Taste Dysfunction in Head and Neck Cancer: Pathophysiology and Clinical Management—A Comprehensive Review
Abstract
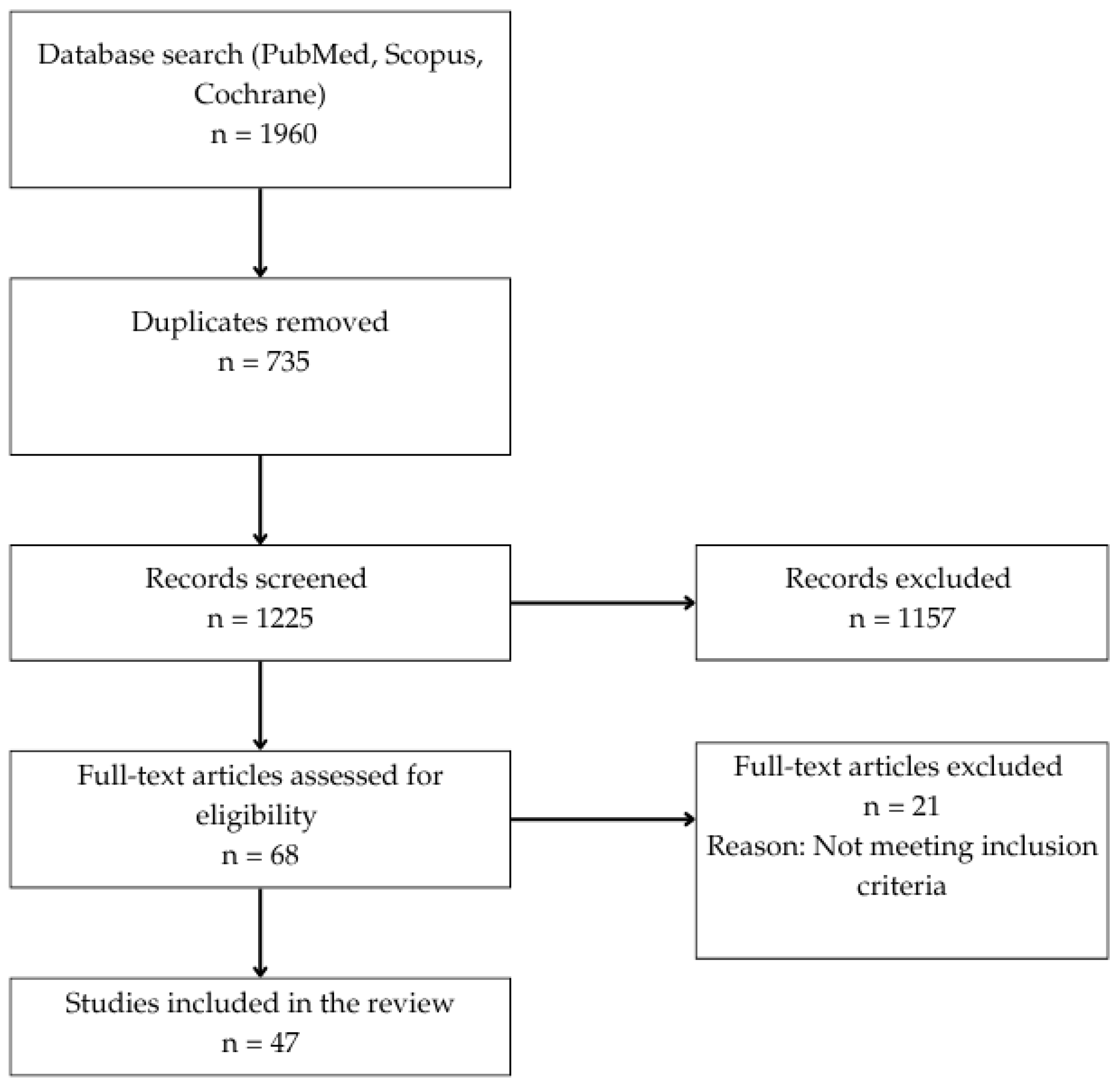
1. Introduction
2. Materials and Methods
2.1. Objective and Review Design
2.2. Literature Search Strategy
2.3. Study Selection Process
2.4. Eligibility Criteria
2.5. Data Extraction and Synthesis
2.6. Quality Considerations
3. Results
3.1. Taste Dysfunction Caused by Cancer Itself
Surgical Resection as an Independent Contributor to Taste Dysfunction
3.2. Taste Dysfunction Caused by Chemotherapy
3.3. Taste and Salivary Dysfunction Caused by Radiotherapy
3.4. Clinical Management of Taste Dysfunction in Oropharyngeal Cancer Patients
3.4.1. Dietary and Nutritional Interventions
Nutritional Supplements
3.4.2. Pharmacological Treatments
3.4.3. Non-Pharmacological Interventions
Surgical Reconstruction and Gustatory Preservation
Complementary and Integrative Therapies
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
- The following search strategy was used in PubMed to identify relevant studies published between 1 January 2015 and 28 February 2025, focusing on taste dysfunction in patients with head and neck cancer (HNC):
- The search combined MeSH terms and free-text keywords as follows:
- (“Head and Neck Neoplasms” [MeSH Terms] OR “Oropharyngeal Neoplasms” [MeSH Terms] OR “oral cancer” OR “head and neck cancer” OR “oropharyngeal cancer”) AND (“Taste Disorders” [MeSH Terms] OR dysgeusia OR ageusia OR “taste dysfunction”) AND (“2015/01/01” [Date—Publication]: “2025/02/28” [Date—Publication]) AND (English [Language]).
- The following filters were applied: (1) Species (Humans); (2) language (English); (3) publication date (2015–2025).
- This search strategy was designed to ensure the inclusion of studies relevant to gustatory dysfunction in patients affected by head and neck cancer, with no limitations regarding study design.
References
- Pugnaloni, S.; Vignini, A.; Borroni, F.; Sabbatinelli, J.; Alia, S.; Fabri, M.; Taus, M.; Mazzanti, L.; Berardi, R. Modifications of taste sensitivity in cancer patients: A method for the evaluations of dysgeusia. Support. Care Cancer 2020, 28, 1173–1181. [Google Scholar] [CrossRef]
- Murtaza, B.; Hichami, A.; Khan, A.S.; Ghiringhelli, F.; Khan, N.A. Alteration in taste perception in cancer: Causes and strategies of treatment. Front. Physiol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Fábián, T.; Beck, A.; Fejérdy, P.; Hermann, P.; Fábián, G. Molecular mechanisms of taste recognition: Considerations about the role of saliva. Int. J. Mol. Sci. 2015, 16, 5945–5974. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Moore, A.; Yarom, N.; Popovtzer, A. The effect of radiotherapy on taste sensation in head and neck cancer patients–a prospective study. Radiat. Oncol. 2020, 15, 144. [Google Scholar] [CrossRef]
- Togni, L.; Mascitti, M.; Vignini, A.; Alia, S.; Sartini, D.; Barlattani, A.; Emanuelli, M.; Santarelli, A. Treatment-related dysgeusia in oral and oropharyngeal cancer: A comprehensive review. Nutrients 2021, 13, 3325. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. (Eds.) WHO Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017; Volume 9. [Google Scholar]
- Hong, J.H.; Omur-Ozbek, P.; Stanek, B.T.; Dietrich, A.M.; Duncan, S.E.; Lee, Y.W.; Lesser, G. Taste and odor abnormalities in cancer patients. J. Support. Oncol. 2009, 7, 8. [Google Scholar]
- Tharakan, T.; Piccirillo, J.F.; Miller, B.; Reed, D.R.; Kallogjeri, D.; Paniello, R.; Puram, S.V.; Jackson, R.S. Acute taste dysfunction in oropharyngeal cancer patients after transoral robotic surgery. Laryngoscope 2023, 133, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Rosati, D.; Mastino, P.; Romeo, M.; De Soccio, G.; Pentangelo, D.; Petrella, C.; Barbato, C.; Minni, A. Taste and smell alterations (TSAs) in cancer patients. Diseases 2024, 12, 130. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O′Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 version). In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; Joanna Briggs Institute: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 15 March 2025).
- Hannon, M.; Shaw, A.; Connolly, M.; Davies, A. Taste disturbance in patients with advanced cancer: A scoping review of clinical features and complications. Support. Care Cancer 2023, 31, 562. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Barasch, A. Taste disorders in cancer patients: Pathogenesis, and approach to assessment and management. Oral Oncol. 2010, 46, 77–81. [Google Scholar] [CrossRef]
- Riantiningtyas, R.R.; Dougkas, A.; Bredie, W.L.P.; Kwiecien, C.; Bruyas, A.; Philouze, P.; Giboreau, A.; Carrouel, F. Investigating oral somatosensory perception and oral symptoms of head and neck cancer patients: Insights on eating behaviour. Support. Care Cancer 2024, 32, 320. [Google Scholar] [CrossRef]
- Singh, A.G.; Dani, M.; Sinha, S.; Shetty, R.; Joshi, P.; Nair, S.; Chaturvedi, P. A prospective study to evaluate the association of sensory dysregulation and adverse pathologic features in oral tongue cancers. J. Maxillofac. Oral Surg. 2024. Available online: https://link.springer.com/10.1007/s12663-024-02410-2 (accessed on 20 March 2025).
- Lilja, M.; Markkanen-Leppänen, M.; Viitasalo, S.; Saarilahti, K.; Lindford, A.; Lassus, P.; Mäkitie, A. Olfactory and gustatory functions after free flap reconstruction and radiotherapy for oral and pharyngeal cancer: A prospective follow-up study. Eur. Arch. Otorhinolaryngol. 2018, 275, 959–966. [Google Scholar] [CrossRef]
- Uí Dhuibhir, P.; Barrett, M.; O’Donoghue, N.; Gillham, C.; El Beltagi, N.; Walsh, D. Self-reported and objective taste and smell evaluation in treatment-naive solid tumour patients. Support. Care Cancer 2020, 28, 2389–2396. [Google Scholar] [CrossRef]
- Cunha, M.D.D.; Terto, D.D.S.; Diniz, J.; Assis, R.B. Assessment of the gustatory function in patients with advanced oral cavity and oropharyngeal cancer. CoDAS 2020, 32, e20190122. [Google Scholar] [CrossRef]
- Abbas, S.A.; Tariq, M.U.U.; Raheem, A.; Saeed, J.; Hashmi, S.S.; Karim, M.; Nizam, M. Assessment of factors affecting quality of life in oral squamous cell carcinoma patients using University of Washington Quality of Life Questionnaire. Cureus 2019, 11, e5049. [Google Scholar] [CrossRef]
- Snyder, D.J.; Bartoshuk, L.M. Oral sensory nerve damage: Causes and consequences. Rev. Endocr. Metab. Disord. 2016, 17, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.Y.; Jiang, R.S.; Wang, C.P.; Wang, C.C.; Liu, S.A. Dysgeusia after comprehensive treatment for oral cavity cancer. Laryngoscope 2025, 135, 2869–2875. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Goda, M.; Fujimoto, C.; Kanno, K.; Nobe, M.; Kitamura, Y.; Abe, K.; Kawai, M.; Matsumoto, H.; Sakai, T.; et al. Effects of chemotherapy on gene expression of lingual taste receptors in patients with head and neck cancer: Effect of chemotherapy on taste receptor mRNA. Laryngoscope 2016, 126, E103–E109. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Crary, M.A.; Madhavan, A.; Gregorio, D.C.; Im, I.; Ross, S.E.; Carnaby, G.D. Dysphagia and oral morbidities in chemoradiation-treated head and neck cancer patients. Dysphagia 2018, 33, 739–748. [Google Scholar] [CrossRef]
- Epstein, J.B.; Villines, D.; Epstein, G.L.; Smutzer, G. Oral examination findings, taste and smell testing during and following head and neck cancer therapy. Support. Care Cancer 2020, 28, 4305–4311. [Google Scholar] [CrossRef]
- Palmieri, M.; Sarmento, D.J.S.; Falcão, A.P.; Martins, V.A.O.; Brandão, T.B.; Morais-Faria, K.; Ribeiro, A.C.P.; Hasséus, B.; Giglio, D.; Braz-Silva, P.H. Frequency and evolution of acute oral complications in patients undergoing radiochemotherapy treatment for head and neck squamous cell carcinoma. Ear Nose Throat J. 2021, 100 (Suppl. 5), 449S–455S. [Google Scholar] [CrossRef] [PubMed]
- Galitis, E.; Droukas, V.; Tzakis, M.; Psarras, V.; Galiti, D.; Kyrodimos, E.; Trichas, M.; Psyrri, A.; Papadogeorgakis, N.; Kouri, M.; et al. Trismus and reduced quality of life in patients with oral squamous cell carcinoma, who received post-operative radiotherapy alone or combined with chemotherapy. Forum Clin. Oncol. 2017, 8, 29–36. [Google Scholar] [CrossRef][Green Version]
- Malta, C.E.N.; De Lima Martins, J.O.; Carlos, A.C.A.M.; Freitas, M.O.; Magalhães, I.A.; De Vasconcelos, H.C.A.; de Lima Silva-Fernandes, I.J.; de Barros Silva, P.G. Risk factors for dysgeusia during chemotherapy for solid tumors: A retrospective cross-sectional study. Support. Care Cancer 2022, 30, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Messing, B.P.; Ward, E.C.; Lazarus, C.; Ryniak, K.; Maloney, J.; Thompson, C.B.; Kramer, E. Longitudinal comparisons of a whole-mouth taste test to clinician-rated and patient-reported outcomes of dysgeusia postradiotherapy in patients with head and neck cancer and associations with oral intake. Head Neck 2021, 43, 2159–2177. [Google Scholar] [CrossRef] [PubMed]
- Moroney, L.B.; Helios, J.; Ward, E.C.; Crombie, J.; Pelecanos, A.; Burns, C.L.; Spurgin, A.L.; Blake, C.; Kenny, L.; Chua, B.; et al. Helical intensity-modulated radiotherapy with concurrent chemotherapy for oropharyngeal squamous cell carcinoma: A prospective investigation of acute swallowing and toxicity patterns. Head Neck 2018, 40, 1955–1966. [Google Scholar] [CrossRef]
- Sio, T.T.; Lin, H.K.; Shi, Q.; Gunn, G.B.; Cleeland, C.S.; Lee, J.J.; Hernandez, M.; Blanchard, P.; Thaker, N.G.; Phan, J.; et al. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: First comparative results of patient-reported outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1107–1114. [Google Scholar] [CrossRef]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T.C. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol. Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Vempati, P.; Halthore, A.N.; Teckie, S.; Rana, Z.; Gogineni, E.; Antone, J.; Zhang, H.; Marrero, M.; Beadle, K.; Frank, D.K.; et al. Phase I trial of dose-escalated stereotactic radiosurgery (SRS) boost for unfavorable locally advanced oropharyngeal cancer. Radiat. Oncol. 2020, 15, 278. [Google Scholar] [CrossRef]
- Barbosa Da Silva, J.L.; Doty, R.L.; Miyazaki, J.V.M.K.; Borges, R.; Pinna, F.D.R.; Voegels, R.L.; Fornazieri, M.A. Gustatory disturbances occur in patients with head and neck cancer who undergo radiotherapy not directed to the oral cavity. Oral Oncol. 2019, 95, 115–119. [Google Scholar] [CrossRef]
- Morelli, I.; Desideri, I.; Romei, A.; Scoccimarro, E.; Caini, S.; Salvestrini, V.; Becherini, C.; Livi, L.; Bonomo, P. Impact of radiation dose on patient-reported acute taste alteration in a prospective observational study cohort in head and neck squamous cell cancer (HNSCC). Radiol. Med. 2023, 128, 1571–1579. [Google Scholar] [CrossRef]
- Stankevice, D.; Fjaeldstad, A.W.; Ovesen, T. Isolated taste disorders in patients referred to a flavor clinic with taste and smell loss. Brain Behav. 2021, 11, e02071. [Google Scholar] [CrossRef]
- Jin, S.; Lu, Q.; Sun, Y.; Xiao, S.; Zheng, B.; Pang, D.; Yang, P. Nutrition impact symptoms and weight loss in head and neck cancer during radiotherapy: A longitudinal study. BMJ Support. Palliat. Care 2021, 1, 17–24. [Google Scholar] [CrossRef]
- Negi, P.; Kingsley, P.A.; Thomas, M.; Sachdeva, J.; Srivastava, H.; Kalra, B. Pattern of gustatory impairment and its recovery after head and neck irradiation. Iran. J. Otorhinolaryngol. 2017, 29, 319–327. [Google Scholar] [PubMed]
- Martini, S.; Iorio, G.C.; Arcadipane, F.; Olivero, F.; Silvetti, P.; Rampino, M.; Garzino Demo, P.; Fasolis, M.; Pecorari, G.; Airoldi, M.; et al. Prospective assessment of taste impairment and nausea during radiotherapy for head and neck cancer. Med. Oncol. 2019, 36, 44. [Google Scholar] [CrossRef]
- Jin, S.; Lu, Q.; Jin, S.; Zhang, L.; Cui, H.; Li, H. Relationship between subjective taste alteration and weight loss in head and neck cancer patients treated with radiotherapy: A longitudinal study. Eur. J. Oncol. Nurs. 2018, 37, 43–50. [Google Scholar] [CrossRef]
- Kırca, K.; Kutlutürkan, S. Symptoms of patients with head and neck cancers undergoing radiotherapy. Eur. J. Cancer Care 2017, 26, e12584. [Google Scholar] [CrossRef]
- Alfaro, R.; Crowder, S.; Sarma, K.P.; Arthur, A.E.; Pepino, M.Y. Taste and smell function in head and neck cancer survivors. Chem. Senses 2021, 46, bjab026. [Google Scholar] [CrossRef] [PubMed]
- Mathlin, J.; Courtier, N.; Hopkinson, J. Taste changes during radiotherapy for head and neck cancer. Radiography 2023, 29, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Camacho, M.; Gonella, S.; Ghosh, S.; Kubrak, C.; Scrimger, R.A.; Chu, K.P.; Wismer, W.V. The impact of taste and smell alterations on quality of life in head and neck cancer patients. Qual. Life Res. 2016, 25, 1495–1504. [Google Scholar] [CrossRef]
- Sapir, E.; Tao, Y.; Feng, F.; Samuels, S.; El Naqa, I.; Murdoch-Kinch, C.A.; Feng, M.; Schipper, M.; Eisbruch, A. Predictors of dysgeusia in patients with oropharyngeal cancer treated with chemotherapy and intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 354–361. [Google Scholar] [CrossRef]
- Gamper, E.M.; Zabernigg, A.; Wintner, L.M.; Giesinger, J.M.; Oberguggenberger, A.; Kemmler, G.; Sperner-Unterweger, B.; Holzner, B. Coming to your senses: Detecting taste and smell alterations in chemotherapy patients. A systematic review. J. Pain Symptom Manag. 2012, 44, 880–895. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, N.; Zhang, Y.; Yan, J.; Chen, L.; He, H.; Sun, S.; Zhang, Y.; Zhang, M. Prevalence and risk factors of chemotherapy-induced taste alterations among cancer patients: A systematic review and meta-analysis. Eur. J. Oncol. Nurs. 2025, 74, 102735. [Google Scholar] [CrossRef]
- García-Chías, B.; Figuero, E.; Castelo-Fernández, B.; Cebrián-Carretero, J.L.; Cerero-Lapiedra, R. Prevalence of oral side effects of chemotherapy and its relationship with periodontal risk: A cross-sectional study. Support. Care Cancer 2019, 27, 3479–3490. [Google Scholar] [CrossRef]
- Wang, A.; Duncan, S.E.; Lesser, G.J.; Ray, W.K.; Dietrich, A.M. Effect of lactoferrin on taste and smell abnormalities induced by chemotherapy: A proteome analysis. Food Funct. 2018, 9, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Zabernigg, A.; Gamper, E.M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Sevryugin, O.; Kasvis, P.; Vigano, M.; Vigano, A. Taste and smell disturbances in cancer patients: A scoping review of available treatments. Support. Care Cancer 2021, 29, 49–66. [Google Scholar] [CrossRef]
- Kaizu, M.; Komatsu, H.; Yamauchi, H.; Sumitani, M.; Doorenbos, A.Z. Characteristics of taste alterations in people receiving taxane-based chemotherapy and their association with appetite, weight, and quality of life. Support. Care Cancer 2021, 29, 5103–5114. [Google Scholar] [CrossRef]
- Amézaga, J.; Alfaro, B.; Ríos, Y.; Larraioz, A.; Ugartemendia, G.; Urruticoechea, A.; Tueros, I. Assessing taste and smell alterations in cancer patients undergoing chemotherapy according to treatment. Support. Care Cancer 2018, 26, 4077–4086. [Google Scholar] [CrossRef] [PubMed]
- Gunn, L.; Gilbert, J.; Nenclares, P.; Soliman, H.; Newbold, K.; Bhide, S.; Wong, K.H.; Harrington, K.; Nutting, C. Taste dysfunction following radiotherapy to the head and neck: A systematic review. Radiother. Oncol. 2021, 157, 130–140. [Google Scholar] [CrossRef]
- Deshpande, T.S.; Blanchard, P.; Wang, L.; Foote, R.L.; Zhang, X.; Frank, S.J. Radiation-related alterations of taste function in patients with head and neck cancer: A systematic review. Curr. Treat. Options Oncol. 2018, 19, 72. [Google Scholar] [CrossRef]
- Kiss, N.; Symons, K.; Hewitt, J.; Davis, H.; Ting, C.; Lee, A.; Boltong, A.; Tucker, R.M.; Tan, S.Y. Taste function in adults undergoing cancer radiotherapy or chemotherapy, and implications for nutrition management: A systematic review. J. Acad. Nutr. Diet. 2021, 121, 278–304. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.J.; Myers, J.N.; Frank, S.J.; Aponte-Wesson, R.A.; Otun, A.O.; Nogueras-González, G.M.; Li, Y.; Geng, Y.; Chambers, M.S. The effects of zinc on radiation-induced dysgeusia: A systematic review and meta-analysis. Support. Care Cancer 2020, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Cancer Treat. Rev. 2010, 36, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Khan, A.H.; Safdar, J.; Siddiqui, S.U. Efficacy of zinc sulfate on concurrent chemoradiotherapy induced taste alterations in oral cancer patients: A double blind randomized controlled trial. Pak. J. Med. Sci. 2019, 35, 624–629. Available online: http://www.pjms.org.pk/index.php/pjms/article/view/503 (accessed on 25 March 2025). [CrossRef]
- Hoppe, C.; Kutschan, S.; Dörfler, J.; Büntzel, J.; Büntzel, J.; Huebner, J. Zinc as a complementary treatment for cancer patients: A systematic review. Clin. Exp. Med. 2021, 21, 297–313. [Google Scholar] [CrossRef]
- Lesser, G.J.; Irby, M.B.; Taylor, R.C.; Snavely, A.; Case, D.; Wang, A.; Dietrich, A.; Duncan, S. Lactoferrin supplementation for taste and smell abnormalities among patients receiving cancer chemotherapy. Support. Care Cancer 2022, 30, 2017–2025. [Google Scholar] [CrossRef]
- Shono, H.; Tsutsumi, R.; Beppu, K.; Matsushima, R.; Watanabe, S.; Fujimoto, C.; Kanamura, R.; Ohnishi, H.; Kondo, E.; Azuma, T.; et al. Dietary supplementation with monosodium glutamate suppresses chemotherapy-induced downregulation of the T1R3 taste receptor subunit in head and neck cancer patients. Nutrients 2021, 13, 2921. [Google Scholar] [CrossRef]
- Thorne, T.; Olson, K.; Wismer, W. A state-of-the-art review of the management and treatment of taste and smell alterations in adult oncology patients. Support. Care Cancer 2015, 23, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- López-Plaza, B.; Gil, Á.; Menéndez-Rey, A.; Bensadon-Naeder, L.; Hummel, T.; Feliú-Batlle, J.; Palma-Milla, S. Effect of regular consumption of a miraculin-based food supplement on taste perception and nutritional status in malnourished cancer patients: A triple-blind, randomized, placebo-controlled clinical trial. Nutrients 2023, 15, 4639. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, E.; Doweck, I.; Schiff, E.; Samuels, N. Exploring an integrative patient-tailored complementary medicine approach for chemotherapy-induced taste disorders. Explore 2018, 14, 289–294. [Google Scholar] [CrossRef]
- Heiser, C.; Hofauer, B.; Scherer, E.; Schukraft, J.; Knopf, A. Liposomal treatment of xerostomia, odor, and taste abnormalities in patients with head and neck cancer. Head Neck 2016, 38 (Suppl. 1). ahead of print. [Google Scholar]
- Epstein, J.B.; De Andrade E Silva, S.M.; Epstein, G.L.; Leal, J.H.S.; Barasch, A.; Smutzer, G. Taste disorders following cancer treatment: Report of a case series. Support. Care Cancer 2019, 27, 4587–4595. [Google Scholar] [CrossRef]
- Dalbem Paim, É.; Costa Batista Berbert, M.; Gonzales Zanella, V.; Beatris Martins, V.; Edler Macagnan, F. Effects of transcutaneous electrical nerve stimulation on the salivary flow of patients with hyposalivation induced by radiotherapy in the head and neck region—A randomised clinical trial. J. Oral Rehabil. 2019, 46, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, P.; Gong, L.; Xu, L.; Zhang, J.; Zheng, J.; Zhang, D.; Tian, T.; Wang, P. Construction and clinical evaluation of a new customized bite block used in radiotherapy of head and neck cancer. Cancer Radiother. 2019, 23, 125–131. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Xia, R.; Shen, Y.; Zheng, Z.; Sun, J. Modification of the anterior-posterior tongue rotation flap for oral tongue reconstruction. Head Neck 2020, 42, 3769–3775. [Google Scholar] [CrossRef]
- Li, W.; Zhang, P.; Li, R.; Liu, Y.; Kan, Q. Radial free forearm flap versus pectoralis major pedicled flap for reconstruction in patients with tongue cancer: Assessment of quality of life. Med. Oral 2016. online ahead of print. [Google Scholar]
- Yuan, Y.; Zhang, P.; He, W.; Li, W. Comparison of oral function: Free anterolateral thigh perforator flaps versus vascularized free forearm flap for reconstruction in patients undergoing glossectomy. J. Oral Maxillofac. Surg. 2016, 74, 1500.e1–1500.e6. [Google Scholar] [CrossRef]
- Yue, J.; Zhuo, S.; Zhang, H.; Liu, X.; Zhang, W. Long-term quality of life measured by the University of Washington QoL questionnaire (version 4) in patients with oral cancer treated with or without reconstruction with a microvascular free flap. Br. J. Oral Maxillofac. Surg. 2018, 56, 475–481. [Google Scholar] [CrossRef]
- Djaali, W.; Simadibrata, C.L.; Nareswari, I. Acupuncture therapy in post-radiation head-and-neck cancer with dysgeusia. Med. Acupunct. 2020, 32, 157–162. [Google Scholar] [CrossRef]
- El Mobadder, M.; Farhat, F.; El Mobadder, W.; Nammour, S. Photobiomodulation therapy in the treatment of oral mucositis, dysphagia, oral dryness, taste alteration, and burning mouth sensation due to cancer therapy: A case series. Int. J. Environ. Res. Public Health 2019, 16, 4505. [Google Scholar] [CrossRef] [PubMed]
- Yangchen, K.; Siddharth, R.; Singh, S.; Singh, R.; Aggarwal, H.; Mishra, N.; Tripathi, S.; Srivastava, K.; Verma, T.; Kumar, P. A pilot study to evaluate the efficacy of cerrobend shielding stents in preventing adverse radiotherapeutic effects in buccal carcinoma patients. J. Cancer Res. Ther. 2016, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.M.; Rosalen, P.L.; Fernandes, D.T.; Dias-Neto, E.; Alencar, S.M.; Bueno-Silva, B.; Alves, F.A.; Lopes, M.A. Brazilian organic propolis for prevention and treatment of radiation-related oral acute toxicities in head and neck cancer patients: A double-blind randomized clinical trial. Front. Pharmacol. 2022, 13, 973255. [Google Scholar] [CrossRef] [PubMed]
- Sardellitti, L.; Bortone, A.; Filigheddu, E.; Serralutzu, F.; Milia, E.P. Xerostomia: From pharmacological treatments to traditional medicine—An overview on the possible clinical management and prevention using systemic approaches. Curr. Oncol. 2023, 30, 4412–4426. [Google Scholar] [CrossRef]
- Pingili, S.; Ahmed, J.; Sujir, N.; Shenoy, N.; Ongole, R. Evaluation of malnutrition and quality of life in patients treated for oral and oropharyngeal cancer. Sci. World J. 2021, 2021, 1–6. [Google Scholar] [CrossRef]
| Ref. | Study Design | Sample Size (n) | Treatment | Taste Assessment | Results |
|---|---|---|---|---|---|
| Singh et al., 2024 [16] | Prospective observational | 100 HNC patients | None | NIH Taste Intensity Test (sweet, salty, sour, bitter, umami, baseline only) | Sweet/Salty taste loss correlated with perineural invasion. |
| Lilja et al., 2018 [17] | Prospective longitudinal | 44 HNC patients | Tumor resection + free flap + RT | EGM (electrogustometry) at baseline, 6 weeks, 3 months, 6 months, and 12 months | Persistent taste loss on tumor side; mild recovery at 12 months. |
| Uí Dhuibhir et al., 2019 [18] | Prospective observational | 30 patients with mixed solid tumors, including HNC | None | Taste strips (detection threshold, baseline only) + self-report (taste/smell abnormality) | 74% had taste/smell abnormalities; taste more affected; no HNC-specific data. |
| Cunha et al., 2020 [19] | Cross-sectional observational | 31 HNC patients | None | Taste strips (4 concentrations for sweet, salty, and sour and 3 for bitter; 15 points total), baseline only | Hypogeusia in 39%; bitter taste most affected (80.6%). |
| Abbas et al., 2019 [20] | Cross-sectional observational | 59 HNC patients | Surgery ± RT ± CT | UW-QOL v4 (taste domain) | Taste scores significantly lower in advanced-stage tumors (p = 0.045). |
| Tsutsumi et al., 2016 [23] | Prospective observational (molecular focus) | 26 HNC patients | CT+ RT | mRNA expression (T1R1, T1R2, T1R3, T2R5) + whole-mouth gustatory test (umami, sweet, bitter) | ↓ *T1R3 and ↑ *T2R5 linked to dysgeusia and phantogeusia. |
| Ihara et al., 2018 [24] | Prospective cohort | 30 HNC patients | CRT | gLMS (intensity rating for sweet, salty, sour, bitter; baseline vs. 6 weeks vs. 3 months) | ↓ in all tastes at 6 weeks; partial recovery by 3 months. |
| Epstein et al., 2020 [25] | Prospective longitudinal | 10 HNC patients | IMRT ± CT | Edible strips, taste drops, CTCAE v4.0, STTA; baseline vs. 6 weeks post-RT and up to 24 months | Altered taste in all; partial recovery, persistent in some up to 2 years. |
| Palmieri et al., 2021 [26] | Prospective observational | 20 HNC patients | RT + CT | NCI Common Toxicity Criteria (weekly for 6 weeks) | Dysgeusia onset at week 2; peaked at week 5; partial recovery post-treatment. |
| Galitis et al., 2017 [27] | Longitudinal observational | 10 HNC patients | Post-operative RT or chemoradiotherapy | EORTC QLQ-C30 and H&N35 (baseline, end-RT, 3 months post-RT)) | Dysgeusia in 88% post-RT; persistent in 50% at 3-month follow-up. |
| Malta et al., 2021 [28] | Cross-sectional retrospective | 514 HNC patients | CT ± RT | CTCAE v5.0 (self-reported dysgeusia grade ≥ 2) | Dysgeusia associated with cisplatin and radiotherapy. |
| Messing et al., 2021 [29] | Prospective longitudinal | 28 HNC patients | RT ± CT ± surgery | Whole-mouth taste test, CiTAS, HNSC (baseline, week 2, week 4, 1, 3, 6 months) | Taste improved by 6 months; persistent dysgeusia correlated with oral dose and xerostomia. |
| Moroney et al., 2018 [30] | Prospective observational | 76 HNC patients | Helical IMRT + CT | CTCAE v4.0 grading for dysgeusia; weekly during RT and post-RT (2, 4, 12 weeks) | Grade 2 dysgeusia in 97.4%; 26.4% had persistent symptoms at 12 weeks. |
| Sio et al., 2016 [31] | Prospective comparative | 81 HNC patients | CT + IMPT or IMRT | MDASI-HN questionnaire (acute, subacute, chronic); baseline | IMPT reduced subacute dysgeusia compared with IMRT. |
| Chen 2015 [32] | Longitudinal observational | 77 HNC patients | RT or CCRT | MSS-moo (taste change assessed at baseline, 4, and 8 weeks during RT) | Taste change peaked at 8 weeks; severity linked to dose, regimen, smoking. |
| Vempati et al., 2020 [33] | Prospective Phase I trial | 34 HNC patients | IMRT + SRS boost + CT | CTCAE v4.03 for toxicity grading; MDASI-HN questionnaire | Acute dysgeusia in 88%; peaked at 9 months; 7% had grade 2 at 24 mo; none grade 3–4. |
| Asif et al., 2020 [4] | Prospective | 21 HNC patients | IMRT/VMAT | Taste strips (4 tastes) and EORTC QoL (baseline, mid-RT, end-RT, 1-, 3-, and 12-month follow-up) | Sweet/salty taste declined during RT; recovered by 1 month. |
| Barbosa et al., 2019 [34] | Prospective | 56 HNC patients | RT | Modified global gustatory test + self-reported qualitative changes (baseline, end-RT, 3 months, 6 months) | Severe taste loss post-RT; recovery by 3–6 months; 14% had qualitative distortions. |
| Morelli et al., 2023 [35] | Prospective observational | 31 HNC patients | IMRT ± CT | CiTAS + EORTC QLQ-C30 + HN43 (baseline, 3 weeks post-RT, 3 months post-RT) | Peak dysgeusia at 3 wks post-RT; correlated with dose to salivary glands. |
| Riantiningtyas et al., 2024 [15] | Cross-sectional | 30 HNC patients | RT ± surgery ± CT | Self-reported sensory perception, oral symptoms, and eating behavior questionnaire (post-treatment) | 53% reported altered taste; linked to food aversion and eating difficulties. |
| Stankevice et al., 2021 [36] | Retrospective observational study | 19 HNC patients | RT | TDT, FPT, and EGM for objective gustatory threshold and localization assessment | Persistent dysgeusia reported in two patients treated with RT. |
| Jin et al., 2020 [37] | Longitudinal | 117 HNC patients | IMRT ± surgery ± CT | HNSC checklist for subjective taste change and dietary interference (baseline, mid-RT, post-RT) | Taste change in 93% post-RT; linked to weight loss and dry mouth. |
| Negi et al., 2017 [38] | Prospective | 27 HNC patients | 3D-CRT + CT | Forced three-choice stimulus drop technique (weekly during RT, monthly post-RT up to 6 months) | Bitter taste most affected; no full recovery by 6 months. |
| Martini et al., 2019 [39] | Prospective | 31 HNC patients | VMAT RT ± surgery ± CT | CiTAS questionnaire weekly during RT and at 1 week, 1 month, and 6 months post-RT | Taste worsened during RT; phantogeusia improved, but hypogeusia persisted. |
| Jin et al., 2018 [40] | Longitudinal | 114 HNC patients | IMRT ± surgery ± CT | Single-item STA assessment and CiTAS Scale at baseline, mid-treatment, post-treatment, and 1–2 months follow-up | STA in 92% post-RT; only CiTAS “discomfort” sub-scale predicted weight loss. |
| Kırca & Kutlutürkan, 2016 [41] | Descriptive, Longitudinal | 47 HNC patients | RT | MSAS taste item assessed at mid-RT, end-RT, and 1-month post-RT | Taste change is frequent and distressing; associated with dry mouth, sores. |
| Alfaro et al., 2021 [42] | Cross-sectional | 40 HNC patients | RT ± CT ± surgery | Regional (tip of the tongue) and whole-mouth taste tests using the gLMS Scale; cross-sectional assessment | Localized taste dysfunction detected at the tip of the tongue (sweet, salty, bitter stimuli) despite preserved whole-mouth taste perception. |
| Mathlin et al., 2023 [43] | Prospective | 61 HNC patients | VMAT RT ± CT | MDASI-HN questionnaire at week 1 and week 4 of RT; supplementary coping questions for dysgeusia at week 4 | 97% reported taste changes; worse with chewing; more frequent in females. |
| Alvarez-Camacho et al., 2016 [44] | Longitudinal | 160 HNC patients | RT ± CT ± surgery | Self-report: CCS + UW-QoL (pre, post, 2.5 mo) | Taste/Smell changes predicted worse QoL post-treatment. |
| Sapir 2016 [45] | Prospective longitudinal | 73 HNC patients | CRT via IMRT | Patient-reported taste (HNQOL, UWQOL) + unstimulated/stimulated salivary flow; baseline to 12 months | Dysgeusia in 50% (1 mo), 23% (12 months); correlated with oral cavity dose. |
| Ref. | Study Design | Sample Size (n) | Intervention/Treatment | Outcome Measures | Results |
|---|---|---|---|---|---|
| Shono et al. (2021) [63] | Randomized controlled cohort study (non-blinded) | 51 HNC patients | Monosodium glutamate (MSG) (2.7 g/day) during chemoradiotherapy | T1R3 gene expression, VAS for dysgeusia, daily energy intake | MSG preserved T1R3 expression, improved taste and calorie intake. |
| López-Plaza et al. (2023) [65] | Randomized, placebo-controlled, triple-blind trial (pilot) | 31 malnourished cancer patients (HNC included) | Miraculin-based supplement (standard/high dose) vs. placebo | Taste acuity (electrogustometry), dietary intake, QoL | Standard dose improved taste, intake, QoL; no adverse events. |
| Khan et al. (2019) [60] | Double-blind randomized controlled trial | 70 HNC patients | Zinc sulphate 50 mg TID vs. placebo during and after CCRT | Detection and recognition thresholds for 4 tastes | No significant benefit overall; some improvement in sweet and sour recognition. |
| Ben-Arye et al. (2018) [66] | Prospective chart-based study | 34 (some patients with HNC, not quantified) | Complementary medicine: sage, carob, wheatgrass, acupuncture, mind–body therapies | ESAS, MYCAW for symptom improvement | 85% reported taste improvement; herbal/acupuncture was the most beneficial. |
| Lesser et al. (2022) [62] | Pilot clinical trial | 26 not quantified, but HNC patients included | Lactoferrin 750 mg/day for 30 days | TSQ (taste, smell, composite scores) | Significant taste/smell improvement at 60 days; partial at 30. |
| Heiser et al. (2016) [67] | Prospective cohort study (pre-post) | 98 HNC patients | Liposomal spray (oral and nasal) for 2 months | Taste strips, smell test, xerostomia questionnaire | Significant improvement in taste, smell, and xerostomia symptoms. |
| Epstein et al. (2019) [68] | Clinical case series | 14 (9 HNC) | Zinc, clonazepam, megestrol acetate, dronabinol, PBMT | STTA, CTCAE, chemical gustometry | 71% of patients reported improvement in taste function |
| Dalbem Paim et al. (2019) [69] | Randomized controlled trial | 68 HNC patients | Transcutaneous Electrical Nerve Stimulation (TENS), 8 sessions | Stimulated salivary flow (SSF), VAS for salivation, QoL (UW-QOL) | TENS significantly improved SSF, self-perceived saliva flow, and QoL up to 6 months. |
| Feng et al., 2019 [70] | Prospective | 60 HNC patients | IMRT ± CT; bite block | Clinical observation (presence of dysgeusia at end-RT; with vs. without bite block) | Bite block prevented dysgeusia; reduced mucosal dose. |
| Lu et al., 2020 [71] | Prospective case series | 21 HNC patients | Surgical resection + modified anterior–posterior tongue rotation flap | UW-QOL v4 (self-reported taste domain; 12–24 months follow-up) | All patients reported normal taste post-op. Flap preserved tongue length and symmetry. Excellent outcomes also for swallowing, chewing, and speech. |
| Li et al., 2016 [72] | Retrospective comparative study | 41 HNC patients | Surgical resection + reconstruction with RFFF or PMMF | UW-QOL v4 (self-reported taste domain; ≥12 months follow-up) | No significant difference in taste function between RFFF and PMMF (p = 0.673). |
| Yuan et al., 2016 [73] | Prospective observational study | 67 HNC patients | Surgical resection + reconstruction with ALTFF or RFFF | UW-QOL v4 (self-reported taste domain; 6- and 12-months follow-up) | Taste improved at 12 months; no difference between flaps. |
| Yue et al., 2018 [74] | Prospective observational study | 139 HNC patients | Tumor resection ± immediate reconstruction with free flap | UW-QOL v4 (self-reported taste domain; ≥12 months follow-up) | Taste was among the worst domains; no difference by treatment group. |
| Djali et al., 2020 [75] | Case report | 65-year-old man with stage I laryngeal SCC post-RT | Acupuncture (body points, auricular battlefield acupuncture, wrist balancing method); 12 sessions, 2×/week | Clinical observation (VAS) | Full taste recovery and pain reduction (VAS 4 → 1) after 12 sessions. |
| El Mobadder et al., 2019 [76] | Case series | 3 cancer patients with different diagnoses; 1 HNC patient. | Photobiomodulation therapy (635 nm diode laser; 10 sessions on tongue dorsum and lateral surfaces) | ISO 3972:2011 (sip and spit test for 5 basic tastes) | Taste score improved from 0/5 to 5/5 after 10 PBM sessions. |
| Yangchen et al., 2016 [77] | Pilot controlled cohort study (not RCT) | 24 HNC patients | Cerrobend shielding stent during radiotherapy vs. no stent (control) | RTOG 0435 Scale: multiple oral side effects including taste alteration assessed at 1 and 3 months | No significant difference in taste alteration between groups. |
| Fernandes et al., 2022 [78] | Double-blind RCT | 60 HNC Patients | Brazilian organic propolis spray vs. placebo (6×/day, during RT) | NCI CTCAE (weekly dysgeusia score) | Lower dysgeusia in propolis group (not significant); ↓ candidiasis, IL-1β, TNF-α. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardellitti, L.; Filigheddu, E.; Mastandrea, G.; Di Palma, A.; Milia, E.P. Taste Dysfunction in Head and Neck Cancer: Pathophysiology and Clinical Management—A Comprehensive Review. Biomedicines 2025, 13, 1853. https://doi.org/10.3390/biomedicines13081853
Sardellitti L, Filigheddu E, Mastandrea G, Di Palma A, Milia EP. Taste Dysfunction in Head and Neck Cancer: Pathophysiology and Clinical Management—A Comprehensive Review. Biomedicines. 2025; 13(8):1853. https://doi.org/10.3390/biomedicines13081853
Chicago/Turabian StyleSardellitti, Luigi, Enrica Filigheddu, Giorgio Mastandrea, Armando Di Palma, and Egle Patrizia Milia. 2025. "Taste Dysfunction in Head and Neck Cancer: Pathophysiology and Clinical Management—A Comprehensive Review" Biomedicines 13, no. 8: 1853. https://doi.org/10.3390/biomedicines13081853
APA StyleSardellitti, L., Filigheddu, E., Mastandrea, G., Di Palma, A., & Milia, E. P. (2025). Taste Dysfunction in Head and Neck Cancer: Pathophysiology and Clinical Management—A Comprehensive Review. Biomedicines, 13(8), 1853. https://doi.org/10.3390/biomedicines13081853








