Additionally, a higher prevalence of family history of kidney stones in the normocitraturic group was a counterintuitive finding. While this may be due to sampling variation, reporting bias, or small subgroup sizes, it invites further exploration of non-citrate-related mechanisms of genetic inheritance in urolithiasis [
23,
24].
The absence of rs11568476 across the entire cohort raises questions regarding potential genotyping errors, as highlighted by one reviewer. However, our laboratory used validated protocols (Roche™ LightCycler 480, High Pure Template Kit, Roche Diagnostics GmbH, Mannheim, Germany), and internal controls confirmed procedural integrity. Moreover, melting curve analyses consistently showed wild-type signatures, making an analytical artifact unlikely. Nonetheless, replication in independent cohorts is warranted [
25].
From a clinical standpoint, our findings suggest that rs11568476 is unlikely to serve as a useful genetic marker for hypocitraturia screening in Turkish stone formers. Given its rarity, routine genotyping for this variant appears to have limited utility in this population. Broader sequencing or SNP array-based approaches may be more informative, especially when combined with phenotyping for dietary habits, acid-base status, and renal function [
26,
27].
Therapeutically, the identification of individuals with hypocitraturia remains important regardless of genotype. Potassium citrate supplementation remains the cornerstone of treatment. However, novel approaches—including modulation of NaDC1 expression or function via SGLT2 inhibitors or lithium citrate—represent emerging strategies, and understanding genetic regulators of citrate transport could improve personalization of such interventions [
28,
29].
Despite its contributions, our study has several limitations. The relatively small sample size and lack of a healthy control group reduce statistical power and generalizability. The cross-sectional design prevents causal inference, and the evaluation of a single polymorphism limits the genetic scope. Furthermore, certain environmental and dietary variables that could impact citrate metabolism were not rigorously controlled or quantified. Methodologically, potential errors in 24 h urine collection and the lack of multiple measurements may have influenced citrate classification. Finally, the absence of rs11568476 may reflect a true population-specific rarity, but an undetected technical issue, although unlikely, cannot be completely ruled out.
5. Conclusions
This study investigated the presence and potential clinical relevance of the rs11568476 (V477M) polymorphism in the SLC13A2 gene, which encodes the sodium-dependent dicarboxylate cotransporter 1 (NaDC1)—a key membrane transporter involved in renal citrate handling. Contrary to our initial hypothesis, none of the 90 Turkish individuals with calcium-containing kidney stones exhibited this polymorphism, regardless of their urinary citrate status.
This negative finding is important, as it highlights potential ethnic or population-specific differences in the genetic architecture of hypocitraturia. The absence of rs11568476 in our cohort suggests that this variant is likely rare or absent in the Turkish population and does not contribute significantly to the pathogenesis of hypocitraturia in this context. These results are aligned with the limited data available in the literature and further emphasize the need for population-specific genetic screening strategies in nephrolithiasis.
In addition, the significantly higher serum uric acid levels in the hypocitraturic group, along with the unexpected distribution of family history, indicate that multifactorial and potentially non-genetic mechanisms may underlie hypocitraturia and stone formation in these patients. These findings may warrant broader metabolic and genetic evaluation beyond single SNP analysis.
From a methodological standpoint, this study is strengthened by rigorous biochemical characterization and validated genotyping, yet limitations such as the modest sample size and lack of broader SNP screening should be acknowledged. Future research should consider expanding the genetic scope to include other functional variants of SLC13A2, such as rs11567842, and exploring additional genes involved in citrate metabolism and renal tubular transport.
In conclusion, while rs11568476 may have functional relevance in other populations, its absence in our cohort suggests limited utility as a biomarker for hypocitraturia or calcium stone disease in Turkish patients. Larger-scale, multiethnic studies integrating genomic, metabolomic, and clinical data will be crucial to fully elucidate the genetic landscape of hypocitraturia and guide personalized prevention and treatment strategies in urolithiasis.
Author Contributions
Conceptualization, D.B. and E.B.; methodology, D.B. and S.D.; software, Y.Ş. and E.B.; validation, D.B., A.Y., S.D. and A.T.; formal analysis, D.B. and M.A.K.; investigation, S.D., E.B., A.Y. and A.T.; resources, D.B., E.B., Y.Ş. and M.A.K.; data curation, E.B. and Y.Ş.; writing—original draft preparation, D.B. and E.B.; writing—review and editing, D.B., A.Y., S.D., E.B., Y.Ş., M.A.K., A.Y. and A.T.; visualization, D.B. and E.B.; supervision, D.B. and A.T.; project administration, D.B., A.Y. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research and/or authorship of this article.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and ethical approval for this study was obtained from the Clinical Research Ethics Committee of Düzce University Faculty of Medicine (decision No. 2015/135, Date: 3 March 2015). This study was conducted in accordance with the Declaration of Helsinki, and the confidentiality of patient data was fully protected.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AE1 | Anion Exchanger 1 (encoded by SLC4A1 gene) |
| ALDOB | Aldolase B, Fructose-Bisphosphate (gene) |
| ATP6V1B1 | ATPase H⁺ Transporting V1 Subunit B1 (gene) |
| CA2 | Carbonic Anhydrase II (gene) |
| CI | Confidence Interval |
| COS-7 | Cell line derived from African green monkey kidney tissue |
| LiC | Lithium Carbonate |
| LiCit | Lithium Citrate |
| mg/dl | Milligrams per Deciliter |
| mg/1.73 m2/24 h | Milligrams per 1.73 square meters per 24 h |
| NaDC1 | Sodium-dependent Dicarboxylate Cotransporter 1 |
| NBCe1-A | Electrogenic Sodium Bicarbonate Cotransporter 1-A (SLC4A4) |
| OR | Odds Ratio |
| PCT | Proximal Convoluted Tubule |
| PCR | Polymerase Chain Reaction |
| PST-MR | Proximal Straight Tubule-Medullary Ray |
| SLC13A2 | Solute Carrier Family 13 Member 2 (gene) |
| SNP | Single Nucleotide Polymorphism |
| SPSS | Statistical Package for the Social Sciences |
| Tm | Melting Temperature |
References
- Phillips, R.; Hanchanale, V.S.; Myatt, A.; Somani, B.; Nabi, G.; Biyani, C.S. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst. Rev. 2015, 2015, Cd010057. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, A.; Memik, Ö.; Düzenli, S.; Tekin, A. The Association Between Sodium Citrate Cotransporter (NaDC-1) Gene Polymorphism and Urinary Citrate Excretion in Patients with Calcium-containing Kidney Stones. J. Urol. Surg. 2023, 10, 290–294. [Google Scholar] [CrossRef]
- Zuckerman, J.M.; Assimos, D.G. Hypocitraturia: Pathophysiology and medical management. Rev. Urol. 2009, 11, 134–144. [Google Scholar] [PubMed]
- Sloan, M.; Borofsky, M.S. Hypocitraturia: Diagnosis and Treatment. Urol. Clin. N. Am. 2025, 52, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Castellani, D.; Giulioni, C. Dietary management of hypocitraturia in children with urolithiasis: Results from a systematic review. World J. Urol. 2023, 41, 1243–1250. [Google Scholar] [CrossRef]
- Ganesan, C.; Thomas, I.C.; Montez-Rath, M.E.; Chertow, G.M. Hypocitraturia and Risk of Bone Disease in Patients with Kidney Stone Disease. J. Bone Miner. Res. Plus 2023, 7, e10786. [Google Scholar] [CrossRef]
- Lee, H.W.; Handlogten, M.E.; Osis, G.; Clapp, W.L.; Wakefield, D.N.; Verlander, J.W.; Weiner, I.D. Expression of sodium-dependent dicarboxylate transporter 1 (NaDC1/SLC13A2) in normal and neoplastic human kidney. Am. J. Physiol. Ren. Physiol. 2017, 312, F427–F435. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Yu, Z.; Wu, D.; Lv, Y.; Shi, S.; Zhu, H. Sodium dicarboxylate cotransporter-1 expression in renal tissues and its role in rat experimental nephrolithiasis. J. Nephrol. 2004, 17, 34–42. [Google Scholar]
- Osis, G.; Webster, K.L.; Harris, A.N.; Lee, H.W.; Chen, C.; Fang, L.; Romero, M.F.; Khattri, R.B.; Merritt, M.E.; Verlander, J.W. Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A. Am. J. Physiol. Ren. Physiol. 2019, 317, F489–F501. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Baldini, N.; Ulivieri, F.M.; Caudarella, R. Role of citrate in pathophysiology and medical management of bone diseases. Nutrients 2019, 11, 2576. [Google Scholar] [CrossRef]
- Udomsilp, P.; Saepoo, S.; Ittiwut, R.; Shotelersuk, V.; Dissayabutra, T.; Boonla, C. rs11567842 SNP in SLC13A2 gene associates with hypocitraturia in Thai patients with nephrolithiasis. Genes Genom. 2018, 40, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Pajor, A.M.; Sun, N.N. Single nucleotide polymorphisms in the human Na+-dicarboxylate cotransporter affect transport activity and protein expression. Am. J. Physiol. Ren. Physiol. 2010, 299, F704–F711. [Google Scholar] [CrossRef] [PubMed]
- Praditsap, O.; Sawasdee, N.; Pungsrinont, T.; Sritippayawan, S.; Ahsan, N.; Yenchitsomanus, P.-t.; Rungroj, N. Genetic heterogeneity of kidney stone disease in northeastern Thai patients. Genom. Genet. 2022, 15, 1–15. [Google Scholar]
- Basulto-Martínez, M.; Peña-Espinoza, B.; Valdez-Ortiz, R.; Escalante-Sosa, R.; Flores-Tapia, J.P.; Menjivar, M. High prevalence of hypocitraturia in stone formers from the Maya region of Yucatan, Mexico. Arch. Med. Res. 2022, 53, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Ullah, A.; Ahmad, J.; Hamid, S. The prevalence of incidentally detected urolithiasis in subjects undergoing computerized tomography. Cureus 2020, 12, e10374. [Google Scholar] [CrossRef]
- Subasi, B.; Gökçe, İ.; Delil, K.; Alpay, H. Vitamin D receptor gene polymorphisms in children with kidney stone disease. Turk. J. Pediatr. 2017, 59, 404–409. [Google Scholar] [CrossRef]
- Caudarella, R.; Vescini, F. Urinary citrate and renal stone disease: The preventive role of alkali citrate treatment. Arch. Ital. Urol. Androl. 2009, 81, 182–187. [Google Scholar]
- Peerapen, P.; Thongboonkerd, V. Kidney Stone Prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef]
- Elshamaa, M.F.; Fadel, F.I. Genetic polymorphisms in CLDN14 (rs219780) and ALP (rs1256328) genes are associated with risk of nephrolithiasis in Egyptian children. Turk. J. Urol. 2021, 47, 73–80. [Google Scholar] [CrossRef]
- Reddy, S.K.; Shaik, A.B.; Bokkisam, S. Effect of potassium magnesium citrate and vitamin B-6 prophylaxis for recurrent and multiple calcium oxalate and phosphate urolithiasis. Korean J. Urol. 2014, 55, 411. [Google Scholar] [CrossRef][Green Version]
- Krader, C.G. Hypocitraturia leading metabolic factor in kids with stones. Urol. Times 2012, 40, 12. [Google Scholar]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular biological and clinical understanding of the pathophysiology and treatments of hyperuricemia and its association with metabolic syndrome, cardiovascular diseases and chronic kidney disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ge, J.; Han, W.; Wang, D.; Zhao, Y.; Shen, Y.; Chen, J.; Chen, D.; Wu, J.; Shen, N.; et al. Risk factors for kidney stone disease recurrence: A comprehensive meta-analysis. BMC Urol. 2022, 22, 62. [Google Scholar] [CrossRef]
- Bokhari, A.; Alghamdi, A.A.M.; Khushayl, A.M.A.; Alaklabi, S.N.A.; Albarrak, S.K.A.; Aldarwish, H.A. Prevalence and Risk Factors of Renal Stones Among the Bisha Population, Saudi Arabia. Cureus 2023, 15, e40090. [Google Scholar] [CrossRef]
- Cho, M.H.; Ciulla, D.; Klanderman, B.J.; Raby, B.A.; Silverman, E.K. High-resolution melting curve analysis of genomic and whole-genome amplified DNA. Clin. Chem. 2008, 54, 2055–2058. [Google Scholar] [CrossRef]
- Dika, Ž.; Živko, M.; Kljajić, M.; Jelaković, B. SGLT2 Inhibitors and Their Effect on Urolithiasis: Current Evidence and Future Directions. J. Clin. Med. 2024, 13, 6017. [Google Scholar] [CrossRef]
- Sisakht, A.R.; Kamkar, P.; Darzi, M.M.; Madani, M.H.; Arismani, R.J.; Gharebakhshi, F.; Behi, M.; Mardanparvar, H.; Haghighi10, R. The effect of SGLT2 inhibitors on kidney stones; a systematic review and meta-analysis. J. Ren. Inj. Prev. 2024, e32292. [Google Scholar] [CrossRef]
- Inoue, K.; Zhuang, L.; Maddox, D.M.; Smith, S.B.; Ganapathy, V. Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J. Biol. Chem. 2002, 277, 39469–39476. [Google Scholar] [CrossRef]
- He, Y.; Chen, X.; Yu, Z. The change of human Na+/dicarboxylate co-transporter 1 expression in the kidney and its relationship with pathogenesis of nephrolithiasis. Zhonghua Yi Xue Za Zhi 2001, 81, 1066–1069. [Google Scholar] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).