Prenatal Management of Spinal Muscular Atrophy in the Era of Genetic Screening and Emerging Opportunities in In Utero Therapy
Abstract
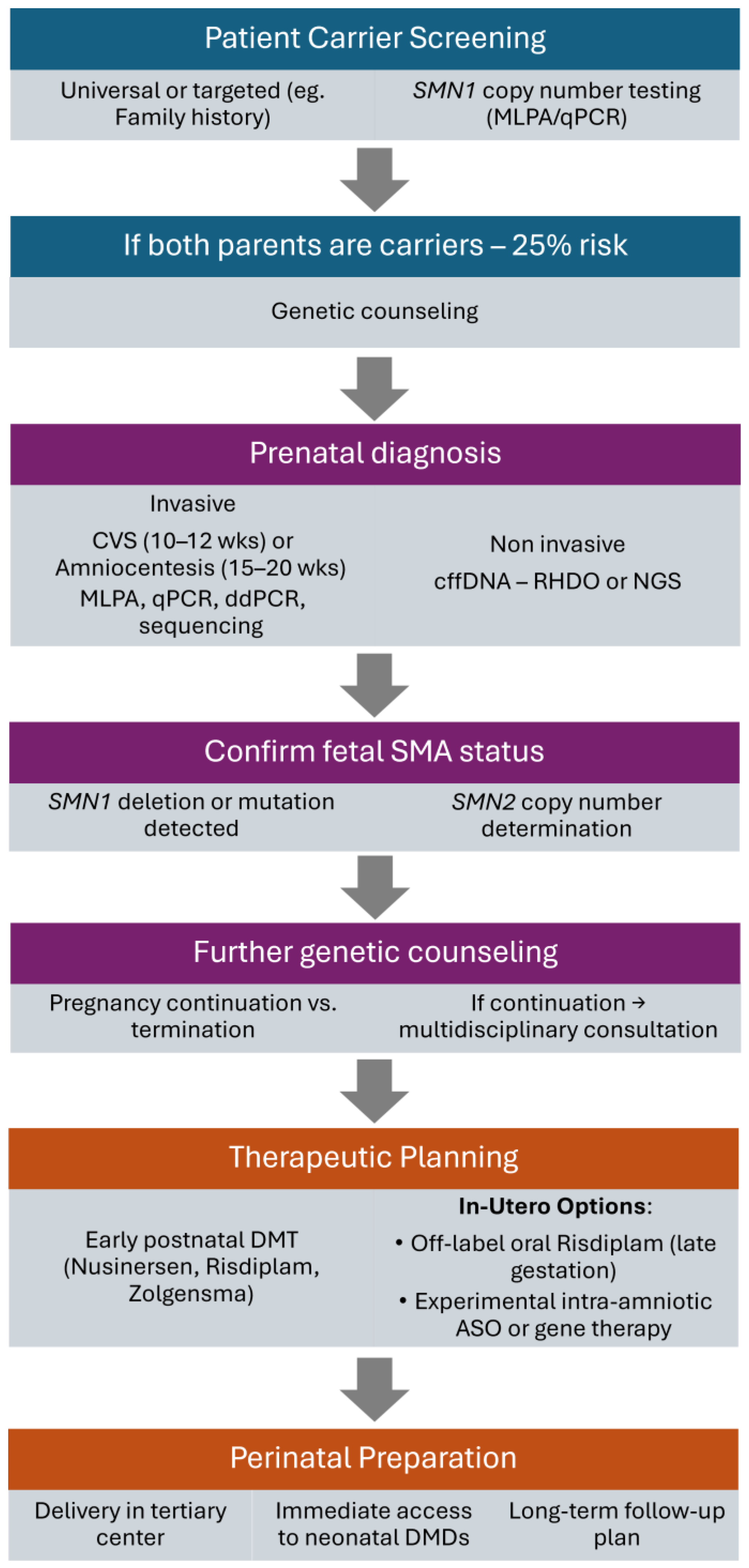
1. Introduction
2. Prenatal Testing for SMA—Benefits vs. Risks
2.1. Invasive Methods
2.1.1. Chorionic Villus Sampling
2.1.2. Amniocentesis
2.1.3. Molecular Diagnostic Methods Following Invasive Sampling
2.2. Non-Invasive Methods
2.2.1. Liquid Biopsy
2.2.2. Molecular Diagnostic Methods Following Non-Invasive Sampling
2.3. Increasing Clinical Potential of Prenatal Testing
3. Advances in In Utero Therapies: Clinical Innovations, Genetic Interventions, and Health System Perspectives
3.1. Prenatal Intervention in Spinal Muscular Atrophy: A New Frontier in Fetal Therapy
3.2. In Utero Gene Editing: Correcting Genetic Disorders Before Birth
3.3. Comparative Approaches to In Utero Therapies in Genetic Diseases: Lessons from In Utero Enzyme Replacement Therapy for Lysosomal Storage Disorders
3.4. Pharmacoeconomic and Health Technology Assessment Perspectives
4. Regulatory and Ethical Considerations in Fetal Therapy
5. Future Considerations and Recommendations for Research
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASOs | Antisense Oligonucleotides |
| cffDNA | Cell-free Fetal DNA |
| CNS | Central Nervous System |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CVS | Chorionic Villus Sampling |
| ddPCR | Digital Droplet PCR |
| DMDs | Disease-Modifying Drugs |
| EMA | European Medicines Agency |
| ERT | Enzyme Replacement Therapy |
| EXIT | Ex Utero Intrapartum Treatment |
| FDA | Food and Drug Administration |
| FTCs | Fetal Therapy Centers |
| MLPA | Multiplex Ligation-Dependent Probe Amplification |
| MPS | Mucopolysaccharidosis |
| NGS | Next-Generation Sequencing |
| NIPT | Non-Invasive Prenatal Testing |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| RHDO | Relative Haplotype Dosage |
| SMA | Spinal Muscular Atrophy |
| SMN | Survival Motor Neuron |
| SMN1 | Survival Motor Neuron 1 (gene) |
| SMN2 | Survival Motor Neuron 2 (gene) |
References
- Darras, B.T.; Markowitz, J.A.; Monani, U.R.; De Vivo, D.C. Chapter 8-Spinal Muscular Atrophies. In Neuromuscular Disorders of Infancy, Childhood, and Adolescence, 2nd ed.; Darras, B.T., Jones, H.R., Ryan, M.M., De Vivo, D.C., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 117–145. ISBN 978-0-12-417044-5. [Google Scholar]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, Incidence and Carrier Frequency of 5q-Linked Spinal Muscular Atrophy—a Literature Review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
- Linear Graph-601627-SURVIVAL OF MOTOR NEURON 2; SMN2-OMIM-(OMIM.ORG). Available online: https://www.omim.org/graph/linear/601627 (accessed on 10 July 2025).
- Bürglen, L.; Lefebvre, S.; Clermont, O.; Burlet, P.; Viollet, L.; Cruaud, C.; Munnich, A.; Melki, J. Structure and Organization of the Human Survival Motor Neurone (SMN) Gene. Genomics 1996, 32, 479–482. [Google Scholar] [CrossRef]
- Savad, S.; Ashrafi, M.R.; Samadaian, N.; Heidari, M.; Modarressi, M.-H.; Zamani, G.; Amidi, S.; Younesi, S.; Amin, M.M.T.; Saadati, P.; et al. A Comprehensive Overview of SMN and NAIP Copy Numbers in Iranian SMA Patients. Sci. Rep. 2023, 13, 3202. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Fahmy, N.A.; El-Bagoury, N.M.; Eissa, N.R.; Sharaf-Eldin, W.E.; Issa, M.Y.; Zaki, M.S.; Essawi, M.L. MLPA Analysis for Molecular Diagnosis of Spinal Muscular Atrophy and Correlation of 5q13.2 Genes with Disease Phenotype in Egyptian Patients. Egypt. J. Med. Hum. Genet. 2022, 23, 156. [Google Scholar] [CrossRef]
- Chen, T.-H. New and Developing Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Talbot, K.; Tizzano, E.F. The Clinical Landscape for SMA in a New Therapeutic Era. Gene Ther. 2017, 24, 529–533. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Finkel, R.S. Spinal Muscular Atrophy: A Changing Phenotype beyond the Clinical Trials. Neuromuscul. Disord. 2017, 27, 883–889. [Google Scholar] [CrossRef]
- Watihayati, M.S.; Fatemeh, H.; Marini, M.; Atif, A.B.; Zahiruddin, W.M.; Sasongko, T.H.; Tang, T.H.; Zabidi-Hussin, Z.; Nishio, H.; Zilfalil, B.A. Combination of SMN2 Copy Number and NAIP Deletion Predicts Disease Severity in Spinal Muscular Atrophy. Brain Dev. 2009, 31, 42–45. [Google Scholar] [CrossRef]
- Farrar, M.A.; Carey, K.A.; Paguinto, S.-G.; Kasparian, N.A.; De Abreu Lourenço, R. “The Whole Game Is Changing and You’ve Got Hope”: Australian Perspectives on Treatment Decision Making in Spinal Muscular Atrophy. Patient 2020, 13, 389–400. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Spinraza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spinraza (accessed on 12 June 2025).
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Day, J.W.; Finkel, R.S.; Chiriboga, C.A.; Connolly, A.M.; Crawford, T.O.; Darras, B.T.; Iannaccone, S.T.; Kuntz, N.L.; Peña, L.D.M.; Shieh, P.B.; et al. Onasemnogene Abeparvovec Gene Therapy for Symptomatic Infantile-Onset Spinal Muscular Atrophy in Patients with Two Copies of SMN2 (STR1VE): An Open-Label, Single-Arm, Multicentre, Phase 3 Trial. Lancet Neurol. 2021, 20, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Muntoni, F.; Baranello, G.; Masson, R.; Boespflug-Tanguy, O.; Bruno, C.; Corti, S.; Daron, A.; Deconinck, N.; Servais, L.; et al. Onasemnogene Abeparvovec Gene Therapy for Symptomatic Infantile-Onset Spinal Muscular Atrophy Type 1 (STR1VE-EU): An Open-Label, Single-Arm, Multicentre, Phase 3 Trial. Lancet Neurol. 2021, 20, 832–841. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Zolgensma. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma (accessed on 12 June 2025).
- Darras, B.T.; Masson, R.; Mazurkiewicz-Bełdzińska, M.; Rose, K.; Xiong, H.; Zanoteli, E.; Baranello, G.; Bruno, C.; Vlodavets, D.; Wang, Y.; et al. Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls. N. Engl. J. Med. 2021, 385, 427–435. [Google Scholar] [CrossRef]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and Efficacy of Once-Daily Risdiplam in Type 2 and Non-Ambulant Type 3 Spinal Muscular Atrophy (SUNFISH Part 2): A Phase 3, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Evrysdi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evrysdi (accessed on 12 June 2025).
- Belančić, A.; Faour, A.K.; Gkrinia, E.M.M.; Vitezić, D. A Systematic Review of Economic Evaluations of Orphan Medicines for the Management of Spinal Muscular Atrophy. Br. J. Clin. Pharmacol. 2025, 91, 95–116. [Google Scholar] [CrossRef]
- Philpott, J. Entering the Era of Prenatal Gene Therapies. Pharmaceutical Technology. 2025. Available online: https://www.pharmaceutical-technology.com/features/entering-the-era-of-prenatal-gene-therapies/ (accessed on 14 June 2025).
- Finkel, R.S.; Hughes, S.H.; Parker, J.; Civitello, M.; Lavado, A.; Mefford, H.C.; Mueller, L.; Kletzl, H. Prenatal SMA Risdiplam Study Group Risdiplam for Prenatal Therapy of Spinal Muscular Atrophy. N. Engl. J. Med. 2025, 392, 1138–1140. [Google Scholar] [CrossRef]
- Su, Y.-N.; Hung, C.-C.; Lin, S.-Y.; Chen, F.-Y.; Chern, J.P.S.; Tsai, C.; Chang, T.-S.; Yang, C.-C.; Li, H.; Ho, H.-N.; et al. Carrier Screening for Spinal Muscular Atrophy (SMA) in 107,611 Pregnant Women during the Period 2005–2009: A Prospective Population-Based Cohort Study. PLoS ONE 2011, 6, e17067. [Google Scholar] [CrossRef]
- Hopkins, M.K.; Dugoff, L.; Kuller, J.A. Spinal Muscular Atrophy: Inheritance, Screening, and Counseling for the Obstetric Provider. Obstet. Gynecol. Surv. 2021, 76, 166. [Google Scholar] [CrossRef]
- Sun, Y.; Kong, X.; Zhao, Z.; Zhao, X. Mutation analysis of 419 family and prenatal diagnosis of 339 cases of spinal muscular atrophy in China. BMC Med. Genet. 2020, 21, 133. [Google Scholar] [CrossRef]
- Brambati, B.; Tului, L. Chorionic Villus Sampling and Amniocentesis. Curr. Opin. Obstet. Gynecol. 2005, 17, 197–201. [Google Scholar] [CrossRef]
- Brambati, B.; Simoni, G. DIAGNOSIS OF FETAL TRISOMY 21 IN FIRST TRIMESTER. Lancet 1983, 321, 585–586. [Google Scholar] [CrossRef]
- Wapner, R.J.; Evans, M.I.; Davis, G.; Weinblatt, V.; Moyer, S.; Krivchenia, E.L.; Jackson, L.G. Procedural Risks versus Theology: Chorionic Villus Sampling for Orthodox Jews at Less than 8 Weeks’ Gestation. Am. J. Obstet. Gynecol. 2002, 186, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, Z.; Navaratnam, K.; Mujezinovic, F. Amniocentesis and Chorionic Villus Sampling for Prenatal Diagnosis. Cochrane Database Syst. Rev. 2017, 9, CD003252. [Google Scholar] [CrossRef] [PubMed]
- Zaninović, L.; Katušić Bojanac, A.; Bašković, M. Metode molekularne dijagnostike u prenatalnoj medicini. Med. Flum. 2022, 58, 224–237. [Google Scholar] [CrossRef]
- HeMED-Pregled Genetskih Poremećaja. Available online: https://hemed.hr/Default.aspx?sid=18731 (accessed on 2 June 2025).
- Akolekar, R.; Beta, J.; Picciarelli, G.; Ogilvie, C.; D’Antonio, F. Procedure-Related Risk of Miscarriage Following Amniocentesis and Chorionic Villus Sampling: A Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2015, 45, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.; Birnie, E.; Robles de Medina, P.; Sollie, K.M.; Pajkrt, E.; Bilardo, C.M. Total Pregnancy Loss after Chorionic Villus Sampling and Amniocentesis: A Cohort Study. Ultrasound Obstet. Gynecol. 2017, 49, 599–606. [Google Scholar] [CrossRef]
- Malvestiti, F.; Agrati, C.; Grimi, B.; Pompilii, E.; Izzi, C.; Martinoni, L.; Gaetani, E.; Liuti, M.R.; Trotta, A.; Maggi, F.; et al. Interpreting Mosaicism in Chorionic Villi: Results of a Monocentric Series of 1001 Mosaics in Chorionic Villi with Follow-up Amniocentesis. Prenat. Diagn. 2015, 35, 1117–1127. [Google Scholar] [CrossRef]
- Reilly, K.; Doyle, S.; Hamilton, S.J.; Kilby, M.D.; Mone, F. Pitfalls of Prenatal Diagnosis Associated with Mosaicism. Obstet. Gynaecol. 2023, 25, 28–37. [Google Scholar] [CrossRef]
- Soyman, Z.; Kelekci, S.; Demirel, E.; Ekmekci, E.; Atasever, M. Chorionic Villus Sampling and Preeclampsia & Eclampsia: Coincidence or Not? J. Matern. Fetal Neonatal Med. 2022, 35, 6522–6526. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, X.; Zhang, C.; Wang, Y.; Ma, P.; Hao, S.; Hui, L.; Bai, Y. Analysis of Spinal Muscular Atrophy Carrier Screening Results in 32,416 Pregnant Women and 7,231 Prepregnant Women. Front. Neurol. 2024, 15, 1357476. [Google Scholar] [CrossRef]
- Jindal, A.; Sharma, M.; Karena, Z.V.; Chaudhary, C. Amniocentesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dimri, N.; Baijal, A. Amniocentesis. J. Fetal Med. 2016, 03, 131–135. [Google Scholar] [CrossRef]
- Wolder, D.; Blazuk-Fortak, A.; Michalska, A.; Detka, K.; Świercz, G.; Kaczmarek, P. Perinatal and Delivery Outcomes Following Amniocentesis: A Case-Control Study in the Polish Population. J. Clin. Med. 2025, 14, 309. [Google Scholar] [CrossRef]
- Connolly, K.A.; Eddleman, K.A. Amniocentesis: A Contemporary Review. World J. Obstet. Gynecol. 2016, 5, 58–65. [Google Scholar] [CrossRef]
- Daniilidis, A.; Karydas, H.; Zournatzi, V.; Tantanasis, T.; Giannoulis, C.; Tzafettas, J. A Four-Year Retrospective Study of Amniocentesis: One Centre Experience. Hippokratia 2008, 12, 113–115. [Google Scholar]
- Adrainus Polim, A.; Handayani, N.; Kesumapramudya Nurputra, D.; Melanie Lubis, A.; Sirait, B.; Jakobus, D.; Boediono, A.; Sini, I. Birth of Spinal Muscular Atrophy Unaffected Baby from Genetically At-Risk Parents Following a Pre-Implantation Genetic Screening: A Case Report. Int. J. Reprod. Biomed. 2022, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Khanbazi, A.; Beheshtian, M.; Azad, M.; Akbari Kelishomi, M.; Afroozan, F.; Fatehi, F.; Noudehi, K.; Zamanian Najafabadi, S.; Omrani, M.; Habibi, H.; et al. Comprehensive Copy Number Analysis of Spinal Muscular Atrophy among the Iranian Population. Sci. Rep. 2024, 14, 29880. [Google Scholar] [CrossRef]
- Tuncel, G.; Sanlıdag, B.; Dirik, E.; Baris, T.; Ergoren, M.C.; Temel, S.G. Lessons from Real Life Experience: Importance of In-House Sequencing and Smart Ratio-Based Real-Time PCR Outperform Multiplex Ligation-Dependent Probe Amplification in Prenatal Diagnosis for Spinal Muscular Atrophy: Bench to Bedside Diagnosis. Glob. Med. Genet. 2023, 10, 240–246. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Debski, P.R.; Gewartowski, K.; Bajer, S.; Garstecki, P. Calibration-Free Assays on Standard Real-Time PCR Devices. Sci. Rep. 2017, 7, 44854. [Google Scholar] [CrossRef]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR versus qPCR for Gene Expression Analysis with Low Abundant Targets: From Variable Nonsense to Publication Quality Data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I. A Timely Reminder of Technical Limitations. Microb. Biotechnol. 2016, 9, 435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zou, Y.; Xu, P.; Li, J.; Huang, S.; Gao, M.; Kang, R.; Gao, X.; Gao, Y. Application of droplet digital PCR technology for genetic testing and prenatal diagnosis of spinal muscular atrophy. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2016, 33, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, Z.; Zhou, Y.; Lu, X.; Du, X.; Guo, Q. Comparison of the Accuracy of Multiplex Digital PCR versus Multiplex Ligation-Dependent Probe Amplification in Quantification of the Survival of Motor Neuron Genes Copy Numbers. Clin. Chim. Acta 2024, 553, 117708. [Google Scholar] [CrossRef]
- Vidal-Folch, N.; Gavrilov, D.; Raymond, K.; Rinaldo, P.; Tortorelli, S.; Matern, D.; Oglesbee, D. Multiplex Droplet Digital PCR Method Applicable to Newborn Screening, Carrier Status, and Assessment of Spinal Muscular Atrophy. Clin. Chem. 2018, 64, 1753–1761. [Google Scholar] [CrossRef]
- Yang, W.C.; Zhu, L.; Qiu, Y.M.; Zhou, B.X.; Cheng, J.L.; Wei, C.L.; Chen, H.C.; Li, L.Y.; Fu, X.D.; Fu, J.J. Isolation and Analysis of Cell-Free Fetal DNA from Maternal Peripheral Blood in Chinese Women. Genet. Mol. Res. 2015, 14, 18078–18089. [Google Scholar] [CrossRef]
- Pedini, P.; Graiet, H.; Laget, L.; Filosa, L.; Chatron, J.; Cherouat, N.; Chiaroni, J.; Hubert, L.; Frassati, C.; Picard, C. Qualitative and Quantitative Comparison of Cell-Free DNA and Cell-Free Fetal DNA Isolation by Four (Semi-)Automated Extraction Methods: Impact in Two Clinical Applications: Chimerism Quantification and Noninvasive Prenatal Diagnosis. J. Transl. Med. 2021, 19, 15. [Google Scholar] [CrossRef]
- Parks, M.; Court, S.; Bowns, B.; Cleary, S.; Clokie, S.; Hewitt, J.; Williams, D.; Cole, T.; MacDonald, F.; Griffiths, M.; et al. Non-Invasive Prenatal Diagnosis of Spinal Muscular Atrophy by Relative Haplotype Dosage. Eur. J. Hum. Genet. 2017, 25, 416–422. [Google Scholar] [CrossRef]
- Chen, M.; Lu, S.; Lai, Z.F.; Chen, C.; Luo, K.; Yuan, Y.; Wang, Y.S.; Li, S.Q.; Gao, Y.; Chen, F.; et al. Targeted Sequencing of Maternal Plasma for Haplotype-Based Non-Invasive Prenatal Testing of Spinal Muscular Atrophy. Ultrasound Obstet. Gynecol. 2017, 49, 799–802. [Google Scholar] [CrossRef]
- Hirano, M.; Sahashi, K.; Ichikawa, Y.; Katsuno, M.; Natsume, A. A Rapid and Easy-to-Use Spinal Muscular Atrophy Screening Tool Based on Primers with High Specificity and Amplification Efficiency for SMN1 Combined with Single-Stranded Tag Hybridization Assay. PLoS ONE 2024, 19, e0308179. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.E.; Shao, S.; Zhang, L.; Lianoglou, B.; Belter, L.; Jarecki, J. Investigating Attitudes toward Prenatal Diagnosis and Fetal Therapy for Spinal Muscular Atrophy. Prenat. Diagn. 2022, 42, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, X.; Yang, J.; Dong, W.; Cao, L.; Zeng, X. Carrier Screening and Prenatal Diagnosis for Spinal Muscular Atrophy in Ningde City, Fujian Province. Mol. Genet. Genom. Med. 2025, 13, e70077. [Google Scholar] [CrossRef]
- Zettler, B.; Estrella, E.; Liaquat, K.; Lichten, L. Evolving Approaches to Prenatal Genetic Counseling for Spinal Muscular Atrophy in the New Treatment Era. J. Genet. Couns. 2022, 31, 803–814. [Google Scholar] [CrossRef]
- Goedeker, N.L.; Rogers, A.; Fisher, M.; Arya, K.; Brandsema, J.F.; Farah, H. Outcomes of Early-Treated Infants with Spinal Muscular Atrophy: A Multicenter, Retrospective Cohort Study. Muscle Nerve 2024, 70, 1247–1256. [Google Scholar] [CrossRef]
- Sumner, C.J.; Crawford, T.O. Early Treatment Offers a Lifeline for Infants with SMA. Nat. Med. 2022, 28, 1348–1349. [Google Scholar] [CrossRef]
- Dangouloff, T.; Servais, L. Clinical Evidence Supporting Early Treatment Of Patients With Spinal Muscular Atrophy: Current Perspectives. Ther. Clin. Risk Manag. 2019, 15, 1153–1161. [Google Scholar] [CrossRef]
- Varone, A.; Esposito, G.; Bitetti, I. Spinal Muscular Atrophy in the Era of Newborn Screening: How the Classification Could Change. Front. Neurol. 2025, 16, 1542396. [Google Scholar] [CrossRef]
- Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J. Systematic Review of Presymptomatic Treatment for Spinal Muscular Atrophy. Int. J. Neonatal. Screen. 2024, 10, 56. [Google Scholar] [CrossRef]
- Moon-Grady, A.J.; Baschat, A.; Cass, D.; Choolani, M.; Copel, J.A.; Crombleholme, T.M.; Deprest, J.; Emery, S.P.; Evans, M.I.; Luks, F.I.; et al. Fetal Treatment 2017: The Evolution of Fetal Therapy Centers—A Joint Opinion from the International Fetal Medicine and Surgical Society (IFMSS) and the North American Fetal Therapy Network (NAFTNet). Fetal Diagn. Ther. 2017, 42, 241–248. [Google Scholar] [CrossRef]
- Evans, L.L.; Harrison, M.R. Modern Fetal Surgery—A Historical Review of the Happenings That Shaped Modern Fetal Surgery and Its Practices. Transl. Pediatr. 2021, 10, 1401–1417. [Google Scholar] [CrossRef] [PubMed]
- Herzeg, A.; Almeida-Porada, G.; Charo, R.A.; David, A.L.; Gonzalez-Velez, J.; Gupta, N.; Lapteva, L.; Lianoglou, B.; Peranteau, W.; Porada, C.; et al. Prenatal Somatic Cell Gene Therapies: Charting a Path Toward Clinical Applications (Proceedings of the CERSI-FDA Meeting). J. Clin. Pharmacol. 2022, 62, S36–S52. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.M.; Soliman, M.M.H.; Bagunaid, M.; Radwan, M.A.; Moallem, A.A.; Borah, M.; Almurad, B.M. Intrauterine Fetal Therapy: Past, Current, and Future. Open J. Obstet. Gynecol. 2025, 15, 328–344. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Sung, C.-A.; Shaw, S.W. The Application of Clinical Ultrasound in Fetal Therapy. J. Med. Ultrasound 2021, 29, 1. [Google Scholar] [CrossRef]
- Rossi, A.C.; Vanderbilt, D.; Chmait, R.H. Neurodevelopmental Outcomes After Laser Therapy for Twin–Twin Transfusion Syndrome: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2011, 118, 1145. [Google Scholar] [CrossRef]
- Perrone, E.E.; Deprest, J.A. Fetal Endoscopic Tracheal Occlusion for Congenital Diaphragmatic Hernia: A Narrative Review of the History, Current Practice, and Future Directions. Transl. Pediatr. 2021, 10, 1448–1460. [Google Scholar] [CrossRef]
- Sagar, R.; Walther-Jallow, L.; David, A.L.; Götherström, C.; Westgren, M. Fetal Mesenchymal Stromal Cells: An Opportunity for Prenatal Cellular Therapy. Curr. Stem Cell Rep. 2018, 4, 61–68. [Google Scholar] [CrossRef]
- Kabagambe, S.K.; Jensen, G.W.; Chen, Y.J.; Vanover, M.A.; Farmer, D.L. Fetal Surgery for Myelomeningocele: A Systematic Review and Meta-Analysis of Outcomes in Fetoscopic versus Open Repair. Fetal Diagn. Ther. 2017, 43, 161–174. [Google Scholar] [CrossRef]
- Adzick, N.S. Open Fetal Surgery for Life-Threatening Fetal Anomalies. Semin. Fetal Neonatal Med. 2010, 15, 1–8. [Google Scholar] [CrossRef]
- Konno, H.; Okpaise, O.O.; Sbragia, L.; Tonni, G.; Ruano, R. Perinatal Outcomes of Intrauterine Interventions for Fetal Sacrococcygeal Teratoma Based on Different Surgical Techniques—A Systematic Review. J. Clin. Med. 2024, 13, 2649. [Google Scholar] [CrossRef]
- Spiers, A.; Legendre, G.; Biquard, F.; Descamps, P.; Corroenne, R. Ex Utero Intrapartum Technique (EXIT): Indications, Procedure Methods and Materno-Fetal Complications—A Literature Review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102252. [Google Scholar] [CrossRef]
- Borges, B.; Brown, S.M.; Chen, W.J.; Clarke, M.T.; Herzeg, A.; Park, J.H. Intra-Amniotic Antisense Oligonucleotide Treatment Improves Phenotypes in Preclinical Models of Spinal Muscular Atrophy. Sci. Transl. Med. 2025, 17, eadv4656. [Google Scholar] [CrossRef]
- Rashnonejad, A.; Amini Chermahini, G.; Gündüz, C.; Onay, H.; Aykut, A.; Durmaz, B.; Baka, M.; Su, Q.; Gao, G.; Özkınay, F. Fetal Gene Therapy Using a Single Injection of Recombinant AAV9 Rescued SMA Phenotype in Mice. Mol. Ther. 2019, 27, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, D.E.; Mackenzie, T.C.; Peranteau, W.; Edmonson, S.; Campagnoli, C.; Liu, Y.-L.; Flake, A.W.; High, K.A. Persistent Expression of hF.IX After Tolerance Induction by in Utero or Neonatal Administration of AAV-1-F.IX in Hemophilia B Mice. Mol. Ther. 2007, 15, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Grandinette, S.A.; Wang, X.; Hudson, T.R.; Briseno, K.; Berry, A.M.; Hacker, J.L.; Hsu, A.; Silverstein, R.A.; Hille, L.T.; et al. Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease. N. Engl. J. Med. 2025, 392, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Han, H.; Cranick, M.G.; Zhao, S.; Xu, S.; Yin, B.; Song, H.; Hu, Y.; Clarke, M.T.; Wang, D.; et al. Widespread Gene Editing in the Brain via In Utero Delivery of mRNA Using Acid-Degradable Lipid Nanoparticles. ACS Nano 2024, 18, 30293–30306. [Google Scholar] [CrossRef]
- Herzeg, A.; Borges, B.; Lianoglou, B.R.; Gonzalez-Velez, J.; Canepa, E.; Munar, D.; Young, S.P.; Bali, D.; Gelb, M.H.; Chakraborty, P.; et al. Intrauterine Enzyme Replacement Therapies for Lysosomal Storage Disorders: Current Developments and Promising Future Prospects. Prenat. Diagn. 2023, 43, 1638–1649. [Google Scholar] [CrossRef]
- Chen, H.H.; Sawamoto, K.; Mason, R.W.; Kobayashi, H.; Yamaguchi, S.; Suzuki, Y.; Orii, K.; Orii, T.; Tomatsu, S. Enzyme Replacement Therapy for Mucopolysaccharidoses; Past, Present, and Future. J. Hum. Genet. 2019, 64, 1153–1171. [Google Scholar] [CrossRef]
- Platt, F.M.; d’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Author Correction: Lysosomal Storage Diseases. Nat. Rev. Dis. Primers 2018, 4, 36. [Google Scholar] [CrossRef]
- Blitz, M.J.; Rochelson, B.; Sood, M.; Bialer, M.G.; Vohra, N. Prenatal Sonographic Findings in a Case of Wolman’s Disease. J. Clin. Ultrasound 2018, 46, 66–68. [Google Scholar] [CrossRef]
- Tsai, A.C.; Hung, Y.; Harding, C.; Koeller, D.M.; Wang, J.; Wong, L.C. Next Generation Deep Sequencing Corrects Diagnostic Pitfalls of Traditional Molecular Approach in a Patient with Prenatal Onset of Pompe Disease. Am. J. Med. Genet. Pt. A 2017, 173, 2500–2504. [Google Scholar] [CrossRef]
- Cohen, J.L.; Chakraborty, P.; Fung-Kee-Fung, K.; Schwab, M.E.; Bali, D.; Young, S.P.; Gelb, M.H.; Khaledi, H.; DiBattista, A.; Smallshaw, S.; et al. In Utero Enzyme-Replacement Therapy for Infantile-Onset Pompe’s Disease. New Engl. J. Med. 2022, 387, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, Ö. Current State of In Utero Cell, Enzyme, and Gene Therapy Outlined During Fetal Medicine Congress. Rare Disease Advisor. 2024. Available online: https://www.rarediseaseadvisor.com/reports/current-state-of-in-utero-cell-enzyme-and-gene-therapy-outlined-during-fetal-medicine-congress/ (accessed on 14 June 2025).
- WORLDSymposium. WORLDSymposium 2024 Program. In Proceedings of the WORLDSymposium 2024, San Diego, CA, USA, 4–9 February 2024. [Google Scholar]
- Balijepalli, C.; Gullapalli, L.; Druyts, E.; Yan, K.; Desai, K.; Barakat, S.; Locklin, J. Can Standard Health Technology Assessment Approaches Help Guide the Price of Orphan Drugs in Canada? A Review of Submissions to the Canadian Agency for Drugs and Technologies in Health Common Drug Review. Clin. Outcomes Res. 2020, 12, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Whittal, A.; Meregaglia, M.; Nicod, E. The Use of Patient-Reported Outcome Measures in Rare Diseases and Implications for Health Technology Assessment. Patient 2021, 14, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Iskrov, G.; Miteva-Katrandzhieva, T.; Stefanov, R. Health Technology Assessment and Appraisal of Therapies for Rare Diseases. Adv. Exp. Med. Biol. 2017, 1031, 221–231. [Google Scholar] [CrossRef]
- Zhou, N.; Ji, H.; Li, Z.; Hu, J.; Xie, J.-H.; Feng, Y.-H.; Yuan, N. Influencing Factors of Health Technology Assessment to Orphan Drugs: Empirical Evidence in England, Scotland, Canada, and Australia. Front. Public Health 2022, 10, 861067. [Google Scholar] [CrossRef]
- Nestler-Parr, S.; Korchagina, D.; Toumi, M.; Pashos, C.L.; Blanchette, C.; Molsen, E.; Morel, T.; Simoens, S.; Kaló, Z.; Gatermann, R.; et al. Challenges in Research and Health Technology Assessment of Rare Disease Technologies: Report of the ISPOR Rare Disease Special Interest Group. Value Health 2018, 21, 493–500. [Google Scholar] [CrossRef]
- Green, D.J.; Park, K.; Bhatt-Mehta, V.; Snyder, D.; Burckart, G.J. Regulatory Considerations for the Mother, Fetus and Neonate in Fetal Pharmacology Modeling. Front. Pediatr. 2021, 9, 698611. [Google Scholar] [CrossRef]
- Lederer, C.W.; Koniali, L.; Buerki-Thurnherr, T.; Papasavva, P.L.; La Grutta, S.; Licari, A.; Staud, F.; Bonifazi, D.; Kleanthous, M. Catching Them Early: Framework Parameters and Progress for Prenatal and Childhood Application of Advanced Therapies. Pharmaceutics 2022, 14, 793. [Google Scholar] [CrossRef]
- Benachi, A.; Vivanti, A.J. Ethical Issues in Fetal Therapies of Life-Threatening Malformations. Eur. J. Pediatr. 2024, 184, 110. [Google Scholar] [CrossRef]
- Khoury, M.J.; Bowen, S.; Dotson, W.D.; Drzymalla, E.; Green, R.F.; Goldstein, R.; Kolor, K.; Liburd, L.C.; Sperling, L.S.; Bunnell, R. Health Equity in the Implementation of Genomics and Precision Medicine: A Public Health Imperative. Genet. Med. 2022, 24, 1630–1639. [Google Scholar] [CrossRef]
- Blasco-Pérez, L.; Paramonov, I.; Leno, J.; Bernal, S.; Alias, L.; Fuentes-Prior, P.; Cuscó, I.; Tizzano, E.F. Beyond Copy Number: A New, Rapid, and Versatile Method for Sequencing the Entire SMN2 Gene in SMA Patients. Hum. Mutat. 2021, 42, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Vill, K.; Schwartz, O.; Blaschek, A.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Czibere, L.; et al. Newborn Screening for Spinal Muscular Atrophy in Germany: Clinical Results after 2 Years. Orphanet J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Kimizu, T.; Ida, S.; Okamoto, K.; Awano, H.; Niba, E.T.E.; Wijaya, Y.O.S.; Okazaki, S.; Shimomura, H.; Lee, T.; Tominaga, K.; et al. Spinal Muscular Atrophy: Diagnosis, Incidence, and Newborn Screening in Japan. Int. J. Neonatal Screen. 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.S.; Barone, C.; Putman, R.; Quijano, E.; Gupta, A.; Nguyen, R. Systemic in Utero Gene Editing as a Treatment for Cystic Fibrosis. Proc. Natl. Acad. Sci. USA 2025, 122, e2418731122. [Google Scholar] [CrossRef]
- Brown, S.M.; Ajjarapu, A.S.; Ramachandra, D.; Blasco-Pérez, L.; Costa-Roger, M.; Tizzano, E.F.; Sumner, C.J.; Mathews, K.D. Onasemnogene-Abeparvovec Administration to Premature Infants with Spinal Muscular Atrophy. Ann. Clin. Transl. Neurol. 2024, 11, 3042–3046. [Google Scholar] [CrossRef]
- Peranteau, W.H.; Flake, A.W. The Future of In Utero Gene Therapy. Mol. Diagn. Ther. 2020, 24, 135–142. [Google Scholar] [CrossRef]
- Oechsel, K.F.; Cartwright, M.S. Combination Therapy with Onasemnogene and Risdiplam in Spinal Muscular Atrophy Type 1. Muscle Nerve 2021, 64, 487–490. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Lindner, G.; Chilcott, E.; Finkel, R.S.; Yáñez-Muñoz, R.J. In Utero Therapy for Spinal Muscular Atrophy: Closer to Clinical Translation. Brain 2025, awaf123. [Google Scholar] [CrossRef]
| SMA Type | Age at Symptom Onset | Maximum Motor Function Achieved | Prognosis |
|---|---|---|---|
| 0 | Prenatal/fetal | Nil | Poor; usually die before 6 months |
| 1 | <6 months | Non-sitter | Poor; usually die before 2 years |
| 2 | 7–18 months | Sit independently | Expected to live into their twenties and beyond |
| 3 | >18 months | Walker | Progressive weakness, motor disability, normal lifespan |
| 4 | 10–30 years | Walker | Weakness of the lower extremities, normal lifespan |
| Treatment | Year of EMA Approval | Type of Molecule | Administration Route | Pros | Cons |
|---|---|---|---|---|---|
| Nusirsen | May 2017 | Antisense oligonucleotide | Intrathecal | First approved SMA therapy; proven efficacy in clinical trials (ENDEAR, CHERISH) | Requires repeated lumbar punctures; invasive administration |
| Onasemnogene abeparvovec | May 2020 (conditional); May 2022 (full) | Gene replacement therapy | Intravenous | One-time treatment; targets genetic root cause; early intervention shows strong benefit (START, STR1VE) | High cost; potential liver-related side effects |
| Risdiplam | March 2021 | Oral splicing modifier | Oral | Convenient oral administration; effective across broad age range (FIREFISH, SUNFISH) | Daily dosing required; newest (long-term data still emerging) |
| Sampling Method | Invasiveness | Downstream Method |
|---|---|---|
| Chorionic villus sampling (CSV) | Invasive | MLPA, qPCR, ddPCR |
| Amniocentesis | ||
| Liquid biopsy (cffDNA) | Non-invasive | RHDO, Deep sequencing, NGS |
| Study/Institution | Model | Therapy | Delivery Route | Timing | Key Findings |
|---|---|---|---|---|---|
| St. Jude Children’s Research Hospital [24] | Human fetus | Risdiplam (5 mg/day) | Oral to mother transplacental | Starting at 32 weeks + 5 days gestation until delivery + newborn continued treatment from 8 days of age | Risdiplam detected in amniotic fluid and cord blood; no clinical SMA signs in child at >2 years; some congenital abnormalities were noted that likely occurred before treatment initiation |
| Borges et al. (UCSF & Johns Hopkins) [80] | Mouse (2 models) | ASO | Intra-amniotic injection | Mid-gestation | Significant improvement in SMA phenotype and survival |
| Borges et al. (UCSF & Johns Hopkins) [80] | Fetal lamb (feasibility and safety study) | ASO | Intra-amniotic injection | Mid-to-late gestation | Broad CNS distribution observed; therapeutic brain levels achieved in some cases; delivery route feasible but needs optimization |
| UC Davis & UC Berkeley | Mouse embryo | CRISPR- Cas9 gene editing | Intracerebroventricular injection | E15.5 | >40% gene editing in cortical neurons and >60% in hippocampus; proof of concept for in utero CNS gene editing |
| Rashnonejad et al. [81] | AAV vectors (AAV9-SMN) | Intracerebroventricular injection | E14.5–E15 | Increased median survival rates; histological improvements; study confirmed that prenatal was more effective than postnatal intervention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mežnarić, S.; Belančić, A.; Rački, V.; Vitezić, D.; Mršić-Pelčić, J.; Pilipović, K. Prenatal Management of Spinal Muscular Atrophy in the Era of Genetic Screening and Emerging Opportunities in In Utero Therapy. Biomedicines 2025, 13, 1796. https://doi.org/10.3390/biomedicines13081796
Mežnarić S, Belančić A, Rački V, Vitezić D, Mršić-Pelčić J, Pilipović K. Prenatal Management of Spinal Muscular Atrophy in the Era of Genetic Screening and Emerging Opportunities in In Utero Therapy. Biomedicines. 2025; 13(8):1796. https://doi.org/10.3390/biomedicines13081796
Chicago/Turabian StyleMežnarić, Silvestar, Andrej Belančić, Valentino Rački, Dinko Vitezić, Jasenka Mršić-Pelčić, and Kristina Pilipović. 2025. "Prenatal Management of Spinal Muscular Atrophy in the Era of Genetic Screening and Emerging Opportunities in In Utero Therapy" Biomedicines 13, no. 8: 1796. https://doi.org/10.3390/biomedicines13081796
APA StyleMežnarić, S., Belančić, A., Rački, V., Vitezić, D., Mršić-Pelčić, J., & Pilipović, K. (2025). Prenatal Management of Spinal Muscular Atrophy in the Era of Genetic Screening and Emerging Opportunities in In Utero Therapy. Biomedicines, 13(8), 1796. https://doi.org/10.3390/biomedicines13081796








