Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives
Abstract
1. Introduction
2. Search Strategy
3. Pathophysiology of Cytokine Storm in Sepsis
4. Extracorporeal Cytokine Adsorption: Principles and Mechanisms
5. Extracorporeal Cytokine Adsorption Efficacy: Clinical Evidence
6. Limitations and Controversies
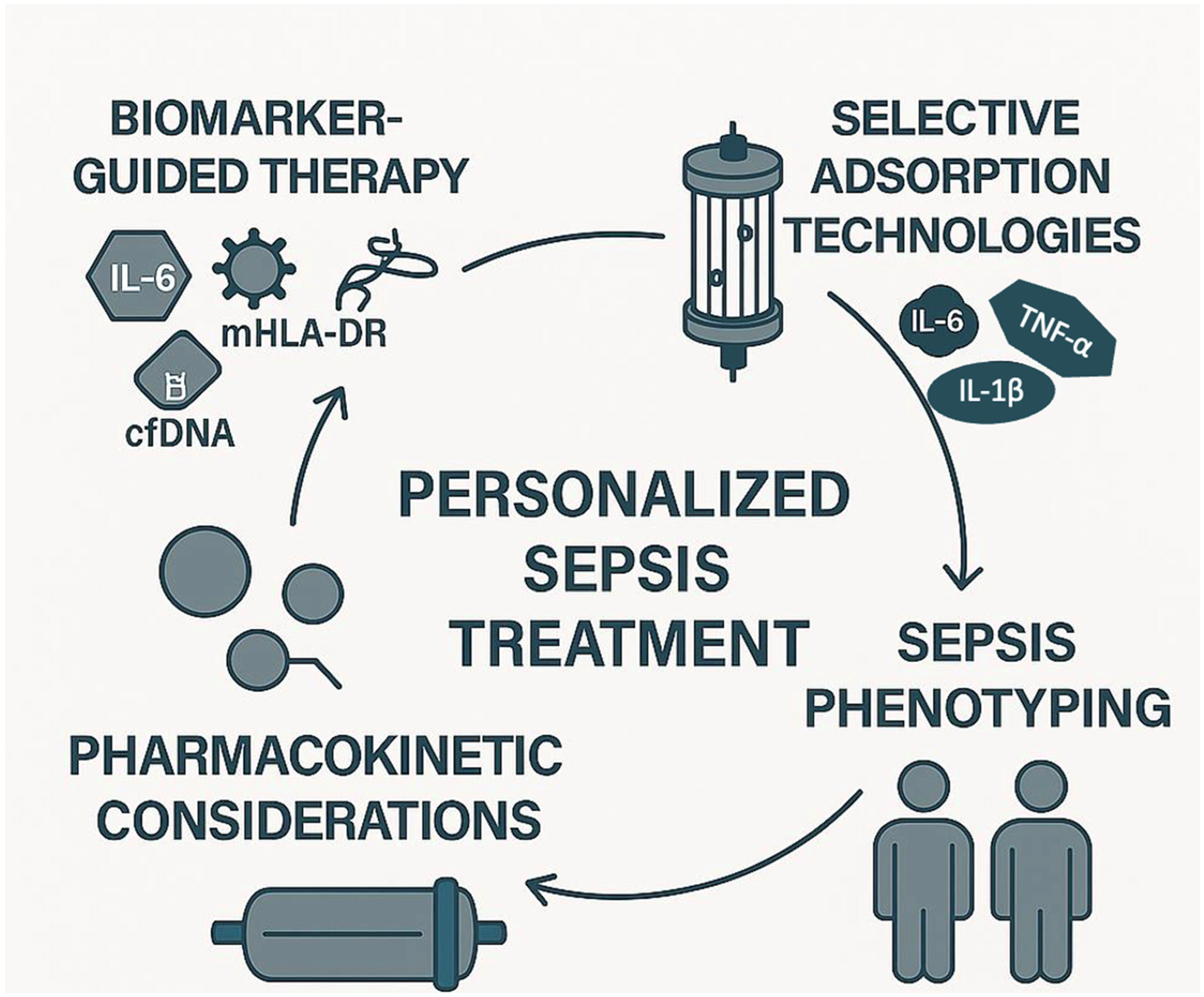
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Guarino, M.; Perna, B.; Cesaro, A.E.; Maritati, M.; Spampinato, M.D.; Contini, C.; De Giorgio, R. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023, 12, 3188. [Google Scholar]
- Meyer, N.J.; Prescott, H.C. Sepsis and Septic Shock. N. Engl. J. Med. 2024, 391, 2133–2146. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar]
- Schouten, M.; Wiersinga, W.J.; Levi, M.; van der Poll, T. Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 2008, 83, 536–545. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar]
- van der Poll, T.; Meijers, J.C. Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis. J. Innate Immun. 2010, 2, 379–380. [Google Scholar]
- Torres, L.K.; Pickkers, P.; van der Poll, T. Sepsis-Induced Immunosuppression. Annu. Rev. Physiol. 2022, 84, 157–181. [Google Scholar]
- Anas, A.A.; Wiersinga, W.J.; de Vos, A.F.; van der Poll, T. Recent insights into the pathogenesis of bacterial sepsis. Neth. J. Med. 2010, 68, 147–152. [Google Scholar]
- Harm, S.; Schildböck, C.; Hartmann, J. Cytokine Removal in Extracorporeal Blood Purification: An in vitro Study. Blood Purif. 2020, 49, 33–43. [Google Scholar]
- Moriyama, K.; Nishida, O. Targeting Cytokines, Pathogen-Associated Molecular Patterns, and Damage-Associated Molecular Patterns in Sepsis via Blood Purification. Int. J. Mol. Sci. 2021, 22, 8882. [Google Scholar]
- Hellman, T.; Uusalo, P.; Järvisalo, M.J. Renal Replacement Techniques in Septic Shock. Int. J. Mol. Sci. 2021, 22, 10238. [Google Scholar]
- Monard, C.; Abraham, P.; Schneider, A.; Rimmelé, T. New Targets for Extracorporeal Blood Purification Therapies in Sepsis. Blood Purif. 2023, 52, 1–7. [Google Scholar]
- Ramasco, F.; Nieves-Alonso, J.; García-Villabona, E.; Vallejo, C.; Kattan, E.; Méndez, R. Challenges in Septic Shock: From New Hemodynamics to Blood Purification Therapies. J. Pers. Med. 2024, 14, 176. [Google Scholar]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Septic Hyperinflammation-Is There a Role for Extracorporeal Blood Purification Techniques? Int. J. Mol. Sci. 2024, 25, 3120. [Google Scholar]
- Becker, S.; Lang, H.; Vollmer Barbosa, C.; Tian, Z.; Melk, A.; Schmidt, B.M.W. Efficacy of CytoSorb®®: A systematic review and meta-analysis. Crit. Care 2023, 27, 215. [Google Scholar]
- Shimizu, T.; Miyake, T.; Kitamura, N.; Tani, M.; Endo, Y. Endotoxin adsorption: Direct hemoperfusion with the polymyxin B-immobilized fiber column (PMX). Transfus. Apher. Sci. 2017, 56, 682–688. [Google Scholar]
- Cecchi, M.; Ulsamer, A.; Villa, G. Oxiris Membrane in Sepsis and Multiple Organ Failure. Contrib. Nephrol. 2023, 200, 55–65. [Google Scholar]
- Li, Y.; Han, M.; Yang, M.; Su, B. Hemoperfusion with the HA330/HA380 Cartridge in Intensive Care Settings: A State-Of-The-Art Review. Blood Purif. 2025, 54, 122–137. [Google Scholar]
- Seffer, M.T.; Kielstein, J.T. Extracorporeal removal of pathogens using a biomimetic adsorber-A new treatment strategy for the intensive care unit: Seraph® 100 Microbind® Affinity Blood Filter and its fields of application. Med. Klin. Intensivmed. Notfallmed. 2025, 120, 290–299. [Google Scholar] [CrossRef]
- Datzmann, T.; Träger, K. Extracorporeal membrane oxygenation and cytokine adsorption. J. Thorac. Dis. 2018, 10 (Suppl. 5), S653–S660. [Google Scholar]
- Wang, G.; He, Y.; Guo, Q.; Zhao, Y.; He, J.; Chen, Y.; Chen, W.; Zhou, Y.; Peng, Z.; Deng, K.; et al. Continuous renal replacement therapy with the adsorptive oXiris filter may be associated with the lower 28-day mortality in sepsis: A systematic review and meta-analysis. Crit. Care 2023, 27, 275. [Google Scholar]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar]
- Jansen, J.; van der Poll, T.; van Deventer, S.J. Endotoxin and the release of tumor necrosis factor receptors. Prog. Clin. Biol. Res. 1994, 388, 383–397. [Google Scholar]
- van der Poll, T.; Lowry, S.F. Tumor necrosis factor in sepsis: Mediator of multiple organ failure or essential part of host defense? Shock 1995, 3, 1–12. [Google Scholar]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar]
- Levi, M.; ten Cate, H.; van der Poll, T.; van Deventer, S.J. Pathogenesis of disseminated intravascular coagulation in sepsis. JAMA 1993, 270, 975–979. [Google Scholar]
- ten Cate, J.W.; van der Poll, T.; Levi, M.; ten Cate, H.; van Deventer, S.J. Cytokines: Triggers of clinical thrombotic disease. Thromb. Haemost. 1997, 78, 415–419. [Google Scholar]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38 (Suppl. 2), S26–S34. [Google Scholar]
- Shoji, H. Extracorporeal endotoxin removal for the treatment of sepsis: Endotoxin adsorption cartridge (Toraymyxin). Ther. Apher. Dial. 2003, 7, 108–114. [Google Scholar]
- Vincent, J.L.; Laterre, P.F.; Cohen, J.; Burchardi, H.; Bruining, H.; Lerma, F.A.; Wittebole, X.; De Backer, D.; Brett, S.; Marzo, D.; et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock 2005, 23, 400–405. [Google Scholar]
- Shimizu, T.; Miyake, T.; Tani, M. History and current status of polymyxin B-immobilized fiber column for treatment of severe sepsis and septic shock. Ann. Gastroenterol. Surg. 2017, 1, 105–113. [Google Scholar]
- Krenn, C.G.; Steltzer, H. Hemoadsorption for blood purification-incomparability of clinically available procedures. Med. Klin. Intensivmed. Notfmed. 2021, 116, 449–453. [Google Scholar]
- Tani, T.; Shimizu, T.; Tani, M.; Shoji, H.; Endo, Y. Anti-endotoxin Properties of Polymyxin B-immobilized Fibers. Adv. Exp. Med. Biol. 2019, 1145, 321–341. [Google Scholar]
- Seffer, M.T.; Cottam, D.; Forni, L.G.; Kielstein, J.T. Heparin 2.0: A New Approach to the Infection Crisis. Blood Purif. 2021, 50, 28–34. [Google Scholar]
- Stewart, I.J.; McCrea, K.; Chawla, L.; Chung, K.K. Adsorption of Pathogens and Blockade of Sepsis Cascade. Contrib. Nephrol. 2023, 200, 123–132. [Google Scholar]
- Seeliger, B.; Stahl, K.; David, S. Extracorporeal techniques for blood purification in sepsis: An update. Internist 2020, 61, 1010–1016. [Google Scholar]
- Chen, G.; Zhou, Y.; Ma, J.; Xia, P.; Qin, Y.; Li, X. Is there a role for blood purification therapies targeting cytokine storm syndrome in critically severe COVID-19 patients? Ren. Fail. 2020, 42, 483–488. [Google Scholar]
- Mehta, Y.; Dixit, S.B.; Zirpe, K.; Sud, R.; Gopal, P.B.; Koul, P.A.; Mishra, V.K.; Ansari, A.S.; Chamle, V.S. Therapeutic Approaches in Modulating the Inflammatory and Immunological Response in Patients With Sepsis, Acute Respiratory Distress Syndrome, and Pancreatitis: An Expert Opinion Review. Cureus 2021, 13, e18393. [Google Scholar]
- Köhler, T.; Schwier, E.; Praxenthaler, J.; Kirchner, C.; Henzler, D.; Eickmeyer, C. Therapeutic Modulation of the Host Defense by Hemoadsorption with CytoSorb®®-Basics, Indications and Perspectives-A Scoping Review. Int. J. Mol. Sci. 2021, 22, 12786. [Google Scholar]
- Klinkmann, G.; Koball, S.; Reuter, D.A.; Mitzner, S. Hemoperfusion with CytoSorb®®: Current Knowledge on Patient Selection, Timing, and Dosing. Contrib. Nephrol. 2023, 200, 17–24. [Google Scholar] [PubMed]
- Gräfe, C.; Weidhase, L.; Liebchen, U.; Weigand, M.A.; Scharf, C. Hemoperfusion in anesthesia and intensive care medicine: Benefits, risks, and evidence for different systems. Anaesthesiologie 2023, 72, 843–851. [Google Scholar] [PubMed]
- Li, Y.; Sun, P.; Chang, K.; Yang, M.; Deng, N.; Chen, S.; Su, B. Effect of Continuous Renal Replacement Therapy with the oXiris Hemofilter on Critically Ill Patients: A Narrative Review. J. Clin. Med. 2022, 11, 6719. [Google Scholar] [PubMed]
- Bellomo, R.; Ankawi, G.; Bagshaw, S.M.; Baldwin, I.; Basu, R.; Bottari, G.; Cantaluppi, V.; Clark, W.; De Rosa, S.; Forni, L.G.; et al. Hemoadsorption: Consensus report of the 30th Acute Disease Quality Initiative workgroup. Nephrol. Dial. Transplant. 2024, 39, 1945–1964. [Google Scholar]
- Diab, M.; Lehmann, T.; Bothe, W.; Akhyari, P.; Platzer, S.; Wendt, D.; Deppe, A.C.; Strauch, J.; Hagel, S.; Günther, A.; et al. Cytokine Hemoadsorption During Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results From a Multicenter Randomized Controlled Trial. Circulation 2022, 145, 959–968. [Google Scholar]
- Mehta, Y.; Paul, R.; Ansari, A.S.; Banerjee, T.; Gunaydin, S.; Nassiri, A.A.; Pappalardo, F.; Premužić, V.; Sathe, P.; Singh, V.; et al. Extracorporeal blood purification strategies in sepsis and septic shock: An insight into recent advancements. World J. Crit. Care Med. 2023, 12, 71–88. [Google Scholar]
- Ruiz-Rodríguez, J.C.; Plata-Menchaca, E.P.; Chiscano-Camón, L.; Ruiz-Sanmartin, A.; Ferrer, R. Blood purification in sepsis and COVID-19: What’s new in cytokine and endotoxin hemoadsorption. J. Anesth. Analg. Crit. Care 2022, 2, 15. [Google Scholar]
- Matson, J.; Lange, P.; Honore, P.M.; Chung, K.K. Adverse outcomes with extracorporeal adsorbent blood treatments in toxic systemic inflammation: A perspective on possible mechanisms. Ann. Intensive Care 2022, 12, 105. [Google Scholar]
- Stahl, K.; Bode, C.; David, S. Extracorporeal Strategies in Sepsis Treatment: Role of Therapeutic Plasma Exchange. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2021, 56, 101–110. [Google Scholar]
- Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar]
- Mariano, F.; Greco’, D.; Depetris, N.; Mella, A.; Sciarrillo, A.; Stella, M.; Berardino, M.; Risso, D.; Gambino, R.; Biancone, L. CytoSorb®® in burn patients with septic shock and Acute Kidney Injury on Continuous Kidney Replacement Therapy is associated with improved clinical outcome and survival. Burns 2024, 50, 1213–1222. [Google Scholar] [PubMed]
- Kogelmann, K.; Hübner, T.; Drüner, M.; Jarczak, D. Impact of CytoSorb Hemoadsorption Therapy on Fluid Balance in Patients with Septic Shock. J. Clin. Med. 2024, 13, 294. [Google Scholar] [PubMed]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef] [PubMed]
- Hawchar, F.; László, I.; Öveges, N.; Trásy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar]
- Kogelmann, K.; Jarczak, D.; Scheller, M.; Drüner, M. Hemoadsorption by CytoSorb in septic patients: A case series. Crit. Care 2017, 21, 74. [Google Scholar]
- Lother, A.; Benk, C.; Staudacher, D.L.; Supady, A.; Bode, C.; Wengenmayer, T.; Duerschmied, D. Cytokine Adsorption in Critically Ill Patients Requiring ECMO Support. Front. Cardiovasc. Med. 2019, 6, 71. [Google Scholar]
- Akil, A.; Ziegeler, S.; Reichelt, J.; Rehers, S.; Abdalla, O.; Semik, M.; Fischer, S. Combined Use of CytoSorb and ECMO in Patients with Severe Pneumogenic Sepsis. Thorac. Cardiovasc. Surg. 2021, 69, 246–251. [Google Scholar]
- Pausch, J.; Mersmann, J.; Bhadra, O.D.; Barten, M.J.; Alassar, Y.A.; Schulte-Uentrop, L.; Reichenspurner, H.; Bernhardt, A.M. Preliminary Experience of Extracorporeal Cytokine Hemoadsorption during Left Ventricular Assist Device Implantation in Cardiogenic Shock Patients. Thorac. Cardiovasc. Surg. 2024, 72, 266–272. [Google Scholar]
- Friesecke, S.; Stecher, S.S.; Gross, S.; Felix, S.B.; Nierhaus, A. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: A prospective single-center study. J. Artif. Organs 2017, 20, 252–259. [Google Scholar]
- Kogelmann, K.; Scheller, M.; Drüner, M.; Jarczak, D. Use of hemoadsorption in sepsis-associated ECMO-dependent severe ARDS: A case series. J. Intensive Care Soc. 2020, 21, 183–190. [Google Scholar]
- Hohn, A.; Malewicz-Oeck, N.M.; Buchwald, D.; Annecke, T.; Zahn, P.K.; Baumann, A. REmoval of cytokines during CArdiac surgery (RECCAS): A randomised controlled trial. Crit. Care 2024, 28, 406. [Google Scholar]
- Heymann, M.; Schorer, R.; Putzu, A. Mortality and adverse events of hemoadsorption with CytoSorb®® in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2022, 66, 1037–1050. [Google Scholar] [PubMed]
- Taccone, F.S.; Brunkhorst, F.M.; Bottari, G.; Hidalgo, J.; Kribben, A.; Teboul, J.L.; Tomescu, D.; Klaus, T.; Scheier, J.; Deliargyris, E.; et al. The COSMOS Registry of CytoSorb Hemoadsorption Therapy in Critically Ill Patients: Protocol for an International, Prospective Registry. JMIR Res. Protoc. 2024, 13, e55880. [Google Scholar] [PubMed]
- Supady, A.; Brodie, D.; Wengenmayer, T. Extracorporeal haemoadsorption: Does the evidence support its routine use in critical care? Lancet Respir. Med. 2022, 10, 307–312. [Google Scholar] [PubMed]
- Honore, P.M.; Hoste, E.; Molnár, Z.; Jacobs, R.; Joannes-Boyau, O.; Malbrain, M.L.N.G.; Forni, L.G. Cytokine removal in human septic shock: Where are we and where are we going? Ann. Intensive Care 2019, 9, 56. [Google Scholar]
- Zhang, H.; Xu, Y.; Huang, X.; Yang, S.; Li, R.; Wu, Y.; Zou, X.; Yu, Y.; Shang, Y. Extracorporeal membrane oxygenation in adult patients with sepsis and septic shock: Why, how, when, and for whom. J. Intensive Med. 2023, 4, 62–72. [Google Scholar]
- Saldaña-Gastulo, J.J.C.; Llamas-Barbarán, M.D.R.; Coronel-Chucos, L.G.; Hurtado-Roca, Y. Cytokine hemoadsorption with CytoSorb®® in patients with sepsis: A systematic review and meta-analysis. Crit. Care Sci. 2023, 35, 217–225. [Google Scholar]
- Rachunek, K.; Krause, M.; Thiel, J.T.; Kolbenschlag, J.; Daigeler, A.; Bury, A. Technical Note: Novel Use of CytoSorb™ Haemadsorption to Provide Wound Healing Support in Case of Severe Burn Trauma via Reduction of Hyperbilirubinaemia. Front. Surg. 2021, 8, 743571. [Google Scholar]
- Albrecht, F.; Schunk, S.; Fuchs, M.; Volk, T.; Geisel, J.; Fliser, D.; Meiser, A. Rapid and Effective Elimination of Myoglobin with CytoSorb®® Hemoadsorber in Patients with Severe Rhabdomyolysis. Blood Purif. 2024, 53, 88–95. [Google Scholar]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb®® sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar]
- Scheier, J.; Nelson, P.J.; Schneider, A.; Colombier, S.; Kindgen-Milles, D.; Deliargyris, E.N.; Nolin, T.D. Mechanistic Considerations and Pharmacokinetic Implications on Concomitant Drug Administration During CytoSorb Therapy. Crit. Care Explor. 2022, 4, e0688. [Google Scholar] [PubMed]
- Akil, A.; Napp, L.C.; Rao, C.; Klaus, T.; Scheier, J.; Pappalardo, F. Use of CytoSorb© Hemoadsorption in Patients on Veno-Venous ECMO Support for Severe Acute Respiratory Distress Syndrome: A Systematic Review. J. Clin. Med. 2022, 11, 5990. [Google Scholar] [PubMed]
- Jakopin, E.; Knehtl, M.; Hojs, N.V.; Bevc, S.; Piko, N.; Hojs, R.; Ekart, R. Treatment of acute kidney injury with continuous renal replacement therapy and cytokine adsorber (CytoSorb®®) in critically ill patients with COVID-19. Ther. Apher. Dial. 2024, 28, 941–950. [Google Scholar] [PubMed]
- Javanbakht, M.; Trevor, M.; Rezaei Hemami, M.; Rahimi, K.; Branagan-Harris, M.; Degener, F.; Adam, D.; Preissing, F.; Scheier, J.; Cook, S.F.; et al. Ticagrelor Removal by CytoSorb®® in Patients Requiring Emergent or Urgent Cardiac Surgery: A UK-Based Cost-Utility Analysis. PharmacoEcon. Open 2020, 4, 307–319. [Google Scholar]
- Malard, B.; Lambert, C.; Kellum, J.A. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med. Exp. 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Liu, C.; Mao, Z.; Qi, S.; Song, R.; Zhou, F. Effectiveness of polymyxin B-immobilized hemoperfusion against sepsis and septic shock: A systematic review and meta-analysis. J. Crit. Care 2021, 63, 187–195. [Google Scholar]
- Waalders, N.J.B.; Kox, M.; Pickkers, P. Haemoadsorption to remove inflammatory mediators in sepsis: Past, present, and future. Intensive Care Med. Exp. 2025, 13, 38. [Google Scholar]
- Monard, C.; Rimmelé, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019, 47 (Suppl. 3), 1–14. [Google Scholar]
- Feng, J.; Zhang, S.; Ai, T.; Wang, L.; Gao, Y.; Li, W.; Zhu, M. Effect of CRRT with oXiris filter on hemodynamic instability in surgical septic shock with AKI: A pilot randomized controlled trial. Int. J. Artif. Organs 2022, 45, 801–808. [Google Scholar]
- Zheng, F.; Wang, Y.L.; Zhou, W.Y.; Zhang, J.; Lu, M.; Pan, N.F.; He, J.; Zhang, Q.; Cao, L.; Wu, J.S.; et al. Continuous renal replacement therapy with adsorbing filter oXiris in the treatment of sepsis associated acute kidney injury: A single-center retrospective observational study. BMC Nephrol. 2024, 25, 456. [Google Scholar]
- Borazjani, R.; Mahmudi-Azer, S.; Taghrir, M.H.; Homaeifar, R.; Dabiri, G.; Paydar, S.; Fard, H.A. Adjunctive hemoperfusion with Resin Hemoadsorption (HA) 330 cartridges improves outcomes in patients sustaining multiple Blunt Trauma: A prospective, quasi-experimental study. BMC Surg. 2023, 23, 148. [Google Scholar]
- An, Y.; Guo, Y.; Zhou, W.; He, Q.; Li, Z.; Sui, X.; Yi, X.; Yi, H. HA380 Hemoperfusion Combined with Continuous Veno-Venous Hemodiafiltration for the Treatment of Septic Shock. Bioengineering 2025, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Smeriglio, A.; Ceresa, F.; Campo, S.; Caruso, D.; Falliti, G.; La Camera, E.; Patané, F.; Trombetta, D.; Monardo, P. Hemoperfusion with Seraph-100 in septic patients removes pathogens and improves clinical outcomes. Sci. Rep. 2025, 15, 17626. [Google Scholar]
- DeLuca, J.P.; Selig, D.J.; Vir, P.; Vuong, C.V.; Della-Volpe, J.; Rivera, I.M.; Park, C.; Levi, B.; Pratt, K.P.; Stewart, I.J. Seraph 100 Microbind Affinity Blood Filter Does Not Clear Antibiotics: An Analysis of Antibiotic Concentration Data from PURIFY-OBS. Blood Purif. 2024, 53, 379–385. [Google Scholar]
- Schmidt, J.J.; Eden, G.; Seffer, M.T.; Winkler, M.; Kielstein, J.T. In vitro elimination of anti-infective drugs by the Seraph®® 100 Microbind®® affinity blood filter. Clin. Kidney J. 2020, 13, 421–424. [Google Scholar]
- Seffer, M.T.; Eden, G.; Engelmann, S.; Kielstein, J.T. Elimination of Staphylococcus aureus from the bloodstream using a novel biomimetic sorbent haemoperfusion device. BMJ Case Rep. 2020, 13, e235262. [Google Scholar]
- Premuzic, V.; Situm, I.; Lovric, D.; Erceg, A.; Karmelic, D.; Mogus, M.; Jurjevic, M.; Nedeljkovic, V.; Mazar, M.; Mihaljevic, S.; et al. Sequential Extracorporeal Blood Purification Is Associated with Prolonged Survival among ICU Patients with COVID-19 and Confirmed Bacterial Superinfection. Blood Purif. 2023, 52, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Eden, G.; Schmidt, J.J.; Büttner, S.; Kümpers, P.; Hafer, C.; Rovas, A.; Koch, B.F.; Schmidt, B.M.W.; Kielstein, J.T. Safety and efficacy of the Seraph®® 100 Microbind®® Affinity Blood Filter to remove bacteria from the blood stream: Results of the first in human study. Crit. Care 2022, 26, 181. [Google Scholar]

| Device | Technology | Clinical Application | Regulatory Approval | Limitations/Side Effects | Recommended Treatment Duration |
|---|---|---|---|---|---|
| CytoSorb® (CytoSorbents Corps) | Hemadsorption 300 mL cartridge with porous polystyrene-divinyl-benzene polymer beads with a highly porous and biocompatible polyvinylpyrrolidone cover (~40,000 m2 surface area); adsorbs cytokines (5–60 kDa), bilirubin, myoglobin, DAMPs, PAMPs, certain drugs [67,68,69,70,71] | Septic shock, cytokine storm, ECMO, CRRT adjunct, drug intoxication [67,72,73,74]. | CE Mark (EU), FDA EUA (USA, COVID-19 only), Approved in >70 countries | Non-selective adsorption may remove beneficial molecules and drugs. Coagulation system interaction- requires anticoagulation during use. Does not remove endotoxins efficiently compared to endotoxin-specific columns [40,71,75]. | 6–24 h per cartridge; may be used repeatedly in 24–72 h cycles |
| Toraymyxin® (PMX-20R) (Toray Industries, Inc.) | Antibiotic Polymyxin B immobilized on polystyrene fibres; targets endotoxins (LPS) [17,34]. | Gram-negative sepsis with high EAA; reduces vasopressor need [76]. | CE Mark, Japan PMDA approved; not FDA approved | Specific to endotoxins; no cytokine removal; benefit debated in some RCTs [77]. | Typically 2 sessions of 2 hours each within 24 h |
| Oxiris® (Baxter) | Three-layer membrane structure: -AN69-based haemofilter: provides the primary renal support by diffusion and convection and adsorbs positively charged cytokines -Heparin grafting: reduce membrane thrombogenicity, minimize treatment interruptions, and support efficient CRRT -PEI coating: enhances hemocompatibility and enables the adsorption of negatively charged endotoxins (LPS) Combines cytokine, endotoxin removal + CRRT [22,78]. | Sepsis with AKI; integrated with CRRT circuits (CVVH, CVVHDF) [78,79,80]. | CE Mark (EU); FDA (USA, COVID-19 only) | Risk of clotting if anticoagulation inadequate; may require high blood flow rates [78]. | Typically replaced every 12-24 h during CRRT sessions |
| HA330/HA380 (Jafron Biomedical Co) | Resin-based hemadsorption (styrene-divinylbenzene copolymer); non-selective cytokine, bilirubin, tryptophan removal via hydrophobic interactions, Van der Waals forces, pore structure (wide range of pore sizes, e.g., 10 kDa to 60 kDa for HA380) [19,77]. | Sepsis, SIRS, hyperinflammatory syndromes (common in China/Asia), integrated with CVVHDF [19,81,82]. | CFDA (China); CE Mark (Europe); Not FDA approved | Non-selective; hypotension during use; limited high-quality data from Western trials [19,77]. | 2–4 h per session; 1–2.5 sessions depending on severity [77] |
| Seraph® 100 Microbind® (ExThera Medical Corporation) | Heparin-coated beads mimic endothelial glycocalyx (~40 m2 surface area); binds pathogens (bacteria, viruses, fungi) and toxins by electrostatic interaction simulating their natural binding site. Negligible clearance of most antibiotics [83,84,85]. | Bloodstream infections (e.g., S. aureus), viral sepsis (e.g., COVID-19); pathogen clearance [86,87,88]. | CE Mark, FDA EUA (USA) | Relevant initial adsorption of aminoglycosides. limited large-scale RCTs [85]. | Up to 5 h per cartridge; max once daily; duration based on pathogen load |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, M.; Costanzini, A.; Luppi, F.; Maritati, M.; Contini, C.; De Giorgio, R.; Spampinato, M.D. Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives. Biomedicines 2025, 13, 1684. https://doi.org/10.3390/biomedicines13071684
Guarino M, Costanzini A, Luppi F, Maritati M, Contini C, De Giorgio R, Spampinato MD. Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives. Biomedicines. 2025; 13(7):1684. https://doi.org/10.3390/biomedicines13071684
Chicago/Turabian StyleGuarino, Matteo, Anna Costanzini, Francesco Luppi, Martina Maritati, Carlo Contini, Roberto De Giorgio, and Michele Domenico Spampinato. 2025. "Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives" Biomedicines 13, no. 7: 1684. https://doi.org/10.3390/biomedicines13071684
APA StyleGuarino, M., Costanzini, A., Luppi, F., Maritati, M., Contini, C., De Giorgio, R., & Spampinato, M. D. (2025). Extracorporeal Cytokine Adsorption in Sepsis: Current Evidence and Future Perspectives. Biomedicines, 13(7), 1684. https://doi.org/10.3390/biomedicines13071684









