Random Insertion Reporter Gimmicks Powered by Cut-and-Paste DNA Transposons
Abstract
1. Introduction
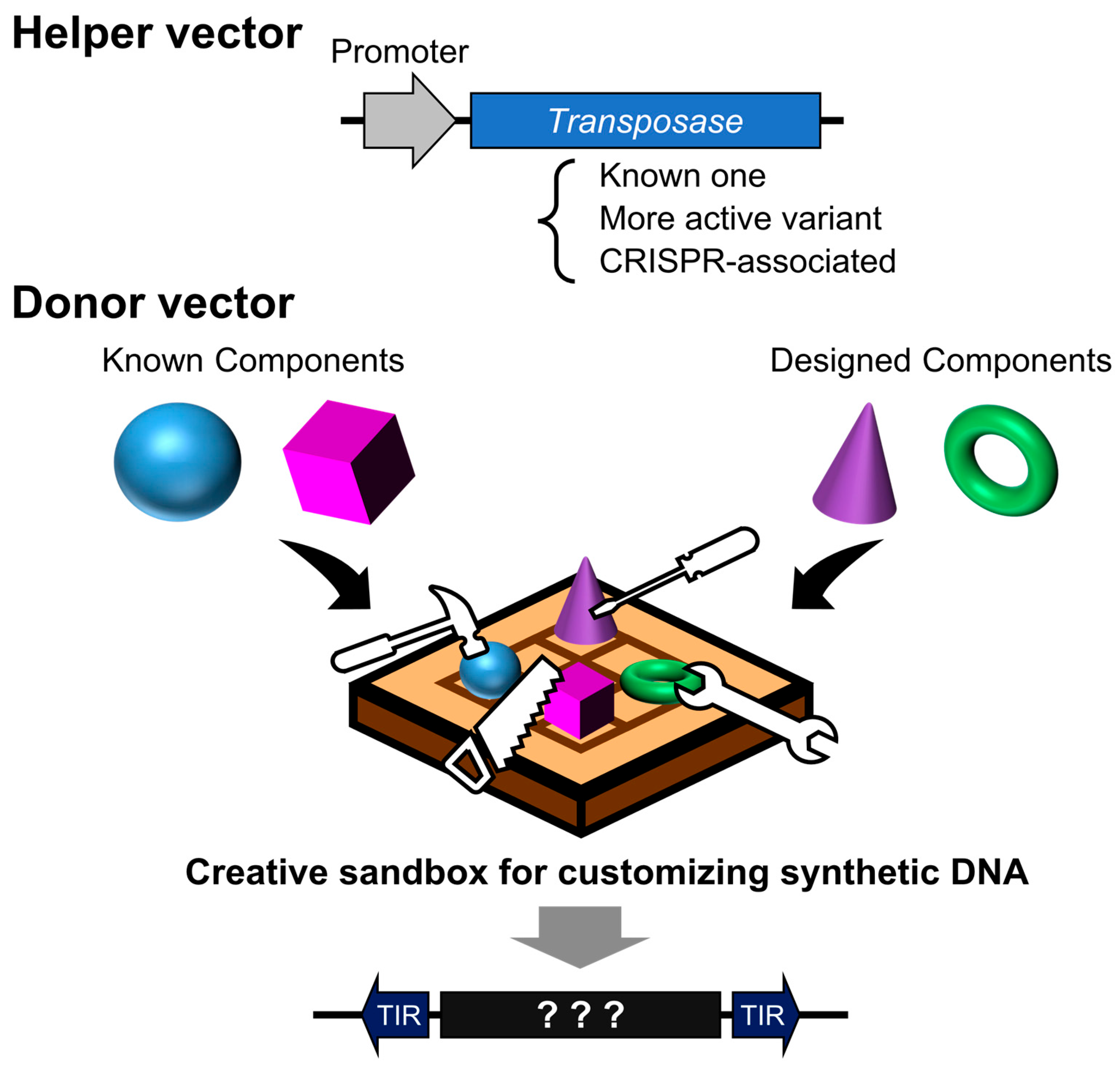
2. Cut-and-Paste DNA Transposons: Structural Overview and Applications in Research
2.1. TIR
2.2. Enzymatic Activity of Transposases Used in Research
2.3. Sequence-Specific Targeting Bias of Transposases
2.4. Improvement of Transposase
3. Randomness and Its Value
3.1. Historical Development and Impact of Transposon Randomness
3.2. Transition to Mammalian Systems
3.3. Transposon Applications in Vertebrate Species: Advances in Mammalian Mutagenesis
4. Gene Screening Utilizing Randomness: Proof-of-Concept Studies Based on Our Transposon-Mediated Approaches
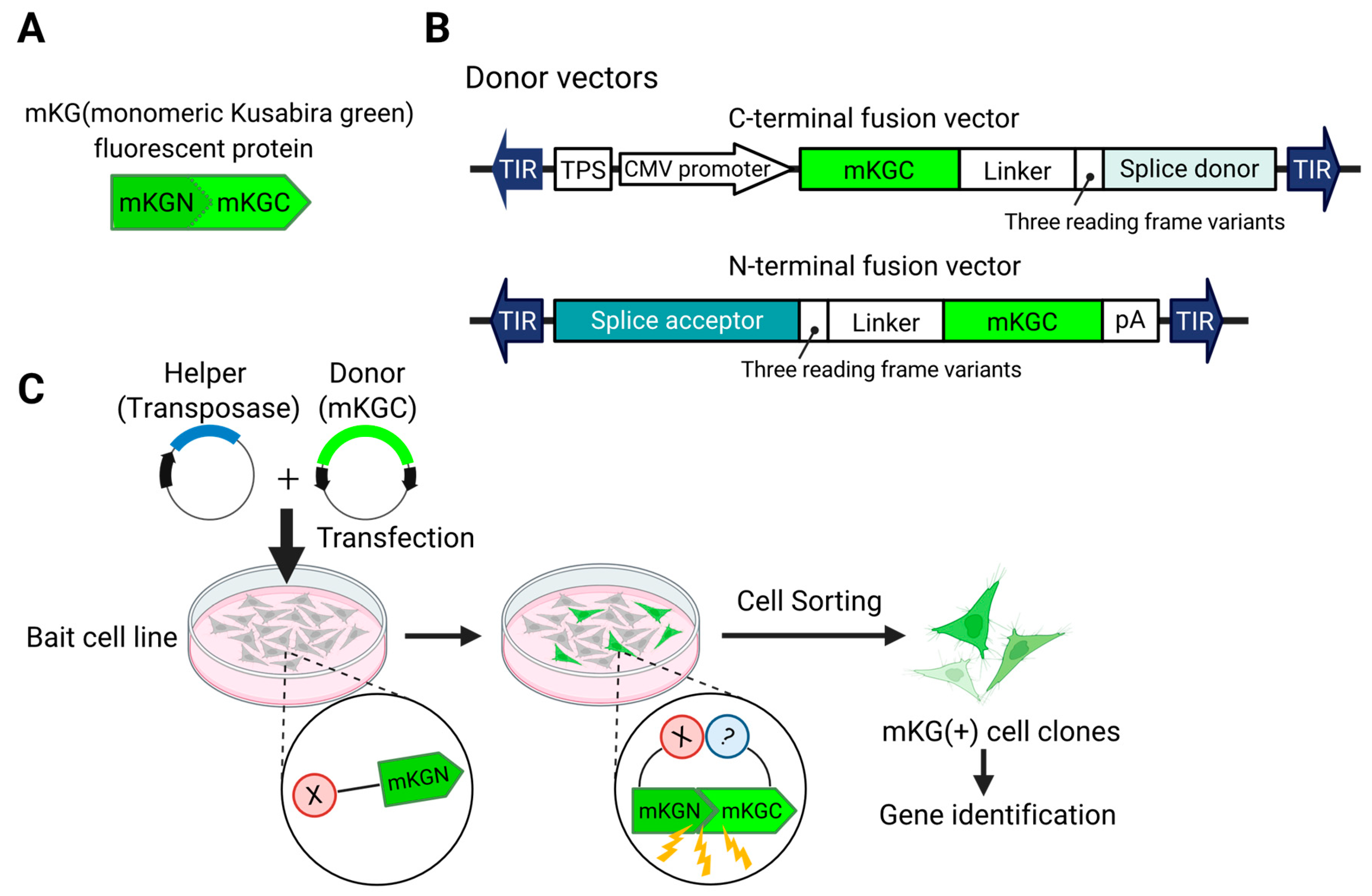
4.1. Fusion of Genes with Reporter Proteins: Applications in Protein–Protein Interaction Analysis
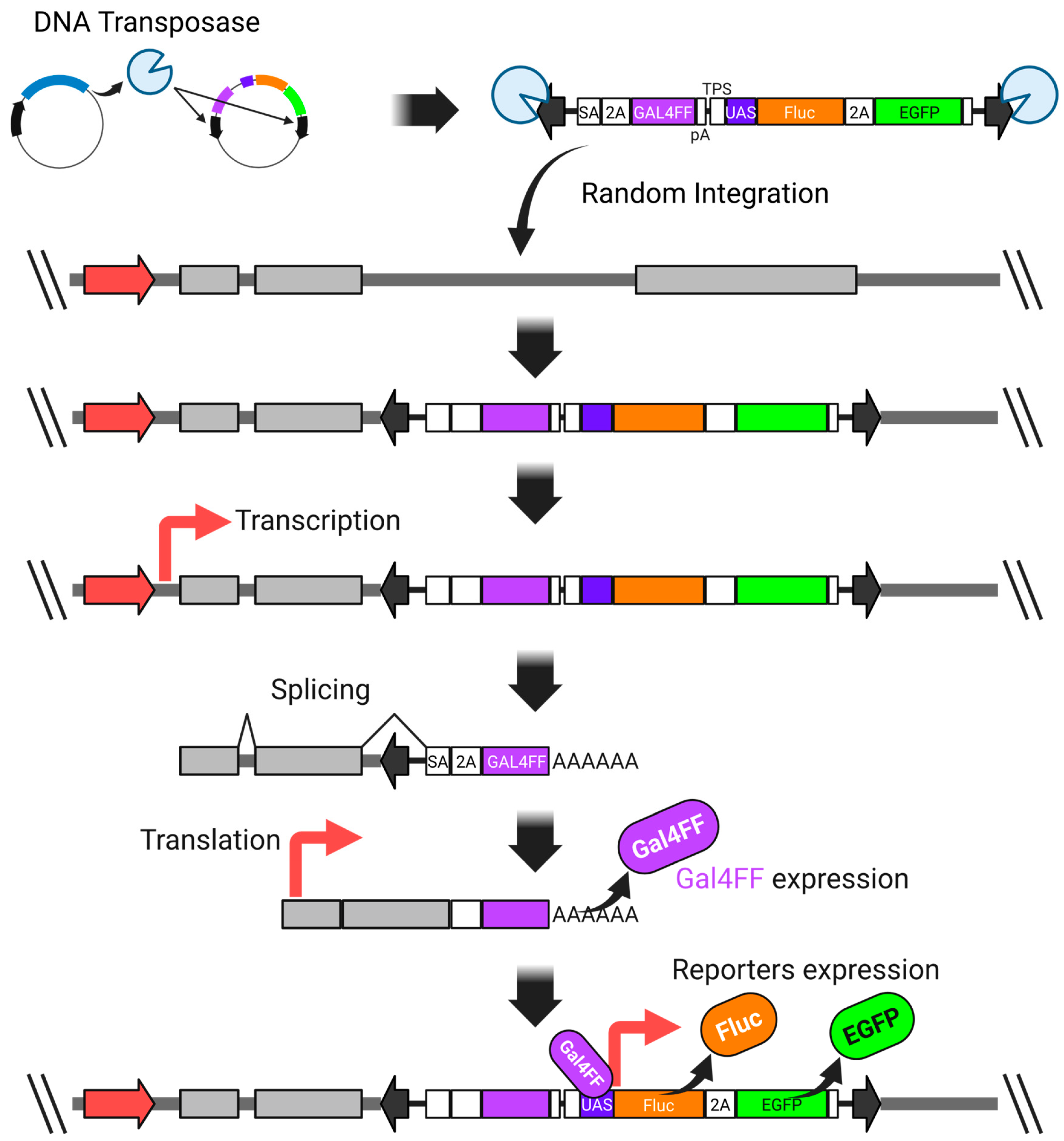
4.2. Fusion of Promoters/Enhancers with Reporter Genes
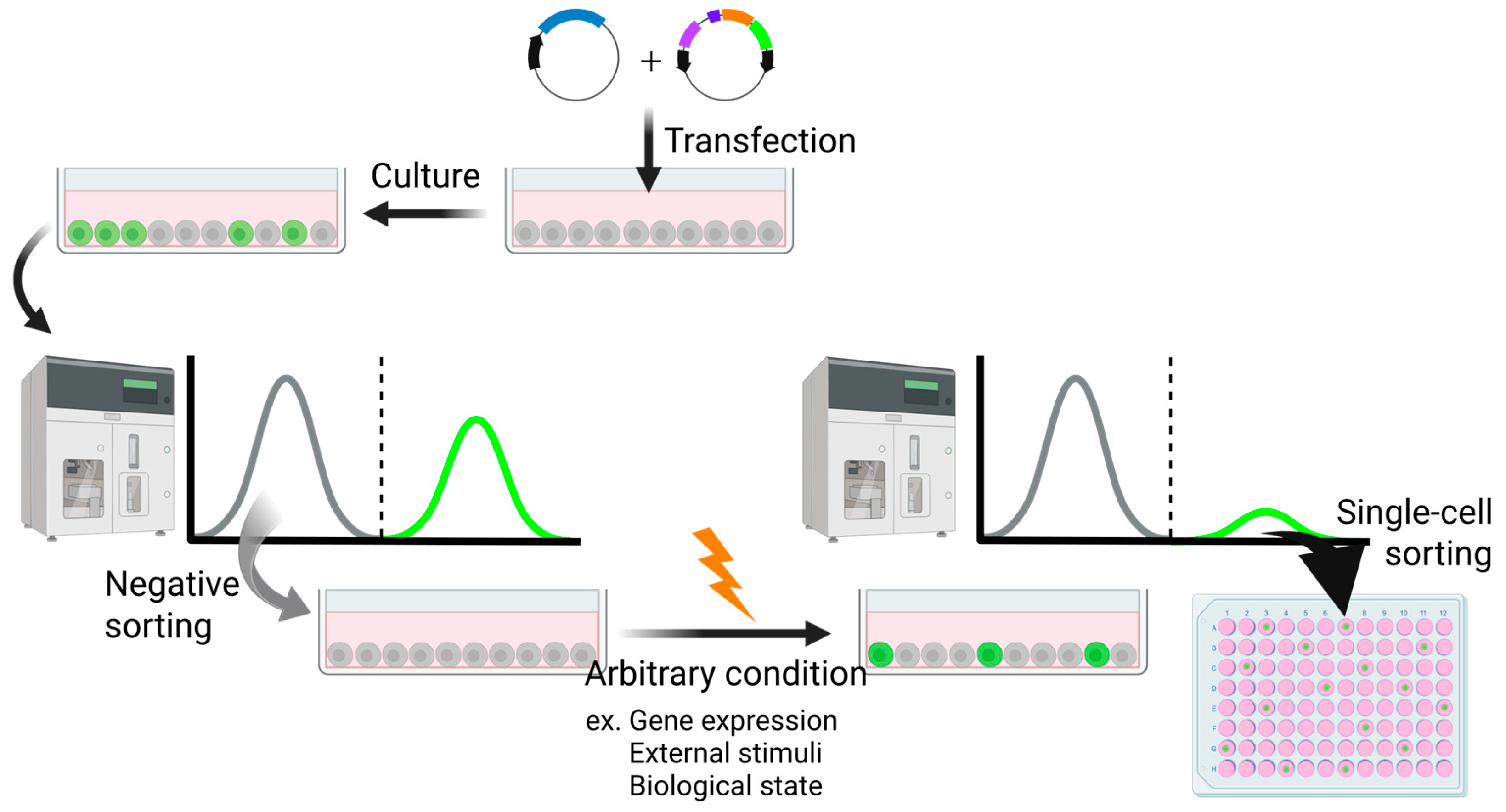
4.3. High-Sensitivity Trap Vector-Based Random Screening
4.3.1. Isolation of c-Myc-Responsive Cells
4.3.2. Isolation of Cells Responsive to ER Stresses
4.3.3. Cross-Pathway Compound Profiling Using Randomly Generated Reporter Cell Lines for Vitamins and Forskolin
4.3.4. Reporter Cells Revealing Hidden Cell Cycle Dynamics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDKRB2 | Bradykinin receptor B2 |
| BiFC | Bimolecular fluorescence complementation |
| BiP | Binding immunoglobulin protein |
| Blm | Bloom syndrome homologue |
| cAMP | Cyclic adenosine monophosphate |
| Cas | CRISPR-associated protein |
| CHO | Chinese hamster ovary |
| c-Myc | Cellular myelocytomatosis oncogene |
| CYP24A1 | Cytochrome P450 family 24 subfamily A member 1 |
| ES | Embryonic stem |
| GFP | Green fluorescent protein |
| LEDLGF | Lens epithelium-derived growth factor |
| LINC-PINT | Long intragenic non-coding RNA p53-induced transcript |
| LTR | Long terminal repeat |
| mKG | Monomeric Kusabira green |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| OSBPL9 | Oxysterol binding protein-like 9 |
| TIR | Terminal inverted repeats |
| TISPL | Transcript induced by stressors from LINC-PINT locus |
| TSKU | Tsukushi, small leucine-rich proteoglycan |
| TSS | Target site sequence |
| UAS | Upstream activating sequence |
| UTR | Untranslated region |
References
- McClintock, B. The origin and behavior of mutable loci in maize. Proc. Natl. Acad. Sci. USA 1950, 36, 344–355. [Google Scholar] [PubMed]
- Jones, R.N. McClintock’s controlling elements: The full story. Cytogenet. Genome Res. 2005, 109, 90–103. [Google Scholar] [PubMed]
- Modzelewski, A.J.; Chong, J.G.; Wang, T.; He, L. Mammalian genome innovation through transposon domestication. Nat. Cell Biol. 2022, 24, 1332–1340. [Google Scholar] [PubMed]
- Sandoval-Villegas, N.; Nurieva, W.; Amberger, M.; Ivics, Z. Contemporary Transposon Tools: A Review and Guide through Mechanisms and Applications of Sleeping Beauty, piggyBac and Tol2 for Genome Engineering. Int. J. Mol. Sci. 2021, 22, 5084. [Google Scholar]
- Balciunas, D.; Wangensteen, K.J.; Wilber, A.; Bell, J.; Geurts, A.; Sivasubbu, S.; Wang, X.; Hackett, P.B.; Largaespada, D.A.; McIvor, R.S.; et al. Harnessing a high cargo-capacity transposon for genetic applications in vertebrates. PLoS Genet. 2006, 2, e169. [Google Scholar]
- Suster, M.L.; Sumiyama, K.; Kawakami, K. Transposon-mediated BAC transgenesis in zebrafish and mice. BMC Genom. 2009, 10, 477. [Google Scholar]
- Li, M.A.; Turner, D.J.; Ning, Z.; Yusa, K.; Liang, Q.; Eckert, S.; Rad, L.; Fitzgerald, T.W.; Craig, N.L.; Bradley, A. Mobilization of giant piggyBac transposons in the mouse genome. Nucleic Acids Res. 2011, 39, e148. [Google Scholar]
- Rostovskaya, M.; Fu, J.; Obst, M.; Baer, I.; Weidlich, S.; Wang, H.; Smith, A.J.; Anastassiadis, K.; Stewart, A.F. Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res. 2012, 40, e150. [Google Scholar]
- Turchiano, G.; Latella, M.C.; Gogol-Döring, A.; Cattoglio, C.; Mavilio, F.; Izsvák, Z.; Ivics, Z.; Recchia, A. Genomic analysis of Sleeping Beauty transposon integration in human somatic cells. PLoS ONE 2014, 9, e112712. [Google Scholar]
- Tagaya, H.; Ishikawa, K.; Hosokawa, Y.; Kobayashi, S.; Ueoka, Y.; Shimada, M.; Ohashi, Y.; Mikami, H.; Yamamoto, M.; Ihara, T.; et al. A method of producing genetically manipulated mouse mammary gland. Breast Cancer Res. 2019, 21, 1. [Google Scholar]
- Darquet, A.M.; Cameron, B.; Wils, P.; Scherman, D.; Crouzet, J. A new DNA vehicle for nonviral gene delivery: Supercoiled minicircle. Gene Ther. 1997, 4, 1341–1349. [Google Scholar] [PubMed]
- Garcia-Garcia, L.; Recalde, S.; Hernandez, M.; Bezunartea, J.; Rodriguez-Madoz, J.R.; Johnen, S.; Diarra, S.; Marie, C.; Izsvák, Z.; Ivics, Z.; et al. Long-Term PEDF Release in Rat Iris and Retinal Epithelial Cells after Sleeping Beauty Transposon-Mediated Gene Delivery. Mol. Ther. Nucleic Acids 2017, 9, 1–11. [Google Scholar] [PubMed]
- Hernandez, M.; Recalde, S.; Garcia-Garcia, L.; Bezunartea, J.; Miskey, C.; Johnen, S.; Diarra, S.; Sebe, A.; Rodriguez-Madoz, J.R.; Pouillot, S.; et al. Preclinical Evaluation of a Cell-Based Gene Therapy Using the Sleeping Beauty Transposon System in Choroidal Neovascularization. Mol. Ther. Methods Clin. Dev. 2019, 15, 403–417. [Google Scholar] [PubMed]
- Walters, A.A.; Kinnear, E.; Shattock, R.J.; McDonald, J.U.; Caproni, L.J.; Porter, N.; Tregoning, J.S. Comparative analysis of enzymatically produced novel linear DNA constructs with plasmids for use as DNA vaccines. Gene Ther. 2014, 21, 645–652. [Google Scholar]
- Bishop, D.C.; Caproni, L.; Gowrishankar, K.; Legiewicz, M.; Karbowniczek, K.; Tite, J.; Gottlieb, D.J.; Micklethwaite, K.P. CAR T Cell Generation by piggyBac Transposition from Linear Doggybone DNA Vectors Requires Transposon DNA-Flanking Regions. Mol. Ther. Methods Clin. Dev. 2020, 17, 359–368. [Google Scholar]
- Lamers, C.H.; Willemsen, R.; van Elzakker, P.; van Steenbergen-Langeveld, S.; Broertjes, M.; Oosterwijk-Wakka, J.; Oosterwijk, E.; Sleijfer, S.; Debets, R.; Gratama, J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 2011, 117, 72–82. [Google Scholar]
- Kawakami, K.; Shima, A. Identification of the Tol2 transposase of the medaka fish Oryzias latipes that catalyzes excision of a nonautonomous Tol2 element in zebrafish Danio rerio. Gene 1999, 240, 239–244. [Google Scholar]
- Wilber, A.; Frandsen, J.L.; Geurts, J.L.; Largaespada, D.A.; Hackett, P.B.; McIvor, R.S. RNA as a source of transposase for Sleeping Beauty-mediated gene insertion and expression in somatic cells and tissues. Mol. Ther. 2006, 13, 625–630. [Google Scholar]
- Wilber, A.; Wangensteen, K.J.; Chen, Y.; Zhuo, L.; Frandsen, J.L.; Bell, J.B.; Chen, Z.J.; Ekker, S.C.; McIvor, R.S.; Wang, X. Messenger RNA as a source of transposase for sleeping beauty transposon-mediated correction of hereditary tyrosinemia type I. Mol. Ther. 2007, 15, 1280–1287. [Google Scholar]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar]
- Kormann, M.S.; Hasenpusch, G.; Aneja, M.K.; Nica, G.; Flemmer, A.W.; Herber-Jonat, S.; Huppmann, M.; Mays, L.E.; Illenyi, M.; Schams, A.; et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011, 29, 154–157. [Google Scholar]
- Galla, M.; Schambach, A.; Falk, C.S.; Maetzig, T.; Kuehle, J.; Lange, K.; Zychlinski, D.; Heinz, N.; Brugman, M.H.; Göhring, G.; et al. Avoiding cytotoxicity of transposases by dose-controlled mRNA delivery. Nucleic Acids Res. 2011, 39, 7147–7160. [Google Scholar] [PubMed]
- Holstein, M.; Mesa-Nuñez, C.; Miskey, C.; Almarza, E.; Poletti, V.; Schmeer, M.; Grueso, E.; Ordóñez Flores, J.C.; Kobelt, D.; Walther, W.; et al. Efficient Non-viral Gene Delivery into Human Hematopoietic Stem Cells by Minicircle Sleeping Beauty Transposon Vectors. Mol. Ther. 2018, 26, 1137–1153. [Google Scholar] [PubMed]
- Kebriaei, P.; Singh, H.; Huls, M.H.; Figliola, M.J.; Bassett, R.; Olivares, S.; Jena, B.; Dawson, M.J.; Kumaresan, P.R.; Su, S.; et al. Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J. Clin. Investig. 2016, 126, 3363–3376. [Google Scholar] [PubMed]
- Hudecek, M.; Izsvak, Z.; Johnen, S.; Renner, M.; Thumann, G.; Ivics, Z. Going non-viral: The Sleeping Beauty transposon system breaks on through to the clinical side. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 355–380. [Google Scholar]
- Hodge, R.; Narayanavari, S.A.; Izsvák, Z.; Ivics, Z. Wide Awake and Ready to Move: 20 Years of Non-Viral Therapeutic Genome Engineering with the Sleeping Beauty Transposon System. Hum. Gene Ther. 2017, 28, 842–855. [Google Scholar]
- Tipanee, J.; Chai, Y.C.; VandenDriessche, T.; Chuah, M.K. Preclinical and clinical advances in transposon-based gene therapy. Biosci. Rep. 2017, 37, BSR20160614. [Google Scholar]
- Monjezi, R.; Miskey, C.; Gogishvili, T.; Schleef, M.; Schmeer, M.; Einsele, H.; Ivics, Z.; Hudecek, M. Enhanced CAR T-cell engineering using non-viral Sleeping Beauty transposition from minicircle vectors. Leukemia 2017, 31, 186–194. [Google Scholar]
- Morero, N.R.; Zuliani, C.; Kumar, B.; Bebel, A.; Okamoto, S.; Guynet, C.; Hickman, A.B.; Chandler, M.; Dyda, F.; Barabas, O. Targeting IS608 transposon integration to highly specific sequences by structure-based transposon engineering. Nucleic Acids Res. 2018, 46, 4152–4163. [Google Scholar]
- Klompe, S.E.; Vo, P.L.H.; Halpin-Healy, T.S.; Sternberg, S.H. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature 2019, 571, 219–225. [Google Scholar]
- Strecker, J.; Ladha, A.; Gardner, Z.; Schmid-Burgk, J.L.; Makarova, K.S.; Koonin, E.V.; Zhang, F. RNA-guided DNA insertion with CRISPR-associated transposases. Science 2019, 365, 48–53. [Google Scholar] [PubMed]
- Saito, M.; Xu, P.; Faure, G.; Maguire, S.; Kannan, S.; Altae-Tran, H.; Vo, S.; Desimone, A.; Macrae, R.K.; Zhang, F. Fanzor is a eukaryotic programmable RNA-guided endonuclease. Nature 2023, 620, 660–668. [Google Scholar] [PubMed]
- Liu, P.; Panda, K.; Edwards, S.A.; Swanson, R.; Yi, H.; Pandesha, P.; Hung, Y.H.; Klaas, G.; Ye, X.; Collins, M.V.; et al. Transposase-assisted target-site integration for efficient plant genome engineering. Nature 2024, 631, 593–600. [Google Scholar]
- Mikkelsen, J.G.; Yant, S.R.; Meuse, L.; Huang, Z.; Xu, H.; Kay, M.A. Helper-Independent Sleeping Beauty transposon-transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo. Mol. Ther. 2003, 8, 654–665. [Google Scholar] [PubMed]
- Mátés, L.; Izsvák, Z.; Ivics, Z. Technology transfer from worms and flies to vertebrates: Transposition-based genome manipulations and their future perspectives. Genome Biol. 2007, 1 (Suppl. S8), S1. [Google Scholar]
- Ivics, Z.; Li, M.A.; Mates, L.; Boeke, J.D.; Nagy, A.; Bradley, A.; Izsvak, Z. Transposon-mediated genome manipulation in vertebrates. Nat. Methods 2009, 6, 415–422. [Google Scholar]
- Muñoz-López, M.; García-Pérez, J.L. DNA transposons: Nature and applications in genomics. Curr. Genom. 2010, 11, 115–128. [Google Scholar]
- Yusa, K. piggyBac Transposon. Microbiol. Spectr. 2015, 3, MDNA3-0028-2014. [Google Scholar]
- Sato, M.; Inada, E.; Saitoh, I.; Watanabe, S.; Nakamura, S. piggyBac-Based Non-Viral In Vivo Gene Delivery Useful for Production of Genetically Modified Animals and Organs. Pharmaceutics 2020, 12, 277. [Google Scholar]
- Zhang, T.; Tan, S.; Tang, N.; Li, Y.; Zhang, C.; Sun, J.; Guo, Y.; Gao, H.; Cai, Y.; Sun, W.; et al. Heterologous survey of 130 DNA transposons in human cells highlights their functional divergence and expands the genome engineering toolbox. Cell 2024, 187, 3741–3760.e30. [Google Scholar]
- Yang, G.; Zhang, F.; Hancock, C.N.; Wessler, S.R. Transposition of the rice miniature inverted repeat transposable element mPing in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 10962–10967. [Google Scholar] [PubMed]
- Hancock, C.N.; Zhang, F.; Wessler, S.R. Transposition of the Tourist-MITE mPing in yeast: An assay that retains key features of catalysis by the class 2 PIF/Harbinger superfamily. Mob. DNA 2010, 1, 5. [Google Scholar] [PubMed]
- Huang, X.; Haley, K.; Wong, M.; Guo, H.; Lu, C.; Wilber, A.; Zhou, X. Unexpectedly high copy number of random integration but low frequency of persistent expression of the Sleeping Beauty transposase after trans delivery in primary human T cells. Hum. Gene Ther. 2010, 21, 1577–1590. [Google Scholar] [PubMed]
- Fraser, M.J.; Ciszczon, T.; Elick, T.; Bauser, C. Precise excision of TTAA-specific lepidopteran transposons piggyBac (IFP2) and tagalong (TFP3) from the baculovirus genome in cell lines from two species of Lepidoptera. Insect Mol. Biol. 1996, 5, 141–151. [Google Scholar]
- Vigdal, T.J.; Kaufman, C.D.; Izsvak, Z.; Voytas, D.F.; Ivics, Z. Common physical properties of DNA affecting target site selection of sleeping beauty and other Tc1/mariner transposable elements. J. Mol. Biol. 2002, 323, 441–452. [Google Scholar]
- Kondrychyn, I.; Garcia-Lecea, M.; Emelyanov, A.; Parinov, S.; Korzh, V. Genome-wide analysis of Tol2 transposon reintegration in zebrafish. BMC Genom. 2009, 10, 418. [Google Scholar]
- Wilson, M.H.; Coates, C.J.; George, A.L., Jr. PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 2007, 15, 139–145. [Google Scholar]
- Galvan, D.L.; Nakazawa, Y.; Kaja, A.; Kettlun, C.; Cooper, L.J.; Rooney, C.M.; Wilson, M.H. Genome-wide mapping of PiggyBac transposon integrations in primary human T cells. J. Immunother. 2009, 32, 837–844. [Google Scholar]
- Ikeda, R.; Kokubu, C.; Yusa, K.; Keng, V.W.; Horie, K.; Takeda, J. Sleeping beauty transposase has an affinity for heterochromatin conformation. Mol. Cell. Biol. 2007, 27, 1665–1676. [Google Scholar]
- Liang, Q.; Kong, J.; Stalker, J.; Bradley, A. Chromosomal mobilization and reintegration of Sleeping Beauty and PiggyBac transposons. Genesis 2009, 47, 404–408. [Google Scholar]
- Huang, X.; Guo, H.; Tammana, S.; Jung, Y.C.; Mellgren, E.; Bassi, P.; Cao, Q.; Tu, Z.J.; Kim, Y.C.; Ekker, S.C.; et al. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2, and piggyBac transposons in human primary T cells. Mol. Ther. 2010, 18, 1803–1813. [Google Scholar] [PubMed]
- Grabundzija, I.; Irgang, M.; Mates, L.; Belay, E.; Matrai, J.; Gogol-Doring, A.; Kawakami, K.; Chen, W.; Ruiz, P.; Chuah, M.K.; et al. Comparative analysis of transposable element vector systems in human cells. Mol. Ther. 2010, 18, 1200–1209. [Google Scholar] [PubMed]
- Meir, Y.J.; Weirauch, M.T.; Yang, H.S.; Chung, P.C.; Yu, R.K.; Wu, S.C. Genome-wide target profiling of piggyBac and Tol2 in HEK 293: Pros and cons for gene discovery and gene therapy. BMC Biotechnol. 2011, 11, 28. [Google Scholar]
- Gogol-Doring, A.; Ammar, I.; Gupta, S.; Bunse, M.; Miskey, C.; Chen, W.; Uckert, W.; Schulz, T.F.; Izsvak, Z.; Ivics, Z. Genome-wide Profiling Reveals Remarkable Parallels Between Insertion Site Selection Properties of the MLV Retrovirus and the piggyBac Transposon in Primary Human CD4(+) T Cells. Mol. Ther. 2016, 24, 592–606. [Google Scholar]
- Yoshida, J.; Akagi, K.; Misawa, R.; Kokubu, C.; Takeda, J.; Horie, K. Chromatin states shape insertion profiles of the piggyBac, Tol2 and Sleeping Beauty transposons and murine leukemia virus. Sci. Rep. 2017, 7, 43613. [Google Scholar]
- Elling, U.; Wimmer, R.A.; Leibbrandt, A.; Burkard, T.; Michlits, G.; Leopoldi, A.; Micheler, T.; Abdeen, D.; Zhuk, S.; Aspalter, I.M.; et al. A reversible haploid mouse embryonic stem cell biobank resource for functional genomics. Nature 2017, 550, 114–118. [Google Scholar]
- Weidhaas, J.B.; Angelichio, E.L.; Fenner, S.; Coffin, J.M. Relationship between retroviral DNA integration and gene expression. J. Virol. 2000, 74, 8382–8389. [Google Scholar]
- Schröder, A.R.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002, 110, 521–529. [Google Scholar]
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003, 300, 1749–1751. [Google Scholar]
- Narezkina, A.; Taganov, K.D.; Litwin, S.; Stoyanova, R.; Hayashi, J.; Seeger, C.; Skalka, A.M.; Katz, R.A. Genome-wide analyses of avian sarcoma virus integration sites. J. Virol. 2004, 78, 11656–11663. [Google Scholar]
- Mitchell, R.S.; Beitzel, B.F.; Schroder, A.R.; Shinn, P.; Chen, H.; Berry, C.C.; Ecker, J.R.; Bushman, F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004, 2, E234. [Google Scholar]
- Maertens, G.; Cherepanov, P.; Pluymers, W.; Busschots, K.; De Clercq, E.; Debyser, Z.; Engelborghs, Y. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J. Biol. Chem. 2003, 278, 33528–33539. [Google Scholar] [PubMed]
- Yant, S.R.; Wu, X.; Huang, Y.; Garrison, B.; Burgess, S.M.; Kay, M.A. High-resolution genome-wide mapping of transposon integration in mammals. Mol. Cell. Biol. 2005, 25, 2085–2094. [Google Scholar] [PubMed]
- Derse, D.; Crise, B.; Li, Y.; Princler, G.; Lum, N.; Stewart, C.; McGrath, C.F.; Hughes, S.H.; Munroe, D.J.; Wu, X. Human T-cell leukemia virus type 1 integration target sites in the human genome: Comparison with those of other retroviruses. J. Virol. 2007, 81, 6731–6741. [Google Scholar]
- Walisko, O.; Schorn, A.; Rolfs, F.; Devaraj, A.; Miskey, C.; Izsvák, Z.; Ivics, Z. Transcriptional activities of the Sleeping Beauty transposon and shielding its genetic cargo with insulators. Mol. Ther. 2008, 16, 359–369. [Google Scholar]
- Wei, M.; Mi, C.L.; Jing, C.Q.; Wang, T.Y. Progress of Transposon Vector System for Production of Recombinant Therapeutic Proteins in Mammalian Cells. Front. Bioeng. Biotechnol. 2022, 10, 879222. [Google Scholar]
- Matasci, M.; Bachmann, V.; Baldi, L.; Hacker, D.L.; De Jesus, M.; Wurm, F.M. CHO cell lines generated by PiggyBac transposition. BMC Proc. 2011, 8 (Suppl. S5), P31. [Google Scholar]
- Balasubramanian, S.; Matasci, M.; Kadlecova, Z.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Rapid recombinant protein production from piggyBac transposon-mediated stable CHO cell pools. J. Biotechnol. 2015, 200, 61–69. [Google Scholar]
- Balasubramanian, S.; Rajendra, Y.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Comparison of three transposons for the generation of highly productive recombinant CHO cell pools and cell lines. Biotechnol. Bioeng. 2016, 113, 1234–1243. [Google Scholar]
- Yusa, K.; Zhou, L.; Li, M.A.; Bradley, A.; Craig, N.L. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl. Acad. Sci. USA 2011, 108, 1531–1536. [Google Scholar]
- Burnight, E.R.; Staber, J.M.; Korsakov, P.; Li, X.; Brett, B.T.; Scheetz, T.E.; Craig, N.L.; McCray, P.B., Jr. A Hyperactive Transposase Promotes Persistent Gene Transfer of a piggyBac DNA Transposon. Mol. Ther. Nucleic Acids 2012, 1, e50. [Google Scholar] [PubMed]
- Wen, W.; Song, S.; Han, Y.; Chen, H.; Liu, X.; Qian, Q. An efficient Screening System in Yeast to Select a Hyperactive piggyBac Transposase for Mammalian Applications. Int. J. Mol. Sci. 2020, 21, 3064. [Google Scholar] [PubMed]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvak, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997, 91, 501–510. [Google Scholar] [PubMed]
- Izsvák, Z.; Ivics, Z.; Plasterk, R.H. Sleeping Beauty, a wide host-range transposon vector for genetic transformation in vertebrates. J. Mol. Biol. 2000, 302, 93–102. [Google Scholar]
- Geurts, A.M.; Yang, Y.; Clark, K.J.; Liu, G.; Cui, Z.; Dupuy, A.J.; Bell, J.B.; Largaespada, D.A.; Hackett, P.B. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol. Ther. 2003, 8, 108–117. [Google Scholar]
- Zayed, H.; Izsvak, Z.; Walisko, O.; Ivics, Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol. Ther. 2004, 9, 292–304. [Google Scholar]
- Mates, L.; Chuah, M.K.; Belay, E.; Jerchow, B.; Manoj, N.; Acosta-Sanchez, A.; Grzela, D.P.; Schmitt, A.; Becker, K.; Matrai, J.; et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 2009, 41, 753–761. [Google Scholar]
- Voigt, F.; Wiedemann, L.; Zuliani, C.; Querques, I.; Sebe, A.; Mátés, L.; Izsvák, Z.; Ivics, Z.; Barabas, O. Sleeping Beauty transposase structure allows rational design of hyperactive variants for genetic engineering. Nat. Commun. 2016, 7, 11126. [Google Scholar]
- Querques, I.; Mades, A.; Zuliani, C.; Miskey, C.; Alb, M.; Grueso, E.; Machwirth, M.; Rausch, T.; Einsele, H.; Ivics, Z.; et al. A highly soluble Sleeping Beauty transposase improves control of gene insertion. Nat. Biotechnol. 2019, 37, 1502–1512. [Google Scholar]
- Wang, S.; Gao, B.; Miskey, C.; Guan, Z.; Sang, Y.; Chen, C.; Wang, X.; Ivics, Z.; Song, C. Passer, a highly active transposon from a fish genome, as a potential new robust genetic manipulation tool. Nucleic Acids Res. 2023, 51, 1843–1858. [Google Scholar]
- Ding, S.; Wu, X.; Li, G.; Han, M.; Zhuang, Y.; Xu, T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 2005, 122, 473–483. [Google Scholar]
- Wang, W.; Lin, C.; Lu, D.; Ning, Z.; Cox, T.; Melvin, D.; Wang, X.; Bradley, A.; Liu, P. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9290–9295. [Google Scholar] [PubMed]
- O’Kane, C.J.; Gehring, W.J. Detection in situ of genomic regulatory elements in Drosophila. Proc. Natl. Acad. Sci. USA 1987, 84, 9123–9127. [Google Scholar] [PubMed]
- Bier, E.; Vaessin, H.; Shepherd, S.; Lee, K.; McCall, K.; Barbel, S.; Ackerman, L.; Carretto, R.; Uemura, T.; Grell, E.; et al. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989, 3, 1273–1287. [Google Scholar] [PubMed]
- Bellen, H.J.; O’Kane, C.J.; Wilson, C.; Grossniklaus, U.; Pearson, R.K.; Gehring, W.J. P-element-mediated enhancer detection: A versatile method to study development in Drosophila. Genes Dev. 1989, 3, 1288–1300. [Google Scholar]
- Wilson, C.; Pearson, R.K.; Bellen, H.J.; O’Kane, C.J.; Grossniklaus, U.; Gehring, W.J. P-element-mediated enhancer detection: An efficient method for isolating and characterizing developmentally regulated genes in Drosophila. Genes Dev. 1989, 3, 1301–1313. [Google Scholar]
- Osborne, B.I.; Baker, B. Movers and shakers: Maize transposons as tools for analyzing other plant genomes. Curr. Opin. Cell Biol. 1995, 7, 406–413. [Google Scholar]
- Fraser, M.J.; Smith, G.E.; Summers, M.D. Acquisition of Host Cell DNA Sequences by Baculoviruses: Relationship Between Host DNA Insertions and FP Mutants of Autographa californica and Galleria mellonella Nuclear Polyhedrosis Viruses. J. Virol. 1983, 47, 287–300. [Google Scholar]
- Cary, L.C.; Goebel, M.; Corsaro, B.G.; Wang, H.G.; Rosen, E.; Fraser, M.J. Transposon mutagenesis of baculoviruses: Analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology 1989, 172, 156–169. [Google Scholar]
- Shinmyo, Y.; Mito, T.; Matsushita, T.; Sarashina, I.; Miyawaki, K.; Ohuchi, H.; Noji, S. piggyBac-mediated somatic transformation of the two-spotted cricket, Gryllus bimaculatus. Dev. Growth Differ. 2004, 46, 343–349. [Google Scholar]
- Bonin, C.P.; Mann, R.S. A piggyBac transposon gene trap for the analysis of gene expression and function in Drosophila. Genetics 2004, 167, 1801–1811. [Google Scholar] [PubMed]
- Luo, G.; Ivics, Z.; Izsvák, Z.; Bradley, A. Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 1998, 95, 10769–10773. [Google Scholar] [PubMed]
- Koga, A.; Suzuki, M.; Inagaki, H.; Bessho, Y.; Hori, H. Transposable element in fish. Nature 1996, 383, 30. [Google Scholar] [PubMed]
- Horn, C.; Offen, N.; Nystedt, S.; Hacker, U.; Wimmer, E.A. piggyBac-based insertional mutagenesis and enhancer detection as a tool for functional insect genomics. Genetics 2003, 163, 647–661. [Google Scholar]
- Dupuy, A.J.; Fritz, S.; Largaespada, D.A. Transposition and gene disruption in the male germline of the mouse. Genesis 2001, 30, 82–88. [Google Scholar]
- Fischer, S.E.; Wienholds, E.; Plasterk, R.H. Regulated transposition of a fish transposon in the mouse germ line. Proc. Natl. Acad. Sci. USA 2001, 98, 6759–6764. [Google Scholar]
- Horie, K.; Kuroiwa, A.; Ikawa, M.; Okabe, M.; Kondoh, G.; Matsuda, Y.; Takeda, J. Efficient chromosomal transposition of a Tc1/mariner- like transposon Sleeping Beauty in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 9191–9196. [Google Scholar]
- Carlson, C.M.; Largaespada, D.A. Insertional mutagenesis in mice: New perspectives and tools. Nat. Rev. Genet. 2005, 6, 568–580. [Google Scholar]
- Collier, L.S.; Largaespada, D.A. Hopping around the tumor genome: Transposons for cancer gene discovery. Cancer Res. 2005, 65, 9607–9610. [Google Scholar]
- Cadiñanos, J.; Bradley, A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 2007, 35, e87. [Google Scholar]
- Collier, L.S.; Adams, D.J.; Hackett, C.S.; Bendzick, L.E.; Akagi, K.; Davies, M.N.; Diers, M.D.; Rodriguez, F.J.; Bender, A.M.; Tieu, C.; et al. Whole-body sleeping beauty mutagenesis can cause penetrant leukemia/lymphoma and rare high-grade glioma without associated embryonic lethality. Cancer Res. 2009, 69, 8429–8437. [Google Scholar] [PubMed]
- Copeland, N.G.; Jenkins, N.A. Harnessing transposons for cancer gene discovery. Nat. Rev. Cancer 2010, 10, 696–706. [Google Scholar]
- Dupuy, A.J.; Akagi, K.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005, 436, 221–226. [Google Scholar] [PubMed]
- Collier, L.S.; Carlson, C.M.; Ravimohan, S.; Dupuy, A.J.; Largaespada, D.A. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 2005, 436, 272–276. [Google Scholar] [PubMed]
- Keng, V.W.; Villanueva, A.; Chiang, D.Y.; Dupuy, A.J.; Ryan, B.J.; Matise, I.; Silverstein, K.A.; Sarver, A.; Starr, T.K.; Akagi, K.; et al. A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nat. Biotechnol. 2009, 27, 264–274. [Google Scholar]
- Dupuy, A.J.; Rogers, L.M.; Kim, J.; Nannapaneni, K.; Starr, T.K.; Liu, P.; Largaespada, D.A.; Scheetz, T.E.; Jenkins, N.A.; Copeland, N.G. A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res. 2009, 69, 8150–8156. [Google Scholar]
- Rad, R.; Rad, L.; Wang, W.; Cadinanos, J.; Vassiliou, G.; Rice, S.; Campos, L.S.; Yusa, K.; Banerjee, R.; Li, M.A.; et al. PiggyBac transposon mutagenesis: A tool for cancer gene discovery in mice. Science 2010, 330, 1104–1107. [Google Scholar]
- Starr, T.K.; Allaei, R.; Silverstein, K.A.; Staggs, R.A.; Sarver, A.L.; Bergemann, T.L.; Gupta, M.; O’Sullivan, M.G.; Matise, I.; Dupuy, A.J.; et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 2009, 323, 1747–1750. [Google Scholar]
- Takeda, H.; Wei, Z.; Koso, H.; Rust, A.G.; Yew, C.C.; Mann, M.B.; Ward, J.M.; Adams, D.J.; Copeland, N.G.; Jenkins, N.A. Transposon mutagenesis identifies genes and evolutionary forces driving gastrointestinal tract tumor progression. Nat. Genet. 2015, 47, 142–150. [Google Scholar]
- Wang, W.; Bradley, A.; Huang, Y. A piggyBac transposon-based genome-wide library of insertionally mutated Blm-deficient murine ES cells. Genome Res. 2009, 19, 667–673. [Google Scholar]
- Koso, H.; Takeda, H.; Yew, C.C.; Ward, J.M.; Nariai, N.; Ueno, K.; Nagasaki, M.; Watanabe, S.; Rust, A.G.; Adams, D.J.; et al. Transposon mutagenesis identifies genes that transform neural stem cells into glioma-initiating cells. Proc. Natl. Acad. Sci. USA 2012, 109, E2998–E3007. [Google Scholar] [PubMed]
- Loeb, K.R.; Hughes, B.T.; Fissel, B.M.; Osteen, N.J.; Knoblaugh, S.E.; Grim, J.E.; Drury, L.J.; Sarver, A.; Dupuy, A.J.; Clurman, B.E. Insertional mutagenesis using the Sleeping Beauty transposon system identifies drivers of erythroleukemia in mice. Sci. Rep. 2019, 9, 5488. [Google Scholar]
- Tang, J.Z.; Carmichael, C.L.; Shi, W.; Metcalf, D.; Ng, A.P.; Hyland, C.D.; Jenkins, N.A.; Copeland, N.G.; Howell, V.M.; Zhao, Z.J.; et al. Transposon mutagenesis reveals cooperation of ETS family transcription factors with signaling pathways in erythro-megakaryocytic leukemia. Proc. Natl. Acad. Sci. USA 2013, 110, 6091–6096. [Google Scholar] [PubMed]
- Elso, C.M.; Chu, E.P.; Alsayb, M.A.; Mackin, L.; Ivory, S.T.; Ashton, M.P.; Bröer, S.; Silveira, P.A.; Brodnicki, T.C. Sleeping Beauty Transposon Mutagenesis as a Tool for Gene Discovery in the NOD Mouse Model of Type 1 Diabetes. G3 2015, 5, 2903–2911. [Google Scholar] [PubMed]
- Miyakura, H.; Fukuda, M.; Enomoto, H.; Ishikawa, K.; Watanabe, S.; Semba, K. A screening system for identifying interacting proteins using biomolecular fluorescence complementation and transposon gene trap. PLoS ONE 2021, 16, e0251240. [Google Scholar]
- Ishikawa, K.; Kobayashi, Y.; Wakabayashi, Y.; Watanabe, S.; Semba, K. A highly sensitive trap vector system for isolating reporter cells and identification of responsive genes. Biol. Methods Protoc. 2018, 3, bpy003. [Google Scholar]
- Ishikawa, K.; Tamamura, S.; Semba, K.; Watanabe, S. Establishment of reporter cells that respond to glucocorticoids by a transposon-mediated promoter-trapping system. Eur. J. Pharm. Sci. 2021, 162, 105819. [Google Scholar]
- Medico, E.; Gambarotta, G.; Gentile, A.; Comoglio, P.M.; Soriano, P. A gene trap vector system for identifying transcriptionally responsive genes. Nat. Biotechnol. 2001, 19, 579–582. [Google Scholar]
- Tanaka, T.S.; Davey, R.E.; Lan, Q.; Zandstra, P.W.; Stanford, W.L. Development of a gene-trap vector with a highly sensitive fluorescent protein reporter system for expression profiling. Genesis 2008, 46, 347–356. [Google Scholar]
- Asakawa, K.; Suster, M.L.; Mizusawa, K.; Nagayoshi, S.; Kotani, T.; Urasaki, A.; Kishimoto, Y.; Hibi, M.; Kawakami, K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. USA 2008, 105, 1255–1260. [Google Scholar]
- Asakawa, K.; Kawakami, K. Targeted gene expression by the Gal4-UAS system in zebrafish. Dev. Growth Differ. 2008, 50, 391–399. [Google Scholar]
- Ogura, E.; Okuda, Y.; Kondoh, H.; Kamachi, Y. Adaptation of GAL4 activators for GAL4 enhancer trapping in zebrafish. Dev. Dyn. 2009, 238, 641–655. [Google Scholar] [PubMed]
- Liu, Z.; Okano, A.; Sanada, E.; Futamura, Y.; Nogawa, T.; Ishikawa, K.; Semba, K.; Li, J.; Li, X.; Osada, H.; et al. Identification of microbial metabolites that accelerate the ubiquitin-dependent degradation of c-Myc. Oncol. Res. 2023, 31, 655–666. [Google Scholar] [PubMed]
- Liu, Z.; Ishikawa, K.; Sanada, E.; Semba, K.; Li, J.; Li, X.; Osada, H.; Watanabe, N. Identification of antimycin A as a c-Myc degradation accelerator via high-throughput screening. J. Biol. Chem. 2023, 299, 105083. [Google Scholar]
- Wakabayashi, Y.; Shimono, A.; Terauchi, Y.; Zeng, C.; Hamada, M.; Semba, K.; Watanabe, S.; Ishikawa, K. Identification of a novel RNA transcript TISPL upregulated by stressors that stimulate ATF4. Gene 2024, 917, 148464. [Google Scholar]
- Ishikawa, K.; Tamamura, S.; Takahashi, N.; Takagi, M.; Semba, K.; Watanabe, S. Isolation of Reporter Cells That Respond to Vitamin A and/or D Using a piggyBac Transposon Promoter-Trapping Vector System. Int. J. Mol. Sci. 2022, 23, 9366. [Google Scholar]
- Kasahara, Y.; Tamamura, S.; Hiyama, G.; Takagi, M.; Nakamichi, K.; Doi, Y.; Semba, K.; Watanabe, S.; Ishikawa, K. Tyrosine Kinase Inhibitor Profiling Using Multiple Forskolin-Responsive Reporter Cells. Int. J. Mol. Sci. 2023, 24, 13863. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasahara, Y.; Semba, K.; Watanabe, S.; Ishikawa, K. Random Insertion Reporter Gimmicks Powered by Cut-and-Paste DNA Transposons. Biomedicines 2025, 13, 1682. https://doi.org/10.3390/biomedicines13071682
Kasahara Y, Semba K, Watanabe S, Ishikawa K. Random Insertion Reporter Gimmicks Powered by Cut-and-Paste DNA Transposons. Biomedicines. 2025; 13(7):1682. https://doi.org/10.3390/biomedicines13071682
Chicago/Turabian StyleKasahara, Yamato, Kentaro Semba, Shinya Watanabe, and Kosuke Ishikawa. 2025. "Random Insertion Reporter Gimmicks Powered by Cut-and-Paste DNA Transposons" Biomedicines 13, no. 7: 1682. https://doi.org/10.3390/biomedicines13071682
APA StyleKasahara, Y., Semba, K., Watanabe, S., & Ishikawa, K. (2025). Random Insertion Reporter Gimmicks Powered by Cut-and-Paste DNA Transposons. Biomedicines, 13(7), 1682. https://doi.org/10.3390/biomedicines13071682




