Impact of Preoperative Weight Loss on Prognosis in Patients with Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment and Data Collection
2.3. Neoadjuvant Therapy, Postoperative Adjuvant Therapy, and Postoperative Follow-Up
2.4. Preoperative Changes in Body Weight and Nutrition Therapy
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics in the Two Groups Classified by Pre-%WL
3.2. Comparison of Pre-%WL and Perioperative Outcomes
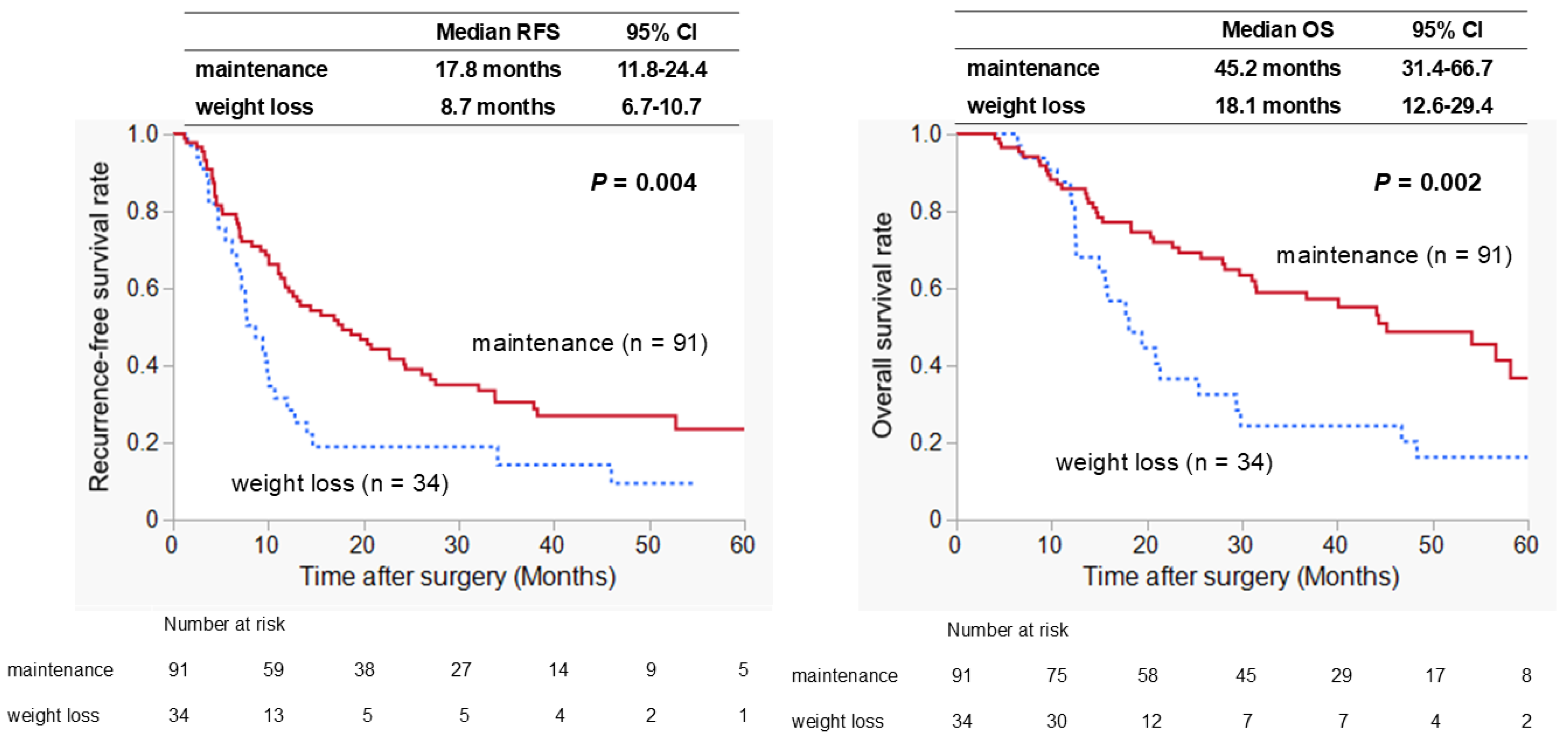
3.3. Correlation Between Pre-%WL and Long-Term Postoperative Outcomes
3.4. Prognostic Factors Associated with RFS and OS
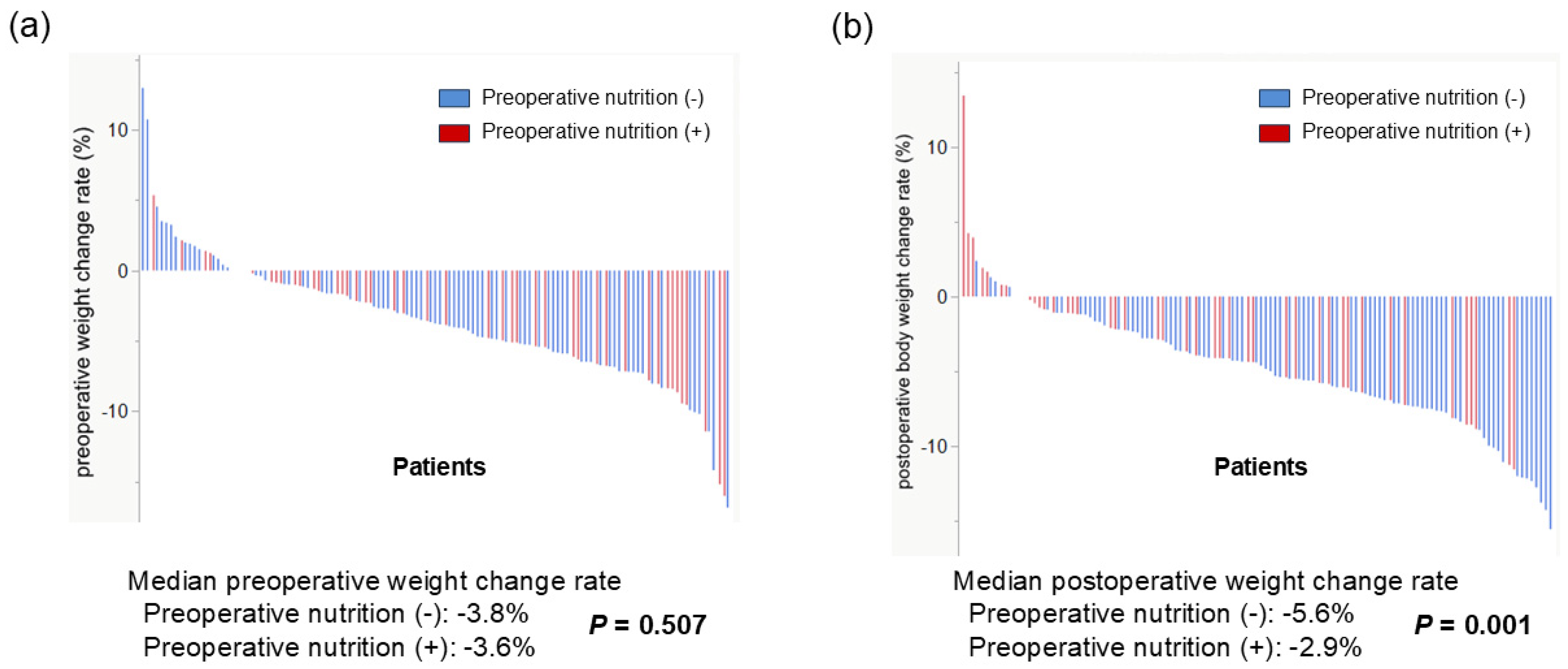
3.5. Effect of Preoperative Nutritional Therapy on Weight Loss Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROC | Receiver operating characteristic curve |
| RFS | Recurrence-free survival |
| PDAC | Pancreatic ductal carcinoma |
| PFS | Progression-free survival |
| OS | Overall survival |
| CRP | C-reactive protein |
| PD | Pancreatoduodenectomy |
| TP | Total pancreatectomy |
| HR | Hazard ratio |
| CI | Confidence interval |
| NAC | Neoadjuvant chemotherapy |
| BCAA | Branched-chain amino acids |
| Pre-%WL | Preoperative weight loss rate |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Carioli, G.; Malvezzi, M.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann. Oncol. 2021, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Xu, Y.; Herzog, K.; Saldia, A.; DeFilippis, E.M.; Li, P.; Allen, P.J.; O’Reilly, E.M.; Kurtz, R.C. Weight loss, diabetes, fatigue, and depression preceding pancreatic cancer. Pancreas 2016, 45, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, T.M.; Villafane-Ferriol, N.; Shah, K.P.; Shah, R.M.; Tran Cao, H.S.; Massarweh, N.N.; Silberfein, E.J.; Choi, E.A.; Hsu, C.; McElhany, A.L.; et al. Nutritional and metabolic derangements in pancreatic cancer and pancreatic resection. Nutrients 2017, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, S.; Krüger, J.; Aghdassi, A.A.; Steveling, A.; Simon, P.; Lerch, M.M.; Mayerle, J. Nutrition in pancreatic cancer: A review. Gastrointest. Tumors 2016, 2, 195–202. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Petzel, M.Q.B.; Zimmers, T.A.; Denlinger, C.S.; Matrisian, L.M.; Picozzi, V.J.; Rahib, L.; Precision Promise Consortium. Pancreas cancer-associated weight loss. Oncologist 2019, 24, 691–701. [Google Scholar] [CrossRef]
- Bachmann, J.; Heiligensetzer, M.; Krakowski-Roosen, H.; Büchler, M.W.; Friess, H.; Martignoni, M.E. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J. Gastrointest. Surg. 2008, 12, 1193–1201. [Google Scholar] [CrossRef]
- Pausch, T.; Hartwig, W.; Hinz, U.; Swolana, T.; Bundy, B.D.; Hackert, T.; Grenacher, L.; Büchler, M.W.; Werner, J. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012, 152 (Suppl 1), S81–S88. [Google Scholar] [CrossRef]
- Trudeau, M.T.; Casciani, F.; Gershuni, V.M.; Maggino, L.; Ecker, B.L.; Lee, M.K.; Roses, R.E.; DeMatteo, R.P.; Fraker, D.L.; Drebin, J.A.; et al. Defining postoperative weight change after pancreatectomy: Factors associated with distinct and dynamic weight trajectories. Surgery 2020, 168, 1041–1047. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chikamoto, A.; Ohmuraya, M.; Abe, S.; Nakagawa, S.; Beppu, T.; Takamori, H.; Hirota, M.; Baba, H. Impact of postoperative weight loss on survival after resection for pancreatic cancer. JPEN J. Parenter. Enteral Nutr. 2015, 39, 598–603. [Google Scholar] [CrossRef]
- Morita, Y.; Sakaguchi, T.; Kitajima, R.; Furuhashi, S.; Kiuchi, R.; Takeda, M.; Hiraide, T.; Shibasaki, Y.; Kikuchi, H.; Konno, H.; et al. Body weight loss after surgery affects the continuity of adjuvant chemotherapy for pancreatic cancer. BMC Cancer 2019, 19, 416. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Nemer, L.; Krishna, S.G.; Shah, Z.K.; Conwell, D.L.; Cruz-Monserrate, Z.; Dillhoff, M.; Guttridge, D.C.; Hinton, A.; Manilchuk, A.; Pawlik, T.M.; et al. Predictors of pancreatic cancer-associated weight loss and nutritional interventions. Pancreas 2017, 46, 1152–1157. [Google Scholar] [CrossRef]
- Kanda, M.; Fujii, T.; Kodera, Y.; Nagai, S.; Takeda, S.; Nakao, A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br. J. Surg. 2011, 98, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yamamoto, J.; Einama, T.; Hoshikawa, M.; Iwasaki, T.; Nakazawa, A.; Takihara, Y.; Tsunenari, T.; Kishi, Y. Preoperative rapid weight loss as a prognostic predictor after surgical resection for pancreatic cancer. Pancreas 2022, 51, 1388–1397. [Google Scholar] [CrossRef]
- Kuwabara, S.; Nakaya, T.; Ishido, K.; Aoki, Y.; Yamamoto, K.; Shoji, Y.; Fukunaga, A.; Ichimura, T.; Manase, H.; Hirano, S. Clinical impact of weight loss during hospitalization on prognosis after pancreatic surgery. Cureus 2024, 16, e69427. [Google Scholar] [CrossRef]
- Doshi, S.; Abad, J.; Wells, A.; Chawla, A. Weight loss during neoadjuvant chemotherapy impacts perioperative outcomes in patients undergoing surgery for pancreatic cancer. Pancreatology 2023, 23, 1020–1027. [Google Scholar] [CrossRef]
- Hue, J.J.; Markt, S.C.; Sugumar, K.; Kyasaram, R.K.; Shanahan, J.; Rothermel, L.D.; Ammori, J.B.; Hardacre, J.M.; Winter, J.M.; Ocuin, L.M. Weight loss during neoadjuvant therapy for pancreatic cancer does not predict poor outcomes. Am. J. Surg. 2022, 223, 927–932. [Google Scholar] [CrossRef]
- Gong, X.; Xuan, Y.; Pang, C.; Dong, C.; Cao, R.; Wei, Z.; Liang, C. DUPAN-2 in pancreatic cancer: Systematic review and meta-analysis. Clin. Chim. Acta 2025, 567, 120080. [Google Scholar] [CrossRef]
- Sasaki, A.; Sakata, K.; Nakano, K.; Tsutsumi, S.; Fujishima, H.; Futsukaichi, T.; Terashi, T.; Ikebe, M.; Bandoh, T.; Utsunomiya, T. DUPAN-2 as a risk factor of early recurrence after curative pancreatectomy for patients with pancreatic ductal adenocarcinoma. Pancreas 2023, 52, e110–e114. [Google Scholar] [CrossRef]
- Rossmeislová, L.; Gojda, J.; Smolková, K. Pancreatic cancer: Branched-chain amino acids as putative key metabolic regulators? Cancer Metastasis Rev. 2021, 40, 1115–1139. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, M.; Harimoto, N.; Araki, K.; Kubo, N.; Watanabe, A.; Igarashi, T.; Ishii, N.; Yamanaka, T.; Hagiwara, K.; Hoshino, K.; et al. Impact of preoperative nutritional support and rehabilitation therapy in patients undergoing pancreaticoduodenectomy. Int. J. Clin. Oncol. 2021, 26, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
| Variables | Weight Maintenance (n = 91) | Weight Loss (n = 34) | p-Value |
|---|---|---|---|
| Age (years) | 73 (42–88) | 73 (44–86) | 0.818 |
| Male sex | 38 (42%) | 20 (59%) | 0.108 |
| Body mass index (kg/m2) | 21.0 (15.6–31.0) | 20.9 (16.8–31.7) | 0.689 |
| Resectability | 1.000 | ||
| Resectable | 81 (89%) | 31 (91%) | |
| Borderline resectable | 10 (11%) | 3 (9%) | |
| Preoperative interval period (days) | 33 (12–667) | 34 (13–129) | 0.615 |
| Preoperative chemotherapy | 25 (27%) | 10 (29%) | 0.826 |
| Parameters | |||
| Albumin (g/dL) | 4.2 (3.2–4.8) | 3.9 (2.3–4.7) | <0.001 * |
| Hemoglobin (g/dL) | 13.2 (7.1–15.9) | 12.4 (8.2–17.3) | 0.069 |
| Lymphocytes (/μL) | 1540 (320–3410) | 1430 (350–2550) | 0.447 |
| CRP (mg/dL) | 0.07 (0.01–3.32) | 0.15 (0.01–18.14) | 0.008 * |
| Zn (µg/dL) | 76 (56–131) | 76 (35–117) | 0.410 |
| CA19-9 (U/mL) | 69 (1–1292) | 82 (1–22472) | 0.739 |
| DUPAN-2 (U/mL) | 81 (25–11706) | 294 (25–16000) | 0.001 * |
| Span-1 (U/mL) | 41.6 (10–1325) | 55.6 (10–5115) | 0.156 |
| Tumor size (mm) | 30 (0–610) | 33 (18–162) | 0.119 |
| Positive lymph node | 59 (65%) | 24 (71%) | 0.671 |
| Preoperative nutritional therapy | 30 (33%) | 15 (44%) | 0.297 |
| Variables | Weight Maintenance (n = 91) | Weight Loss (n = 34) | p-Value |
|---|---|---|---|
| Operative procedures | 0.140 | ||
| Pancreatoduodenectomy/Total pancreatectomy | 55 (60%) | 26 (76%) | |
| Distal pancreatectomy | 36 (40%) | 8 (24%) | |
| Operative time (min) | 446 (188–776) | 526 (211–730) | 0.025 * |
| Blood loss (mL) | 308 (10–2964) | 422 (23–2148) | 0.018 * |
| Postoperative hospitalization (days) | 21 (10–82) | 22 (10–62) | 0.809 |
| Complications (Clavien–Dindo grade ≥ III) | 26 (29%) | 6 (18%) | 0.255 |
| Adjuvant S-1 therapy completion | 45 (49%) | 15 (44%) | 0.689 |
| Factor | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Weight loss | 1.93 | 1.22–3.04 | 0.005 * | 2.07 | 1.28–3.34 | 0.003 * |
| Female sex | 1.05 | 0.69–1.59 | 0.829 | |||
| Borderline resectable | 2.04 | 1.08–3.85 | 0.028 * | 3.72 | 1.85–7.47 | 0.0002 * |
| Neoadjuvant chemotherapy | 1.43 | 0.89–2.31 | 0.142 | |||
| Age ≥ 75 years | 1.26 | 0.76–2.09 | 0.384 | |||
| CA19-9 ≥ 150 U/mL | 1.61 | 1.03–2.50 | 0.035 * | 1.51 | 0.96–2.35 | 0.072 |
| Tumor size ≥ 30 mm | 1.69 | 1.09–2.62 | 0.019 * | 1.27 | 0.82–2.01 | 0.305 |
| Positive lymph node | 1.72 | 1.07–2.75 | 0.025 * | 1.36 | 0.83–2.23 | 0.229 |
| R1 resection | 1.39 | 0.82–2.36 | 0.223 | |||
| PD, TP | 2.14 | 1.32–3.45 | 0.001 * | 2.00 | 1.18–3.41 | 0.012 * |
| Complications (Clavien–Dindo grade ≥ III) | 0.93 | 0.57–1.53 | 0.785 | |||
| S-1 adjuvant therapy < 6 months | 2.95 | 1.91–4.55 | <0.0001 * | 2.94 | 1.86–4.65 | <0.0001 * |
| Factor | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Weight loss | 2.22 | 1.32–3.73 | 0.003 * | 2.55 | 1.48–4.40 | 0.0008 * |
| Male sex | 1.13 | 0.69–1.84 | 0.632 | |||
| Borderline resectable | 1.48 | 0.73–2.99 | 0.280 | |||
| NAC | 1.01 | 0.55–1.88 | 0.964 | |||
| Age ≥ 75 years | 1.12 | 0.67–1.86 | 0.668 | |||
| CA19-9 ≥ 150 U/mL | 0.98 | 0.57–1.67 | 0.933 | |||
| Tumor size ≥ 30 mm | 1.78 | 1.05–3.02 | 0.033 * | 1.32 | 0.76–2.30 | 0.325 |
| Positive lymph node | 1.70 | 0.97–2.96 | 0.064 | 1.51 | 0.84–2.71 | 0.171 |
| R1 resection | 1.47 | 0.81–2.66 | 0.207 | |||
| PD, TP | 1.77 | 1.02–3.09 | 0.044 * | 1.22 | 0.68–2.19 | 0.510 |
| Complications (Clavien–Dindo grade ≥ III) | 0.90 | 0.51–1.59 | 0.729 | |||
| S-1 adjuvant therapy < 6 months | 3.90 | 2.26–6.74 | <0.0001 * | 3.89 | 2.20–6.89 | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukagoshi, M.; Araki, K.; Kubo, N.; Igarashi, T.; Kawai, S.; Hagiwara, K.; Hoshino, K.; Seki, T.; Okuyama, T.; Fukushima, R.; et al. Impact of Preoperative Weight Loss on Prognosis in Patients with Pancreatic Cancer. Biomedicines 2025, 13, 1703. https://doi.org/10.3390/biomedicines13071703
Tsukagoshi M, Araki K, Kubo N, Igarashi T, Kawai S, Hagiwara K, Hoshino K, Seki T, Okuyama T, Fukushima R, et al. Impact of Preoperative Weight Loss on Prognosis in Patients with Pancreatic Cancer. Biomedicines. 2025; 13(7):1703. https://doi.org/10.3390/biomedicines13071703
Chicago/Turabian StyleTsukagoshi, Mariko, Kenichiro Araki, Norio Kubo, Takamichi Igarashi, Shunsuke Kawai, Kei Hagiwara, Kouki Hoshino, Takaomi Seki, Takayuki Okuyama, Ryosuke Fukushima, and et al. 2025. "Impact of Preoperative Weight Loss on Prognosis in Patients with Pancreatic Cancer" Biomedicines 13, no. 7: 1703. https://doi.org/10.3390/biomedicines13071703
APA StyleTsukagoshi, M., Araki, K., Kubo, N., Igarashi, T., Kawai, S., Hagiwara, K., Hoshino, K., Seki, T., Okuyama, T., Fukushima, R., Shoda, T., & Shirabe, K. (2025). Impact of Preoperative Weight Loss on Prognosis in Patients with Pancreatic Cancer. Biomedicines, 13(7), 1703. https://doi.org/10.3390/biomedicines13071703





