High Prevalence of Autosomal Recessive Alport Syndrome in Roma Population of Eastern Slovakia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient Identification and Recruitment
2.3. Genetic Analysis
2.4. Clinical Parameters
2.5. Statistics
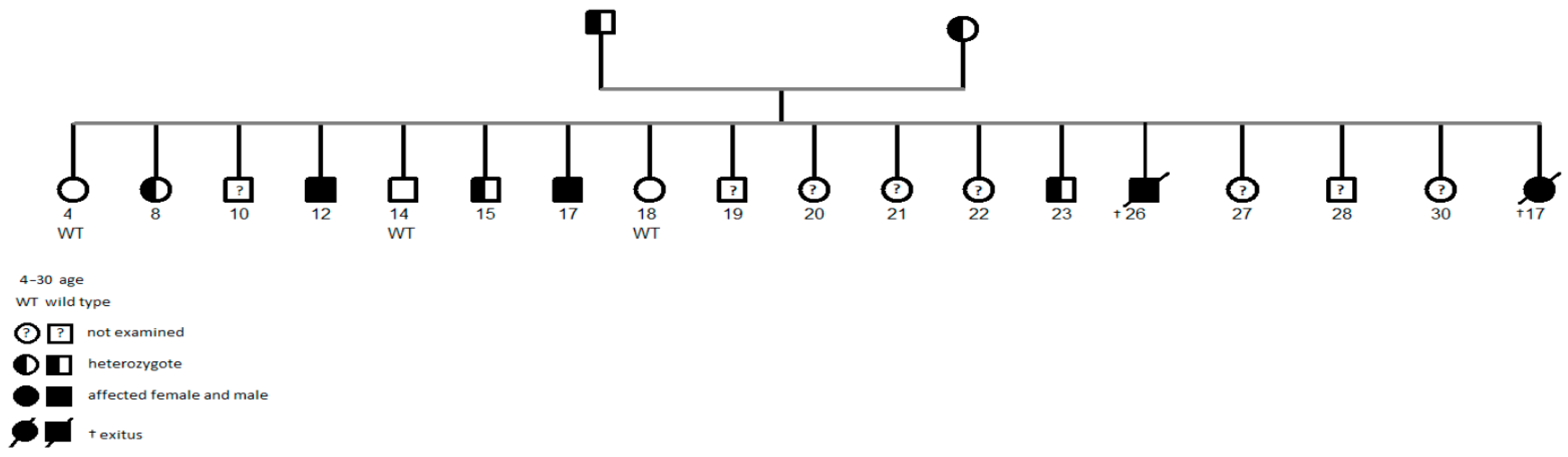
3. Results
3.1. Epidemiology
3.2. Presenting Symptoms of Patients with ARAS Pathogenic Variant c.1598G>A (p.Gly533Asp)
3.3. Growth and Associated Features of Pathogenic Variant c.1598G>A (p.Gly533Asp)
4. Discussion
4.1. Epidemiology
4.2. Clinical Presentation and Progression of Patients with ARAS Pathogenic Variant c.1598G>A (p.Gly533Asp)
4.3. Screening Strategy Implications
4.4. Strengths and Limitations
4.5. Future Directions and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashtan, C.E. Alport syndrome: Achieving early diagnosis and treatment. Am. J. Kidney Dis. 2021, 77, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Fieldhouse, R.; Chan, M.M.; Sadeghi-Alavijeh, O.; Burnett, L.; Izzi, V.; Persikov, A.V.; Gale, D.P.; Storey, H.; Savige, J.; et al. Prevalence Estimates of Predicted Pathogenic COL4A3-COL4A5 Variants in a Population Sequencing Database and Their Implications for Alport Syndrome. J. Am. Soc. Nephrol. 2021, 32, 2273–2290. [Google Scholar] [CrossRef]
- National Organization for Rare Disorders (NORD). Alport Syndrome—Symptoms, Causes, Treatment. 29 October 2024. Available online: https://rarediseases.org/rare-diseases/alport-syndrome/ (accessed on 2 June 2025).
- Orphanet. Alport Syndrome. Orphanet. March 2020. Available online: https://www.orpha.net/en/disease/detail/63 (accessed on 2 June 2025).
- Watson, S.; Padala, S.A.; Hashmi, M.F.; Bush, J.S. Alport Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 14 August 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470419/ (accessed on 2 May 2025).
- Storey, H.; Savige, J.; Sivakumar, V.; Abbs, S.; Flinter, F.A. COL4A3/COL4A4 mutations and features in individuals with autosomal recessive Alport syndrome. J. Am. Soc. Nephrol. 2013, 24, 1945–1954. [Google Scholar] [CrossRef]
- Hertz, J.M.; Thomassen, M.; Storey, H.; Flinter, F. Clinical utility gene card for: Alport syndrome. Eur. J. Hum. Genet. 2012, 20, 84–90. [Google Scholar] [CrossRef]
- Wang, D.; Pan, M.; Li, H.; Li, M.; Li, P.; Xiong, F.; Xiao, H. Four novel mutations identified in the COL4A3, COL4A4 and COL4A5 genes in 10 families with Alport syndrome. BMC Med. Genom. 2024, 17, 181. [Google Scholar] [CrossRef]
- Lim, T.S.T.; Koh, C.T.; Savige, J.; Ng, A.Y.-J.; Ng, J.L.; Chin, H.-L.; Lim, W.K.; Chan, G.C.; Yeo, S.C.; Leow, E.H.M.; et al. Pathogenic variants in the Alport genes are prevalent in the Singapore multiethnic population with highest frequency in the Chinese. Sci. Rep. 2025, 15, 7691. [Google Scholar] [CrossRef]
- de Araújo, W.C.; Falcão, R.M.; Uchoa, R.A.C.; Garcia, C.A.; da Silva, A.Q.B.; Quirino, K.L.M.; Freire-Neto, F.P.; Gurgel, G.P.; Nascimento, P.R.P.; Ferreira, L.C.; et al. Whole exome sequencing shows novel COL4A3 and COL4A4 variants as causes of Alport syndrome in Rio Grande do Norte, Brazil. BMC Genom. 2025, 26, 331. [Google Scholar] [CrossRef]
- Webb, B.; Brandt, T.; Liu, L.; Jalas, C.; Liao, J.; Fedick, A.; Linderman, M.D.; Diaz, G.; Kornreich, R.; Trachtman, H.; et al. A founder mutation in COL4A3 causes autosomal recessive Alport syndrome in the Ashkenazi Jewish population. Clin. Genet. 2014, 86, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pierides, A.; Voskarides, K.; Athanasiou, Y.; Ioannou, K.; Damianou, L.; Arsali, M.; Zavros, M.; Pierides, M.; Vargemezis, V.; Patsias, C.; et al. Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol. Dial. Transplant. 2009, 24, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, K.; Imtiaz, F.; Taibah, K.; Alnufiee, S.; Akhtar, M.; Al-Hazzaa, S.A.; Al-Owain, M. COL4A4-related nephropathy caused by a novel mutation in a large consanguineous Saudi family. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 427–432. [Google Scholar] [CrossRef]
- Plevova, P.; Indrakova, J.; Savige, J.; Kuhnova, P.; Tvrda, P.; Cerna, D.; Hilscherova, S.; Kudrejova, M.; Polendova, D.; Jaklova, R.; et al. A founder COL4A4 pathogenic variant resulting in autosomal recessive Alport syndrome accounts for most genetic kidney failure in Romani people. Front. Med. 2023, 10, 1096869. [Google Scholar] [CrossRef]
- Ena, G.F.; Giménez, A.; Carballo-Mesa, A.; Lišková, P.; e Silva, M.A.C.; Comas, D. The genetic footprint of the European Roma diaspora: Evidence from the Balkans to the Iberian Peninsula. Hum. Genet. 2025, 144, 463–479. [Google Scholar] [CrossRef]
- Kolvek, G.; Rosicova, K.; Rosenberger, J.; Podracka, L.; Stewart, R.E.; Nagyova, I.; Reijneveld, S.A.; van Dijk, J.P. End-stage renal disease among Roma and non-Roma: Roma are at risk. Int. J. Public Health 2012, 57, 751–754. [Google Scholar] [CrossRef]
- Gadalean, F.; Lighezan, D.; Stoian, D.; Schiller, O.; Timar, R.; Timar, B.; Bob, F.; Donciu, M.D.; Munteanu, M.; Mihaescu, A.; et al. The Survival of Roma Minority Patients on Chronic Hemodialysis Therapy—A Romanian Multicenter Survey. PLoS ONE 2016, 11, e0155271. [Google Scholar] [CrossRef]
- Statistical Office of the Slovak Republic. Population and Migration [Internet]; Statistical Office of the Slovak Republic: Bratislava, Slovakia, 2025; Available online: https://infopanel.statistics.sk/population.php?lang=en (accessed on 2 June 2025).
- Mendizabal, I.; Lao, O.; Marigorta, U.M.; Wollstein, A.; Gusmão, L.; Ferak, V.; Ioana, M.; Jordanova, A.; Kaneva, R.; Kouvatsi, A.; et al. Reconstructing the Population History of European Romani from Genome-wide Data. Curr. Biol. 2012, 22, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.H.; Kent, J.O.; Wittwer, C.T. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics 2007, 8, 597–608. [Google Scholar] [CrossRef]
- Erali, M.; Voelkerding, K.V.; Wittwer, C.T. High resolution melting applications for clinical laboratory medicine. Exp. Mol. Pathol. 2008, 85, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Grievink, H.; Stowell, K.M. Identification of ryanodine receptor 1 single nucleotide polymorphisms by high-resolution melting using the light-cycler 480 system. Anal. Biochem. 2008, 374, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Ševčíková, Ľ.; Nováková, J.; Hamade, J. Percentilové Grafy a Antropometrické Ukazovatele: Telesný vývoj detí a Mládeže v SR; Úrad verejného zdravotníctva: Bratislava, Slovakia, 2004; pp. 16–103. [Google Scholar]
- Voskarides, K.; Patsias, C.; Pierides, A.; Deltas, C. COL4A3 founder mutations in Greek Cypriot families with thin basement membrane nephropathy and focal segmental glomerulosclerosis dating from around 18th century. Genet. Test. 2008, 12, 273–278. [Google Scholar] [CrossRef]
- Kalaydjieva, L.; Gresham, D.; Calafell, F. Genetic studies of the Roma (Gypsies): A review. BMC Med. Genet. 2001, 2, 5. [Google Scholar] [CrossRef]
- Malarska, M.; Moczulska, H.; Pachniak, P.; Gadzalska, K.; Jakiel, P.; Gorządek, M.; Juścińska, E.; Pietrusiński, M.; Mazerant, M.; Pukajło-Marczyk, A.; et al. Phenotype-genotype correlations in patients with Alport syndrome from the Polish population. J. Nephrol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.S.L.; Yamamura, T.; Shenoy, M.; Stuart, H.M.; Lennon, R. Detection of Alport gene variants in children and young people with persistent haematuria. Pediatr. Nephrol. 2024, 40, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Savige, J.; Lipska-Zietkiewicz, B.S.; Watson, E.; Hertz, J.M.; Deltas, C.; Mari, F.; Hilbert, P.; Plevova, P.; Byers, P.; Cerkauskaite, A.; et al. Guidelines for Genetic Testing and Management of Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2022, 17, 143–154. [Google Scholar] [CrossRef]
- Gomes, A.M.; Reis, C.F.; Beirão, I.; Malheiro, J.; Lemos, C.; Lopes, D.; Ferreira, G.; Santos, H.; Leão, M.; Almeida, C. Alport syndrome family screening and management—Experience of a tertiary center. Nephrol. Dial. Transplant. 2024, 39 (Suppl. S1), gfae069-0404-1468. [Google Scholar] [CrossRef]
- Gross, O.; Licht, C.; Anders, H.J.; Hoppe, B.; Beck, B.; Tönshoff, B.; Höcker, B.; Wygoda, S.; Ehrich, J.H.; Pape, L.; et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012, 81, 494–501. [Google Scholar] [CrossRef]
- Christodoulaki, V.; Kosma, K.; Marinakis, N.M.; Tilemis, F.-N.; Stergiou, N.; Kampouraki, A.; Kapogiannis, C.; Karava, V.; Mitsioni, A.; Mila, M.; et al. Alport Syndrome: Clinical Utility of Early Genetic Diagnosis in Children. Genes 2024, 15, 1016. [Google Scholar] [CrossRef]
- Chavez, E.; Rodriguez, J.; Drexler, Y.; Fornoni, A. Novel Therapies for Alport Syndrome. Front. Med. 2022, 9, 848389. [Google Scholar] [CrossRef]
- Mahrous, N.N.; Jamous, Y.F.; Almatrafi, A.M.; Fallatah, D.I.; Theyab, A.; Alanati, B.H.; Alsagaby, S.A.; Alenazi, M.K.; Khan, M.I.; Hawsawi, Y.M. A Current Landscape on Alport Syndrome Cases: Characterization, Therapy and Management Perspectives. Biomedicines 2023, 11, 2762. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Q.; Xie, J. Exploration of Gene Therapy for Alport Syndrome. Biomedicines 2024, 12, 1159. [Google Scholar] [CrossRef]
- Huang, H.-X.; Tsai, I.-J.; Greenbaum, L.A. Alport syndrome: Expanding diagnosis and treatment. Pediatr. Neonatol. 2025, 66 (Suppl. S1), S13–S17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Böckhaus, J.; Wang, F.; Wang, S.; Rubel, D.; Gross, O.; Ding, J. Genotype–phenotype correlations and nephroprotective effects of RAAS inhibition in patients with autosomal recessive Alport syndrome. Pediatr. Nephrol. 2021, 36, 2719–2730. [Google Scholar] [CrossRef]
- Lujinschi, Ș.N.; Sorohan, B.M.; Obrișcă, B.; Vrabie, A.; Lupușoru, G.; Achim, C. Genotype–Phenotype Correlations in Alport Syndrome—A Single-Center Experience. Genes 2024, 15, 593. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Kandemir, N.; Saat, H. Expanding the genotype–phenotype correlations in Alport syndrome: Novel mutations, digenic inheritance, and genetic modifiers. Egypt. J. Med. Hum. Genet. 2023, 24, 59. [Google Scholar] [CrossRef]
- Yamamura, T.; Horinouchi, T.; Nagano, C.; Omori, T.; Sakakibara, N.; Aoto, Y.; Ishiko, S.; Nakanishi, K.; Shima, Y.; Nagase, H.; et al. Genotype-phenotype correlations influence the response to angiotensin-targeting drugs in Japanese patients with male X-linked Alport syndrome. Kidney Int. 2020, 98, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, V.; Caparali, E.B.; Shojaei, A.; Ricardo, S.; Barua, M. Alport Syndrome: Clinical Spectrum and Therapeutic Advances. Kidney Med. 2023, 5, 100631. [Google Scholar] [CrossRef] [PubMed]
- Nozu, K.; Takaoka, Y.; Kai, H.; Takasato, M.; Yabuuchi, K.; Yamamura, T.; Horinouchi, T.; Sakakibara, N.; Ninchoji, T.; Nagano, C.; et al. Genetic background, recent advances in molecular biology, and development of novel therapy in Alport syndrome. Kidney Res. Clin. Pract. 2020, 39, 402–413. [Google Scholar] [CrossRef]

| Inheritance Pattern | COL4 Chain | Pathogenic Variant | Age at Verification | CKD Stage at Verification | Previous Verification | Ethnicity |
|---|---|---|---|---|---|---|
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 12 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 11 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 5 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 5 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 10 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 8 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 17 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 4 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 24 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 15 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 26 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 20 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 36 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 50 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 11 | 4 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 39 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 35 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 27 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 21 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | n.a. | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 17 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | n.a. | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 15 | 1 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 28 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 32 | 5 | - | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 38 (15) | 5 (3) | KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 35 (20) | 5 (3) | KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 25 (21) | 5 (3) | KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 31 (18) | 5 (3) | KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 8 (8) | 5 (5) | KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 17 (15, 16) | 4 (3, 4) | G + KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 20 (12, 19) | 1 (1, 1) | G + KBx | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 16 (15) | 5 (5) | G | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 4 (3) | 1 (1) | G | R |
| ARAS | Alpha 4 | c.1598G>A (p.Gly533Asp) | 16 (15) | 1 (1) | G | R |
| Digenic | Alpha3 + Alpha4 | c.1598G>A (p.Gly533Asp) c.415G>C (p.Gly139Arg) | 13 (11) | 1 (1) | G | R |
| Digenic | Alpha3 + Alpha4 | c.1598G>A (p.Gly533Asp) c.415G>C (p.Gly139Arg) | 40 | 5 | - | R |
| XLAS | Alpha 5 | c.1871G>A (p.Gly624Asp) | 48 | n.a. | - | Non-R |
| XLAS | Alpha 5 | c.1871G>A (p.Gly624Asp) | 17 | 1 | - | Non-R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koľvek, G.; Klimčáková, L.; Hrčková, G.; Židzik, J.; Podracká, Ľ.; Baltesová, T.; Kubejová, K.; Rosenberger, J.; Barkai, L. High Prevalence of Autosomal Recessive Alport Syndrome in Roma Population of Eastern Slovakia. Biomedicines 2025, 13, 1960. https://doi.org/10.3390/biomedicines13081960
Koľvek G, Klimčáková L, Hrčková G, Židzik J, Podracká Ľ, Baltesová T, Kubejová K, Rosenberger J, Barkai L. High Prevalence of Autosomal Recessive Alport Syndrome in Roma Population of Eastern Slovakia. Biomedicines. 2025; 13(8):1960. https://doi.org/10.3390/biomedicines13081960
Chicago/Turabian StyleKoľvek, Gabriel, Lucia Klimčáková, Gabriela Hrčková, Jozef Židzik, Ľudmila Podracká, Tatiana Baltesová, Kristína Kubejová, Jaroslav Rosenberger, and László Barkai. 2025. "High Prevalence of Autosomal Recessive Alport Syndrome in Roma Population of Eastern Slovakia" Biomedicines 13, no. 8: 1960. https://doi.org/10.3390/biomedicines13081960
APA StyleKoľvek, G., Klimčáková, L., Hrčková, G., Židzik, J., Podracká, Ľ., Baltesová, T., Kubejová, K., Rosenberger, J., & Barkai, L. (2025). High Prevalence of Autosomal Recessive Alport Syndrome in Roma Population of Eastern Slovakia. Biomedicines, 13(8), 1960. https://doi.org/10.3390/biomedicines13081960







