Necroptotic and Apoptotic Pathways in Sepsis: A Comparative Analysis of Pediatric and Adult ICU Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Ethics and Consent
2.5. Laboratory Methods
2.6. Rationale for Marker Selection
- RIPK1, RIPK3, MLKL, and A20 are core components of the necroptosis cascade [3]: RIPK1 initiates necrosome formation via RIPK3 phosphorylation, MLKL executes membrane disruption, and A20 modulates pathway activity by limiting ubiquitination events—and thus inflammatory cell death—during sepsis.
- Caspase-8 was chosen as the apoptosis-related marker due to its dual functionality: It triggers extrinsic apoptosis through FADD activation and simultaneously suppresses necroptosis by cleaving RIPK1 and RIPK3, thus preventing necrosome formation [5]. Therefore, caspase-8 serves as a pivotal “molecular switch” at the intersection of apoptotic and necroptotic signaling [6].
- While caspase-3 is a downstream executioner of apoptosis, it does not reflect the regulatory balance between apoptotic and necroptotic pathways and was therefore not included in this phase of the study [17].
- We included IL-1β and IL-18 because these cytokines are products of inflammasome activation and serve as biomarkers of necroinflammation and pyroptosis—pathways that intersect cytokine-mediated inflammation and programmed necrosis [9]. Experimental models demonstrate that combined inhibition of IL-1β and IL-18 protects against lethal sepsis, underscoring their relevance as downstream effectors of cell–death–induced inflammation. Elevated IL-18 levels, in particular, have been independently associated with sepsis severity and mortality [18].
- In contrast, classical pro-inflammatory cytokines such as TNF-α and IL-6 were excluded from our biomarker panel due to their non-specificity, rapid kinetics, and broad elevation in various inflammatory conditions (e.g., trauma, surgery, and non-septic illness), which limit their usefulness as stable indicators of programmed cell death pathways in critically ill patients [19].
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Laboratory Findings
3.3. Necroptosis Biomarkers
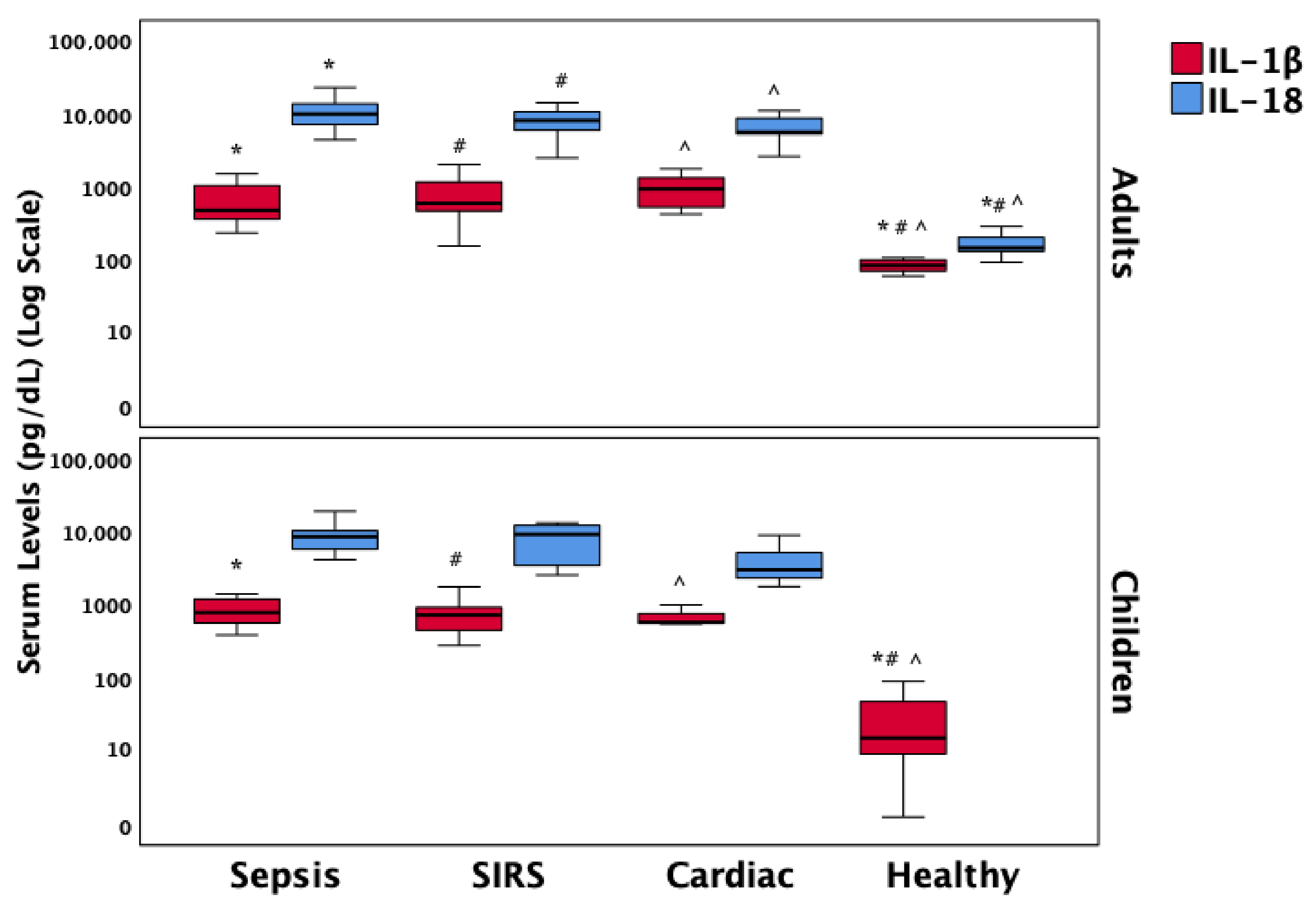
3.4. Biomarker Distribution Across Clinical Groups
3.5. Expression of Necroptosis Pathway Proteins
3.6. Inflammatory Mediators of Necroptosis
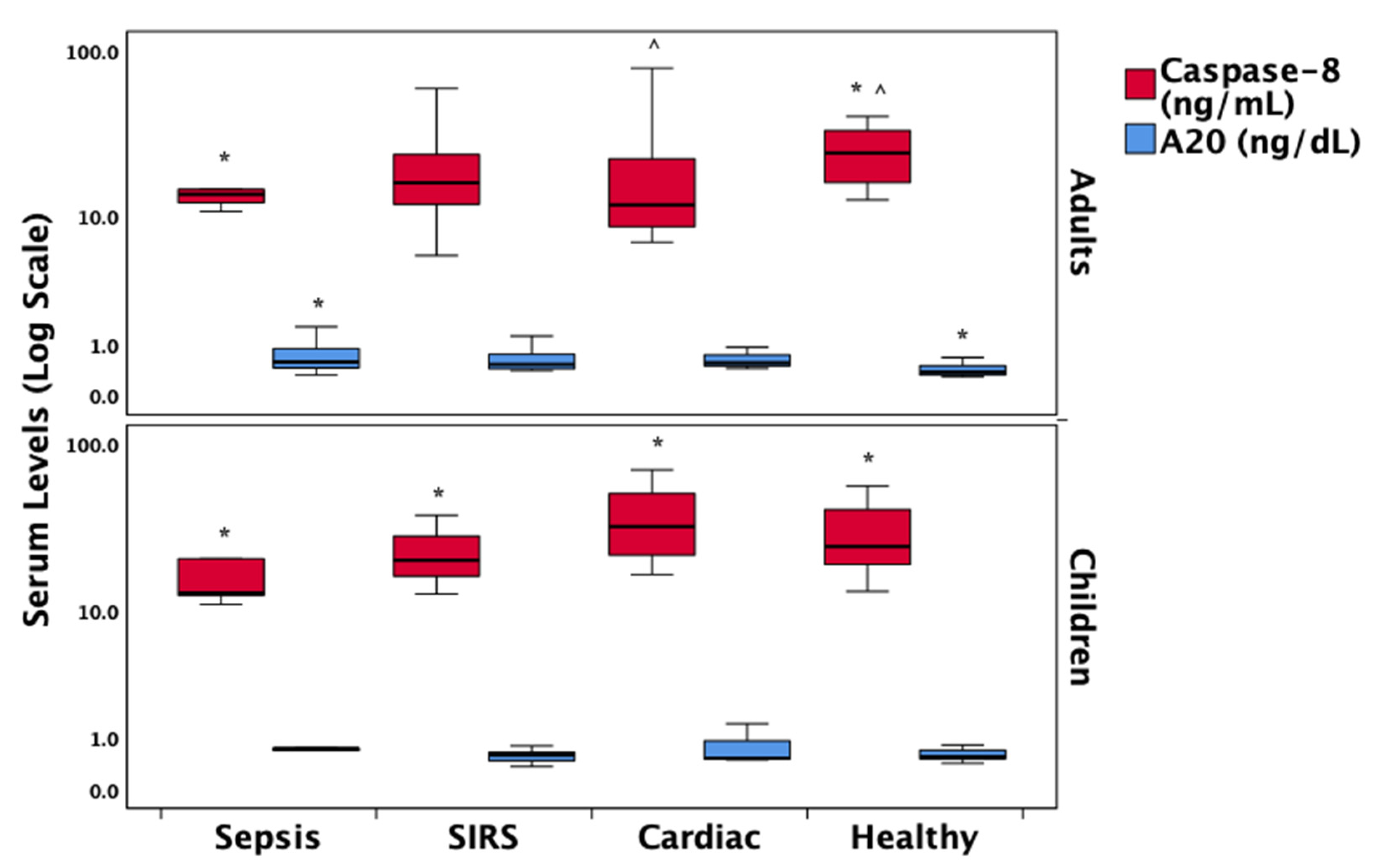
3.7. Regulatory Proteins in the Necroptosis Pathway
3.8. Correlations with Clinical Outcomes
3.9. Independent Associations
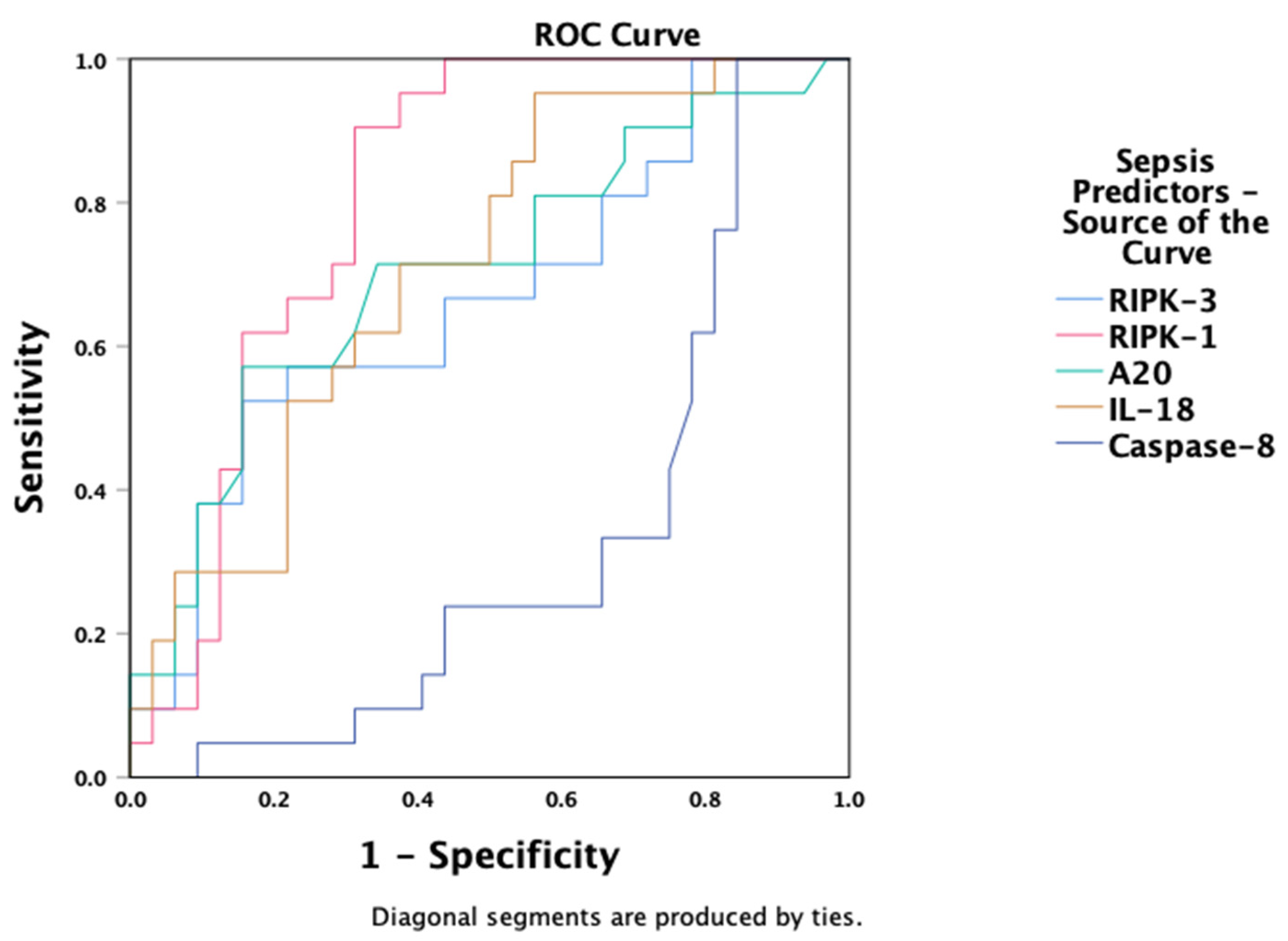
3.10. Predictive Value of Necroptosis Biomarkers
4. Discussion
4.1. Necroptosis Activation in Sepsis
4.2. The Role of Pro-Inflammatory Cytokines IL-1β and IL-18
4.3. A20 as a Regulatory Checkpoint
4.4. Differences Across Age Groups and Disease States
4.5. Prognostic and Therapeutic Implications
4.6. Strengths and Limitations
4.7. Perspective and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| ARDS | Acute Respiratory Distress Syndrome |
| IL | Interleukin |
| SIRS | Systemic Inflammatory Response Syndrome |
| ELISA | Enzyme-linked Immunosorbent Assay |
| APACHE | Acute Physiology and Chronic Health Evaluation Score II |
| SOFA | Sequential Organ Failure Assessment |
| BMI | Body Mass Index |
| RIPK | Receptor-Interacting Protein Kinase |
| MLKL | Mixed Lineage Kinase Domain-Like Protein |
| PeLOD | Pediatric Logistic Organ Dysfunction |
| PRISM | Pediatric Risk of Mortality |
| qSOFA | quick Sepsis-Related Organ Failure Assessment |
| ROC | Receiver Operating Characteristic |
| mTORC1 | Mammalian Target of Rapamycin Complex 1 |
References
- Briassoulis, G.; Briassoulis, P.; Ilia, S.; Miliaraki, M.; Briassouli, E. The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis. Antioxidants 2023, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hu, D.; Sakya, J.; Sun, T.; Wang, D.; Wang, L.; Mao, X.; Su, Z. ABIN-1 Is a Key Regulator in RIPK1-Dependent Apoptosis (RDA) and Necroptosis, and ABIN-1 Deficiency Potentiates Necroptosis-Based Cancer Therapy in Colorectal Cancer. Cell Death Dis. 2021, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, Y.; Yuan, Z.; Zhao, D.; Weng, P.; Li, Y.; Chen, Q.; Zhang, W.; Hu, H.; Yu, C. Hypoxia-Mediated SUMOylation of FADD Exacerbates Endothelial Cell Injury via the RIPK1-RIPK3-MLKL Signaling Axis. Cell Death Dis. 2025, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Miliaraki, M.; Briassoulis, P.; Ilia, S.; Michalakakou, K.; Karakonstantakis, T.; Polonifi, A.; Bastaki, K.; Briassouli, E.; Vardas, K.; Pistiki, A.; et al. Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations. Antioxidants 2022, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Newton, K. RIPK1 and RIPK3: Critical Regulators of Inflammation and Cell Death. Trends Cell Biol. 2015, 25, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Wickliffe, K.E.; Dugger, D.L.; Maltzman, A.; Roose-Girma, M.; Dohse, M.; Kőműves, L.; Webster, J.D.; Dixit, V.M. Cleavage of RIPK1 by Caspase-8 Is Crucial for Limiting Apoptosis and Necroptosis. Nature 2019, 574, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Oshima, S.; Nibe, Y.; Kobayashi, M.; Maeyashiki, C.; Nemoto, Y.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; et al. RIPK3 Regulates P62-LC3 Complex Formation via the Caspase-8-Dependent Cleavage of P62. Biochem. Biophys. Res. Commun. 2015, 456, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Someda, M.; Kuroki, S.; Miyachi, H.; Tachibana, M.; Yonehara, S. Caspase-8, Receptor-Interacting Protein Kinase 1 (RIPK1), and RIPK3 Regulate Retinoic Acid-Induced Cell Differentiation and Necroptosis. Cell Death Differ. 2020, 27, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhu, R.; Dai, Q.; Xu, G.; Zhang, G. The Role and Mechanism of the NLRP3-IL-1β/IL-18 Signaling Axis in the Progression of Sepsis under an Aging Phenotype. Life Sci. 2025, 378, 123812. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, J.; Gao, H.-M.; Xing, Y.-H.; Lin, Z.; Li, H.-J.; Wang, Y.-Q. Necroptosis Regulated Proteins Expression Is an Early Prognostic Biomarker in Patient with Sepsis: A Prospective Observational Study. Oncotarget 2017, 8, 84066–84073. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Im, Y.; Ko, R.-E.; Lee, J.Y.; Park, J.; Jeon, K. Association of Plasma Level of High-Mobility Group Box-1 with Necroptosis and Sepsis Outcomes. Sci. Rep. 2021, 11, 9512. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Lee, J.Y.; Park, J.; Suh, G.Y.; Jeon, K. Association of Plasma Levels of Fas Ligand with Severity and Outcome of Sepsis. Shock. 2021, 56, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Mallarpu, C.S.; Ponnana, M.; Prasad, S.; Singarapu, M.; Kim, J.; Haririparsa, N.; Bratic, N.; Brar, H.; Chelluri, L.K.; Madiraju, C. Distinct Cell Death Markers Identified in Critical Care Patient Survivors Diagnosed with Sepsis. Immunol. Lett. 2021, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Lee, J.Y.; Park, J.; Yang, J.H.; Suh, G.Y.; Jeon, K. Association of Plasma Level of TNF-Related Apoptosis-Inducing Ligand with Severity and Outcome of Sepsis. J. Clin. Med. 2020, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Moerke, C.; Bleibaum, F.; Kunzendorf, U.; Krautwald, S. Combined Knockout of RIPK3 and MLKL Reveals Unexpected Outcome in Tissue Injury and Inflammation. Front. Cell Dev. Biol. 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhong, Y.; Ding, R.; Wang, X.; Xia, F.; Zhang, Q.; Peng, Q. New Insights of Necroptosis and Immune Infiltration in Sepsis-Induced Myocardial Dysfunction from Bioinformatics Analysis through RNA-Seq in Mice. Front. Cell Infect. Microbiol. 2022, 12, 1068324. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, N.M.; Van Opdenbosch, N.; Van Gorp, H.; Martín-Pérez, R.; Zecchin, A.; Vandenabeele, P.; Lamkanfi, M. An Apoptotic Caspase Network Safeguards Cell Death Induction in Pyroptotic Macrophages. Cell Rep. 2020, 32, 107959. [Google Scholar] [CrossRef] [PubMed]
- Eidt, M.V.; Nunes, F.B.; Pedrazza, L.; Caeran, G.; Pellegrin, G.; Melo, D.A.S.; Possuelo, L.; Jost, R.T.; Dias, H.B.; Donadio, M.V.F.; et al. Biochemical and Inflammatory Aspects in Patients with Severe Sepsis and Septic Shock: The Predictive Role of IL-18 in Mortality. Clin. Chim. Acta 2016, 453, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhai, Q. Inflammatory Biomarkers to Predict Clinical Outcomes in Adults after Cardiac Surgery in China: A Prospective Observational Trial. Cytokine 2025, 193, 156987. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kumari, S.; Kim, C.; Van, T.-M.; Wachsmuth, L.; Polykratis, A.; Pasparakis, M. RIPK1 Counteracts ZBP1-Mediated Necroptosis to Inhibit Inflammation. Nature 2016, 540, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Ling, Y.; Yang, W.; Shen, J.; Li, C.; Deng, W.; Liu, W.; Liu, K. Necroptosis Is a Key Mediator of Enterocytes Loss in Intestinal Ischaemia/Reperfusion Injury. J. Cell Mol. Med. 2017, 21, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Gu, L.; Lu, Y.; Zhao, K.; Wu, J.; Wang, H.; Han, J.; Tang, Y.; Lu, B. High Mobility Group Box 1 Enables Bacterial Lipids to Trigger Receptor-Interacting Protein Kinase 3 (RIPK3)-Mediated Necroptosis and Apoptosis in Mice. J. Biol. Chem. 2019, 294, 8872–8884. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Wu, J.; Li, G.; Tao, X.; Lai, K.; Yuan, Y.; Zhang, X.; Zou, Z.; Xu, Y. RIPK3 Collaborates with GSDMD to Drive Tissue Injury in Lethal Polymicrobial Sepsis. Cell Death Differ. 2020, 27, 2568–2585. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fang, Y.; Wu, J.; Chen, H.; Zou, Z.; Zhang, X.; Shao, J.; Xu, Y. RIPK3-MLKL-Mediated Necroinflammation Contributes to AKI Progression to CKD. Cell Death Dis. 2018, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Guida, N.; Laudati, G.; Serani, A.; Mascolo, L.; Molinaro, P.; Montuori, P.; Di Renzo, G.; Canzoniero, L.M.T.; Formisano, L. The Neurotoxicant PCB-95 by Increasing the Neuronal Transcriptional Repressor REST down-Regulates Caspase-8 and Increases Ripk1, Ripk3 and MLKL Expression Determining Necroptotic Neuronal Death. Biochem. Pharmacol. 2017, 142, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Oberst, A.; Dillon, C.P.; Weinlich, R.; McCormick, L.L.; Fitzgerald, P.; Pop, C.; Hakem, R.; Salvesen, G.S.; Green, D.R. Catalytic Activity of the Caspase-8-FLIP(L) Complex Inhibits RIPK3-Dependent Necrosis. Nature 2011, 471, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.P.; Oberst, A.; Weinlich, R.; Janke, L.J.; Kang, T.-B.; Ben-Moshe, T.; Mak, T.W.; Wallach, D.; Green, D.R. Survival Function of the FADD-CASPASE-8-cFLIP(L) Complex. Cell Rep. 2012, 1, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Keshk, W.A.; Ibrahim, M.A.; Shalaby, S.M.; Zalat, Z.A.; Elseady, W.S. Redox Status, Inflammation, Necroptosis and Inflammasome as Indispensable Contributors to High Fat Diet (HFD)-Induced Neurodegeneration; Effect of N-Acetylcysteine (NAC). Arch. Biochem. Biophys. 2020, 680, 108227. [Google Scholar] [CrossRef] [PubMed]
- Sedmaki, K.; Karnam, K.; Sharma, P.; Mahale, A.; Routholla, G.; Ghosh, B.; Prakash Kulkarni, O. HDAC6 Inhibition Attenuates Renal Injury by Reducing IL-1β Secretion and RIP Kinase Mediated Necroptosis in Acute Oxalate Nephropathy. Int. Immunopharmacol. 2022, 110, 108919. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Hou, C.; Zhang, S. miR-425-5p Improves Inflammation and Septic Liver Damage through Negatively Regulating the RIP1-Mediated Necroptosis. Inflamm. Res. 2020, 69, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Perry-Eaddy, M.A.; Faig, W.; Curley, M.A.Q.; Weiss, S.L. Association of Inflammatory Biomarkers with New Functional Morbidity at Hospital Discharge in Children Who Survive Severe Sepsis. Front. Pediatr. 2025, 13, 1519246. [Google Scholar] [CrossRef] [PubMed]
- Mierzchala-Pasierb, M.; Krzystek-Korpacka, M.; Lesnik, P.; Adamik, B.; Placzkowska, S.; Serek, P.; Gamian, A.; Lipinska-Gediga, M. Interleukin-18 Serum Levels in Sepsis: Correlation with Disease Severity and Inflammatory Markers. Cytokine 2019, 120, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.; Dong, G.; Wei, Y.; Xu, Z.; Yang, J. Clinical Value of Serum Interleukin-18 in Neonatal Sepsis Diagnosis and Mortality Prediction. J. Inflamm. Res. 2022, 15, 6923–6930. [Google Scholar] [CrossRef] [PubMed]
- Bottari, G.; Taccone, F.S.; Corrias, A.; Irrera, M.; Currao, P.; Salvagno, M.; Cecchetti, C.; Payen, D. Immunomodulation in Pediatric Sepsis: A Narrative Review. J. Clin. Med. 2025, 14, 2983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wan, L.; Huang, H.; Liao, Z. IL-1β, the First Piece to the Puzzle of Sepsis-Related Cognitive Impairment? Front. Neurosci. 2024, 18, 1370406. [Google Scholar] [CrossRef] [PubMed]
- Carty, M.; Kearney, J.; Shanahan, K.A.; Hams, E.; Sugisawa, R.; Connolly, D.; Doran, C.G.; Muñoz-Wolf, N.; Gürtler, C.; Fitzgerald, K.A.; et al. Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity 2019, 50, 1412–1424.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Ding, Y.; Wu, X.; Li, Y.; Cheng, G.; Wang, N.; Yang, Q.; Zhang, W.; Chen, X.; Liu, X. Pentraxin 3 Promotes the Expression of Pro-Inflammatory Cytokines and the Migration of Macrophages in Myocarditis. BMC Cardiovasc. Disord. 2025, 25, 354. [Google Scholar] [CrossRef] [PubMed]
- Papatheodorou, I.; Blažková, G.; Bosáková, V.; Tomášiková, Z.; Spearing, E.; Klieber, R.; Ostašov, P.; Štíchová, J.; Dvončová, M.; Mýtniková, A.; et al. Redefining the Role of IL-18 in Post-Surgical Recovery and Sepsis: A Key Mediator of Inflammation Resolution. J. Transl. Med. 2025, 23, 728. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-Ubiquitination and Ubiquitin Ligase Domains of A20 Downregulate NF-kappaB Signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Shembade, N.; Ma, A.; Harhaj, E.W. Inhibition of NF-kappaB Signaling by A20 through Disruption of Ubiquitin Enzyme Complexes. Science 2010, 327, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in Inflammation and Autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, T.; Unno, M.; Yoneda, N.; Motomura, Y.; Mochizuki, M.; Sasaki, T.; Pasparakis, M.; Saito, T. RIPK1 Blocks T Cell Senescence Mediated by RIPK3 and Caspase-8. Sci. Adv. 2023, 9, eadd6097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-S.; Yang, Y.; Mou, L.; Xia, X.; Liu, M.; Xu, L.-J.; Liu, R.; Liu, J.-P.; Zhang, H.-Y.; Ao, X.-J.; et al. Melatonin Ameliorates Age-Related Sarcopenia via the Gut-Muscle Axis Mediated by Serum Lipopolysaccharide and Metabolites. J. Cachexia Sarcopenia Muscle 2025, 16, e13722. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.P.; Weinlich, R.; Rodriguez, D.A.; Cripps, J.G.; Quarato, G.; Gurung, P.; Verbist, K.C.; Brewer, T.L.; Llambi, F.; Gong, Y.-N.; et al. RIPK1 Blocks Early Postnatal Lethality Mediated by Caspase-8 and RIPK3. Cell 2014, 157, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Garvin, A.M.; Jackson, M.A.; Korzick, D.H. Inhibition of Programmed Necrosis Limits Infarct Size through Altered Mitochondrial and Immune Responses in the Aged Female Rat Heart. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1434–H1442. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-Alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, M.I.F.J.; Liu, J.; Arslan, F.; den Ouden, K.; van Middelaar, B.J.; Doevendans, P.A.; Sluijter, J.P.G. Inhibition of RIP1-Dependent Necrosis Prevents Adverse Cardiac Remodeling after Myocardial Ischemia-Reperfusion in Vivo. Basic. Res. Cardiol. 2012, 107, 270. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Briassouli, E.; Fitrolaki, D.-M.; Plati, I.; Apostolou, K.; Tavladaki, T.; Spanaki, A.-M. Heat Shock Protein 72 Expressing Stress in Sepsis: Unbridgeable Gap between Animal and Human Studies--a Hypothetical “Comparative” Study. Biomed. Res. Int. 2014, 2014, 101023. [Google Scholar] [CrossRef] [PubMed]
- Spanaki, A.M.; Tavladaki, T.; Dimitriou, H.; Kozlov, A.V.; Duvigneau, J.C.; Meleti, E.; Weidinger, A.; Papakonstantinou, E.; Briassoulis, G. Longitudinal Profiles of Metabolism and Bioenergetics Associated with Innate Immune Hormonal Inflammatory Responses and Amino-Acid Kinetics in Severe Sepsis and Systemic Inflammatory Response Syndrome in Children. JPEN J. Parenter. Enteral Nutr. 2018, 42, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Tavladaki, T.; Spanaki, A.M.; Dimitriou, H.; Kondili, E.; Choulaki, C.; Georgopoulos, D.; Briassoulis, G. Similar Metabolic, Innate Immunity, and Adipokine Profiles in Adult and Pediatric Sepsis Versus Systemic Inflammatory Response Syndrome-A Pilot Study. Pediatr. Crit. Care Med. 2017, 18, e494–e505. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical Inhibitor of Nonapoptotic Cell Death with Therapeutic Potential for Ischemic Brain Injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Chaouhan, H.S.; Vinod, C.; Mahapatra, N.; Yu, S.-H.; Wang, I.-K.; Chen, K.-B.; Yu, T.-M.; Li, C.-Y. Necroptosis: A Pathogenic Negotiator in Human Diseases. Int. J. Mol. Sci. 2022, 23, 12714. [Google Scholar] [CrossRef] [PubMed]
- Pefanis, A.; Bongoni, A.K.; McRae, J.L.; Salvaris, E.J.; Fisicaro, N.; Murphy, J.M.; Ierino, F.L.; Cowan, P.J. Inhibition of RIPK1 or RIPK3 Kinase Activity Post Ischemia-Reperfusion Reduces the Development of Chronic Kidney Injury. Biochem. J. 2025, 482, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Liang, F.; Lou, Z.; Li, Y.; Li, J.; Chen, Y.; Ding, J.; Jiang, B.; Wu, C.; Yu, H.; et al. Necrostatin-1 Alleviates Lung Ischemia-Reperfusion Injury via Inhibiting Necroptosis and Apoptosis of Lung Epithelial Cells. Cells 2022, 11, 3139. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Geng, Z.; Yang, H.; Wei, Y.; Chen, Z.; Jiang, Q.; Chen, Y.; Bi, Y.; Zhang, L. Discovery of Novel Fusidic Acid Derivatives in Mitigating LPS-Induced Acute Liver Injury by Modulating RIPK1. Bioorg Med. Chem. Lett. 2025, 128, 130322. [Google Scholar] [CrossRef] [PubMed]

| Subject Characteristics | Total (n = 88) | Adults (n = 56) | Children (n = 32) | p-Value |
|---|---|---|---|---|
| Gender, n (%) | 0.825 | |||
| 62 (70.5) | 39 (69.6) | 23 (71.9) | |
| 26 (29.5) | 17 (30.4) | 9 (28.1) | |
| Age (years), mean ± SD | 33.1 ± 25 | 47.4 ± 21 | 8.1 ± 5.5 | <0.001 |
| Body weight (kg), mean ± SD | 56.8 ± 33 | 70.6 ± 28 | 32.5 ± 24 | <0.001 |
| BMI (kg/m2), mean ± SD | 23.3 ± 4.9 | 25.5 ± 4.3 | 19.4 ± 2.9 | <0.001 |
| BMI Nutritional Status, n (%) | 0.020 | |||
| 8 (9.1) | 2 (3.6) | 6 (18.8) | |
| 55 (62.5) | 33 (58.9) | 22 (68.8) | |
| 18 (20.5) | 15 (26.8) | 3 (9.4) | |
| 7 (8.0) | 6 (10.7) | 1 (3.1) | |
| Study Group, n (%) | 0.133 | |||
| 23 (26.1) | 17 (30.4) | 6 (18.8) | |
| 29 (33.0) | 15 (26.8) | 14 (43.8) | |
| 19 (21.6) | 15 (26.8) | 4 (12.5) | |
| 17 (19.3) | 9 (16.1) | 8 (25.0) |
| Patients Characteristics | Total (n = 71) | Adults (n = 47) | Children (n = 24) | p-Value |
|---|---|---|---|---|
| Temperature (°C), mean ± SD | 37.9 ± 0.7 | 37.7 ± 0.6 | 38.1 ± 0.9 | 0.072 |
| APACHE II, mean ± SD | 14.9 ± 9.7 | 16.2 ± 11 | 12.6 ± 6.9 | 0.147 |
| SOFA score ≥ 2, n (%) | 44 (62) | 26 (55.3) | 18 (75.0) | 0.106 |
| Comorbidities, n (%) | 31 (35.2) | 29 (51.8) | 2 (6.3) | <0.001 |
| Primary Clinical Diagnoses | 0.007 | |||
| 17 (19.3) | 9 (16.1) | 8 (25.0) | |
| 8 (9.1) | 1 (1.8) | 7 (21.9) | |
| 12 (13.6) | 8 (14.3) | 4 (12.5) | |
| 24 (27.3) | 16 (28.6) | 9 (27.0) | |
| 6 (6.8) | 3 (5.4) | 3 (9.4) | |
| 10 (11.4) | 9 (16.1) | 1 (3.1) | |
| Therapeutic Interventions, n (%) | ||||
| 37 (52.1) | 26 (55.3) | 11 (45.8) | 0.449 |
| 49 (69.0) | 40 (85.1) | 9 (37.5) | <0.001 |
| ICU stay (days), mean ± SD | 7.14 ± 7.5 | 7.37 ± 5.6 | 6.75 ± 9.9 | 0.712 |
| Duration of mechanical ventilation (days) | 5.22 ± 7.2 | 4.20 ± 1.7 | 6.75 ± 11 | 0.306 |
| Duration of vasoactive therapy (days) | 4.1 ± 1.7 | 4.0 ± 1.7 | 6.9 ± 11 | 0.771 |
| Mortality, n (%) | 2 (2.8) | 1 (2.1) | 1 (4.2) | 0.623 |
| Variable | Sepsis | SIRS | Cardiac | Healthy | p-Value |
|---|---|---|---|---|---|
| Participants, n (%) | 23 (26.1) | 29 (33.0) | 19 (21.6) | 17 (19.3) | — |
| Age (years), mean ± SD | 47 ± 30 | 29 ± 26 | 32 ± 21 | 23 ± 12 | 0.012 |
| Body weight (kg), mean ± SD | 52 ± 32 | 55 ± 37 | 77 ± 24 | 43 ± 24 | 0.011 |
| BMI (kg/m2), mean ± SD | 23 ± 4.6 | 23 ± 6.5 | 25 ± 4.2 | 22 ± 1.7 | 0.421 |
| ICU length of stay (days), mean ± SD | 9.7 ± 5.5 | 9.8 ± 9.6 | 5.5 ± 4.0 | — | 0.098 |
| Biomolecules All Subjects | Sepsis (n = 23) | SIRS (n = 29) | Cardiac (n = 19) | Healthy (n = 17) | p-Value ** |
|---|---|---|---|---|---|
| RIPK-1 (ng/mL), median (IQR) | 30.3 (16–47) * | 8.9 (4.6–22) # | 3.7 (2.1–6.1) * | 2.5 (1.7–3.5) *,# | <0.001 |
| RIPK-3 (ng/mL), median (IQR) | 3.8 (1.2–7.0) | 1.9 (0.8–2.9) | 1.1 (0.6–2.7) | 1.5 (0.7–2.2) | 0.085 |
| MLKL (ng/mL), median (IQR) | 6.5 (5.0–7.0) | 6.6 (4.3–7.6) | 6.0 (3.8–6.3) | 5.2 (4.7–6.0) | 0.131 |
| A20 (ng/mL), median (IQR) | 0.72 (0.5–0.9) * | 0.55 (0.4–0.7) | 0.52 (0.5–0.6) | 0.37 (0.3–0.5) * | 0.065 |
| IL-1β (pg/mL), median (IQR) | 561 (387–948) * | 723 (406–940) # | 933 (658–1027) ^ | 84 (70–99) *,#,^ | <0.001 |
| IL-18 (pg/mL), median (IQR) | 9815 (7220–13,504) * | 9033 (3671–12,588) # | 5417 (3133–6990) ^ | 147 (132–206) *,#,^ | <0.001 |
| Caspase-8 (ng/mL), median (IQR) | 13.8 (12–15) * | 17.4 (15–27) | 16.6 (8.9–54) | 24.6 (16–34) * | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briassoulis, G.; Tzermia, K.; Bastaki, K.; Miliaraki, M.; Briassoulis, P.; Damianaki, A.; Kondili, E.; Ilia, S. Necroptotic and Apoptotic Pathways in Sepsis: A Comparative Analysis of Pediatric and Adult ICU Patients. Biomedicines 2025, 13, 1747. https://doi.org/10.3390/biomedicines13071747
Briassoulis G, Tzermia K, Bastaki K, Miliaraki M, Briassoulis P, Damianaki A, Kondili E, Ilia S. Necroptotic and Apoptotic Pathways in Sepsis: A Comparative Analysis of Pediatric and Adult ICU Patients. Biomedicines. 2025; 13(7):1747. https://doi.org/10.3390/biomedicines13071747
Chicago/Turabian StyleBriassoulis, George, Konstantina Tzermia, Kalliopi Bastaki, Marianna Miliaraki, Panagiotis Briassoulis, Athina Damianaki, Eumorfia Kondili, and Stavroula Ilia. 2025. "Necroptotic and Apoptotic Pathways in Sepsis: A Comparative Analysis of Pediatric and Adult ICU Patients" Biomedicines 13, no. 7: 1747. https://doi.org/10.3390/biomedicines13071747
APA StyleBriassoulis, G., Tzermia, K., Bastaki, K., Miliaraki, M., Briassoulis, P., Damianaki, A., Kondili, E., & Ilia, S. (2025). Necroptotic and Apoptotic Pathways in Sepsis: A Comparative Analysis of Pediatric and Adult ICU Patients. Biomedicines, 13(7), 1747. https://doi.org/10.3390/biomedicines13071747







