Colorectal Cancer: Therapeutic Approaches and Their Complications
Abstract
1. Introduction
2. Research Method
3. Results
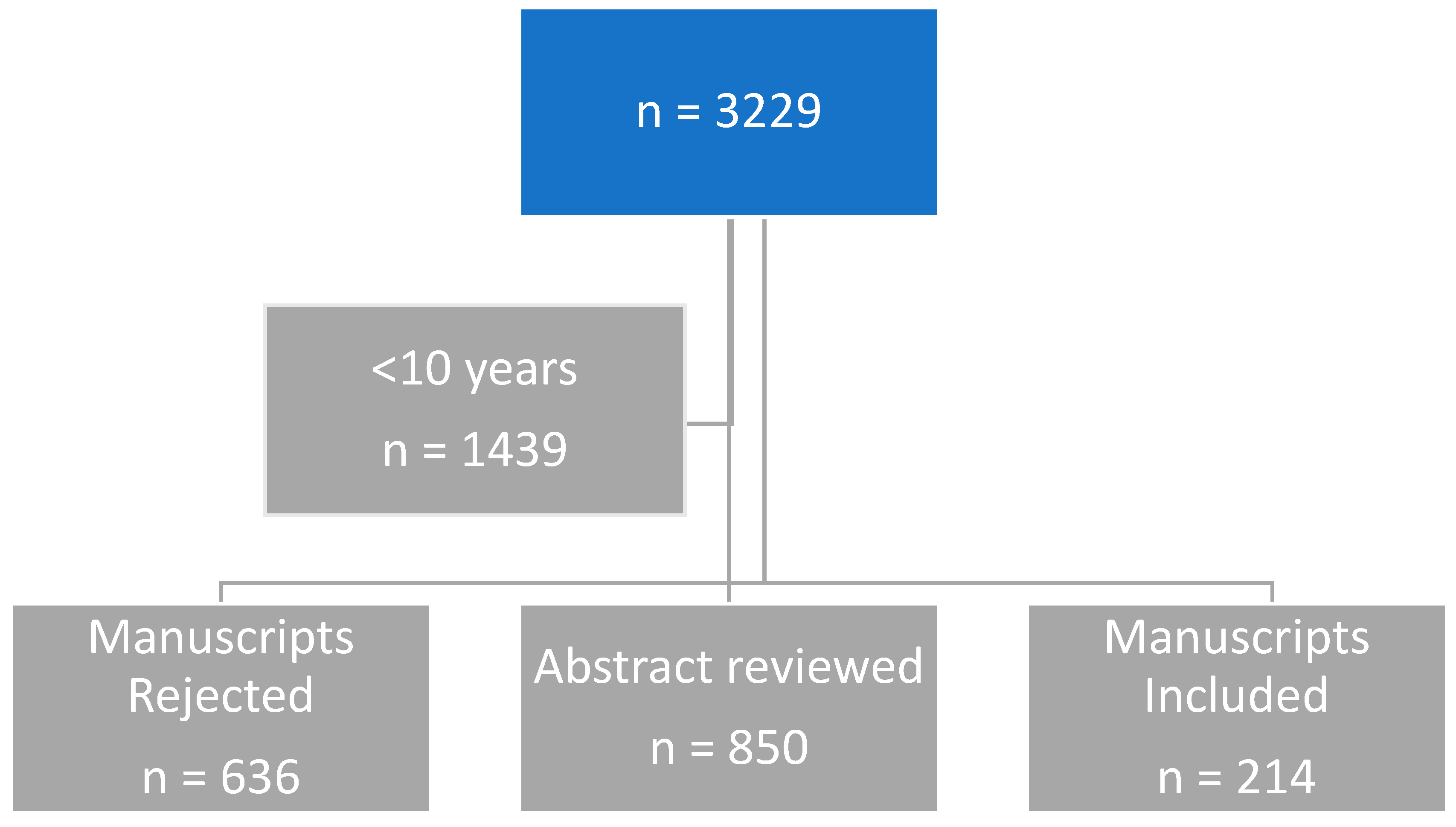
3.1. Search Terms and Articles Found in PubMed Search
3.2. Therapeutic Approaches in Colorectal Cancer Treatment
3.3. Surgical Intervention
3.4. Radiotherapy
3.5. Chemotherapy
3.5.1. Fluoropyrimidines
Intravenous Fluorouracil
Oral Fluoropyrimidines
3.5.2. Topoisomerase Inhibitors (Irinotecan)
3.5.3. Platinum Compounds (Oxaliplatin)
3.5.4. Antimetabolites (Capecitabine)
3.6. Targeted Therapy
3.6.1. Angiogenesis Inhibitors
3.6.2. Epidermal Growth Factor Receptor (EGFR) Inhibitors
3.7. Immunotherapy (Immune Checkpoint Inhibitors)
3.8. Combination Therapies Approach for Colorectal Cancer Treatment
3.8.1. FOLFIRI
3.8.2. FOLFOXIRI
3.8.3. XELIRI
3.9. Personalized Medicine
4. Complications Associated with the Therapeutic Approaches and Their Management
4.1. Complications Associated with Surgery, and Management
4.1.1. Adhesion and Small Bowel Obstructions (SBO)
4.1.2. Thrombosis
4.1.3. Infections
4.1.4. Urogenital Dysfunction
4.2. Complications Associated with Radiotherapy and Management
4.3. Mechanisms Underlying Radiation-Induced Sexual Toxicity
4.4. Complications Associated with Chemotherapy and Management
4.4.1. Chemotherapy-Induced Diarrhea (CID)
4.4.2. Cardiovascular Complications
4.4.3. Neuropathic Complications
4.5. Complications Associated with Targeted Therapy and Management
4.6. Complications Associated with Immunotherapy and Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marley, A.R.; Nan, H. Review Article: Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016, 7, 105–114. [Google Scholar] [PubMed]
- What is Colorectal Cancer? Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/about/what-is-colorectal-cancer.html (accessed on 17 May 2016).
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Ewing, I.; Hurley, J.J.; Josephides, E.; Millar, A. The molecular genetics of colorectal cancer. Frontline Gastroenterol. 2014, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Cancer Facts & Figures 2016. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2016/cancer-facts-and-figures-2016.pdf (accessed on 17 May 2016).
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, Based on 2021 Submission Data (1999–2019): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released in November 2022. Available online: https://www.cdc.gov/cancer/dataviz (accessed on 6 May 2023).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. lobal cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Cerrito, M.G.; Grassilli, E. Identifying Novel Actionable Targets in Colon Cancer. Biomedicines 2021, 9, 579. [Google Scholar] [CrossRef]
- Regenbogen, S.E.; Veenstra, C.M.; Hawley, S.T.; Banerjee, M.; Ward, K.C.; Kato, I.; Morris, A.M. The personal financial burden of complications after colorectal cancer surgery. Cancer 2014, 120, 3074–3081. [Google Scholar] [CrossRef]
- Rutherford, C.; Müller, F.; Faiz, N.; King, M.T.; White, K. Patient-reported outcomes and experiences from the perspective of colorectal cancer survivors: Meta-synthesis of qualitative studies. J. Patient-Rep. Outcomes 2020, 4, 1–19. [Google Scholar] [CrossRef]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.J.; Chen, B.; Kumar, N. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. Hematol. 2016, 86, 232–250. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Gogoi, P.; Kaur, G.; Singh, N.K. Nanotechnology for colorectal cancer detection and treatment. World J. Gastroenterol. 2022, 28, 6497–6511. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Yamashita, K.; Hasegawa, H.; Oshikiri, T.; Hosono, M.; Higashino, N.; Yamamoto, M.; Matsuda, Y.; Kanaji, S.; Nakamura, T.; et al. Recent updates in the surgical treatment of colorectal cancer. Ann. Gastroenterol. Surg. 2018, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kana, S.I.; Essani, K. ImmunoOncolytic Viruses: Emerging Options in the Treatment of Colorectal Cancer. Mol. Diagn. Ther. 2021, 25, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Cremolini, C.; Schirripa, M.; Antoniotti, C.; Moretto, R.; Salvatore, L.; Masi, G.; Falcone, A.; Loupakis, F. First-line chemotherapy for mCRC—A review and evidence-based algorithm. Nat. Rev. Clin. Oncol. 2015, 12, 607. [Google Scholar] [CrossRef]
- Sobrero, A.; Guglielmi, A.; Grossi, F.; Puglisi, F.; Aschele, C. Mechanism of action of fluoropyrimidines: Relevance to the new developments in colorectal cancer chemotherapy. Semin. Oncol. 2000, 27, 72–77. [Google Scholar]
- Van der Jeught, K.; Xu, H.-C.; Li, Y.-J.; Lu, X.-B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Mayer, R.J. Systemic Treatment of Colorectal Cancer. Gastroenterology 2008, 134, 1296131. [Google Scholar] [CrossRef]
- Glimelius, B.; Stintzing, S.; Marshall, J.; Yoshino, T.; De Gramont, A. Metastatic colorectal cancer: Advances in the folate-fluoropyrimidine chemotherapy backbone. Cancer Treat. Rev. 2021, 98, 102218. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Harstrick, A.; Rustum, Y.M. Modulation of fluoropyrimidines: Role of dose and schedule of leucovorin administration. Semin. Oncol. 1992, 19, 10–15. [Google Scholar]
- Ghoshal, K.; Jacob, S.T. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem. Pharmacol. 1997, 53, 1569–1575. [Google Scholar] [CrossRef]
- Gorlick, R.; Banerjee, D. Fluoropyrimidine resistance in colon cancer. Expert Rev. Anticancer Ther. 2002, 2, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Fraile, R.J.; Baker, L.H.; Buroker, T.R.; Horwitz, J.; Vaitkevicius, V.K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980, 40, 2223–2228. [Google Scholar] [PubMed]
- Meta-Analysis Group in Cancer; Piedbois, P.; Rougier, P.; Buyse, M.; Pignon, J.; Ryan, L.; Hansen, R.; Zee, B.; Weinerman, B.; Pater, J.; et al. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J. Clin. Oncol. 1998, 16, 301–308. [Google Scholar]
- Thirion, P.; Michiels, S.; Pignon, J.P.; Buyse, M.; Braud, A.C.; Carlson, R.W.; O’Connell, M.; Sargent, P.; Piedbois, P. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysis. J. Clin. Oncol. 2004, 22, 3766–3775. [Google Scholar] [PubMed]
- Meropol, N.J. Oral fluoropyrimidines in the treatment of colorectal cancer. Eur. J. Cancer 1998, 34, 1509–1513. [Google Scholar] [CrossRef] [PubMed]
- Mathijssen, R.H.; van Alphen, R.J.; Verweij, J.; Loos, W.J.; Nooter, K.; Stoter, G.; Sparreboom, A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 2001, 7, 2182–2194. [Google Scholar]
- Palshof, J.A.; Høgdall, E.V.; Poulsen, T.S.; Linnemann, D.; Jensen, B.V.; Pfeiffer, P.; Tarpgaard, L.S.; Brünner, N.; Stenvang, J.; Yilmaz, M.; et al. Topoisomerase I copy number alterations as biomarker for irinotecan efficacy in metastatic colorectal cancer. BMC Cancer 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Hasegawa, Y.; Ando, Y. Pharmacogenetics of irinotecan: A promoter polymorphism of UGT1A1 gene and severe adverse reactions to irinotecan. Investig. New Drugs 2005, 23, 539–545. [Google Scholar] [CrossRef]
- Sanchez-Dominguez, C.N.; Gallardo-Blanco, H.L.; Salinas-Santander, M.A.; Ortiz-Lopez, R. Uridine 5′-diphospho-glucronosyltrasferase: Its role in pharmacogenomics and human disease. Exp. Ther. Med. 2018, 16, 3. [Google Scholar] [CrossRef]
- Xiao, L.; Zhu, L.; Li, W.; Li, C.; Cao, Y.; Ge, G.; Sun, X. New Insights into SN-38 Glucuronidation: Evidence for the Important Role of UDP Glucuronosyltransferase 1A9. Basic Clin. Pharmacol. Toxicol. 2018, 122, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as firstline treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N. Engl. J. Med. 2000, 343, 905–914. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, R.; Wang, Y.; Liu, X.; Zhang, W.; Zhu, X.; Chen, Z.; Shen, W.; He, Y.; Wang, H.Q.; et al. FOLFIRI (folinic acid, fluorouracil, and irinotecan) increases not efficacy but toxicity compared with single-agent irinotecan as a second-line treatment in metastatic colorectal cancer patients: A randomized clinical trial. Ther. Adv. Med. Oncol. 2022, 14, 17588359211068737. [Google Scholar] [CrossRef]
- Raymond, E.; Chaney, S.G.; Taamma, A.; Cvitkovic, E.L. Oxaliplatin: A review of preclinical and clinical studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, J.; Yue, X.; Wang, J.; Liu, J.; Sun, L.; Kong, D. Expression of the cancer stem cell markers ABCG2 and OCT-4 in right-sided colon cancer predicts recurrence and poor outcomes. Oncotarget 2017, 8, 28463–28470. [Google Scholar] [CrossRef] [PubMed]
- de Sousa EMelo, F.; Colak, S.; Buikhuisen, J.; Koster, J.; Cameron, K.; de Jong, J.H.; Tuynman, J.B.; Prasetyanti, P.R.; Fessler, E.; van den Bergh, S.P.; et al. Methylation of cancer-stem-cell-associated Wnt target genes predicts poor prognosis in colorectal cancer patients. Cell Stem Cell 2011, 9, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Recuero, J.K.; Fitz, J.R.; Pereira, A.A.; Bonamigo, R.R. EGFR inhibitors: Clinical aspects, risk factors and biomarkers for acneiform eruptions and other mucosal and cutaneous adverse effects. An. Bras. Dermatol. 2023, 98, 429. [Google Scholar] [CrossRef]
- Maxwell, P.; Dachs, G.; Gleadle, J.; Nicholls, L.; Harris, A.; Stratford, I.J.; Hankinson, O.; Pugh, C.A.; Ratcliffe, P.J. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA 1997, 94, 8104–8109. [Google Scholar] [CrossRef]
- Jurgensmeier, J.; Schmoll, H.; Robertson, J.; Brooks, L.; Taboada, M.; Morgan, S.R.; Wilson, D.; Hoff, P.M. Prognostic and predictive value of VEGF, SVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy. Br. J. Cancer 2013, 108, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.I.; Fehrenbacher, L.; Hainsworth, J.D.; Heim, W.; Berlin, J.; Holmgren, E.; Hambleton, J.; Novotny, W.F.; Kabbinavar, F. Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J. Clin. Oncol. 2005, 23, 3502–3508. [Google Scholar] [CrossRef]
- Glusker, P.; Recht, L.; Lane, B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N. Engl. J. Med. 2006, 354, 980–982. [Google Scholar] [PubMed]
- Fuchs, C.S.; Marshall, J.; Mitchell, E.; Wierzbicki, R.; Ganju, V.; Jeffery, M.; Schulz, J.; Richards, D.; Soufi-Mahjoubi, R.; Wang, B.; et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-C study. J. Clin. Oncol. 2007, 25, 4779–4786. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nagase, M.; Tamagawa, H.; Ueda, S.; Tamura, T.; Murata, K.; Nakajima, T.E.; Baba, E.; Tsuda, M.; Moriwaki, T.; et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann. Oncol. 2016, 27, 1539–1546. [Google Scholar] [CrossRef]
- Messersmith, W.A.; Hidalgo, M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: Another one or the one? Clin. Cancer Res. 2007, 13, 4664–4666. [Google Scholar] [CrossRef]
- Tabernero, J. The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. 2007, 5, 203–220. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef]
- Ciardiello, F.; Bianco, R.; Damiano, V.; Fontanini, G.; Caputo, R.; Pomatico, G.; Placido, S.D.; Bianco, A.R.; Mendelsohn, J.; Tortora, G. Antiangiogenic and antitumor activity of antiepidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin. Cancer Res. 2000, 6, 3739–3747. [Google Scholar]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J. Gastroenterol. WJG 2014, 20, 3751. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Cappuzzo, F.; Janne, P.A.; Bencardino, K.; Carnaghi, C.; Franklin, W.A.; Roncalli, M.; Crino, L.; Santoro, A.; Varella-Garcia, M. EGFR, HER2, Kras as predictive factors for cetuximab sensitivity in colorectal cancer (abstr). J. Clin. Oncol. 2007, 25, 18S. [Google Scholar] [CrossRef]
- Fan, L.C.; Teng, H.W.; Shiau, C.W.; Tai, W.T.; Hung, M.H.; Yang, S.H.; Jiang, J.K.; Chen, K.F. Regorafenib (Stivarga) pharmacologically targets epithelial-mesenchymal transition in colorectal cancer. Oncotarget 2016, 7, 64136–64147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krasteva, N.; Georgieva, M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics 2022, 14, 1213. [Google Scholar] [CrossRef]
- Tolba, M.F. Revolutionizing the landscape of colorectal cancer treatment: The potential role of immune checkpoint inhibitors. Int. J. Cancer 2020, 147, 2996–3006. [Google Scholar] [CrossRef]
- Halama, N.; Zoernig, I.; Jager, D. Immunotherapy for cancer—Modern immunologic strategiesin oncology. Dtsch. Med. Wochenschr. 2008, 133, 2105–2108. [Google Scholar] [CrossRef]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in colorectal cancer: Current achievements future perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Boland, P.M.; Ma, W.W. Immunotherapy for colorectal cancer. Cancer 2017, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Germano, G.; Lamba, S.; Rospo, G.; Barault, L.; Magrì, A.; Maione, F.; Russo, M.; Crisafulli, G.; Bartolini, A.; Lerda, G.; et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017, 552, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Guo, Y. Combination Therapy to Enhance Cancer Treatment. Ph.D. Thesis, University of California, Merced, CA, USA, 2020. [Google Scholar]
- Cidón, E.U. The challenge of metastatic colorectal cancer. Clin. Med. Insights Oncol. 2010, 4, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Tonini, G.; Vincenzi, B.; Santini, D.; Olzi, D.; Lambiase, A.; Bonini, S. Ocular toxicity related to cetuximab monotherapy in an advanced colorectal cancer patient. J. Natl. Cancer Inst. 2005, 97, 606–607. [Google Scholar] [CrossRef]
- Albain, K.S.; Nag, S.M.; Calderillo-Ruiz, G.; Jordaan, J.P.; Llombart, A.C.; Pluzanska, A.; Rolski, J.; Melemed, A.S.; Reyes-Vidal, J.M.; Sekhon, J.S.; et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J. Clin. Oncol. 2008, 26, 3950–3957. [Google Scholar] [CrossRef]
- Jang, B.; Kwon, H.; Katila, P.; Lee, S.J.; Lee, H. Dual delivery of biological therapeutics for multimodal and synergistic cancer therapies. Adv. Drug Deliv. Rev. 2016, 98, 113–133. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2002, 3, 330–338. [Google Scholar] [CrossRef]
- Benson, A.B., III; Goldberg, R.M. Optimal use of the combination of irinotecan and 5-fluorouracil. Semin. Oncol. 2003, 30, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Szefler, B.; Czeleń, P. Will the interactions of some platinum (II)-Based drugs with B-vitamins reduce their therapeutic effect in cancer patients? Comparison of chemotherapeutic agents such as cisplatin, carboplatin and oxaliplatin—A review. Int. J. Mol. Sci. 2023, 24, 1548. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, P.D.; Sutcliffe, D.F.; Griffith, D.M. Oxaliplatin and its derivatives–An overview. Coord. Chem. Rev. 2023, 497, 215439. [Google Scholar] [CrossRef]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO–78. ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef]
- Rosati, G.; Aprile, G.; Colombo, A.; Cordio, S.; Giampaglia, M.; Cappetta, A.; Porretto, C.M.; De Stefano, A.; Bilancia, D.; Avallone, A. Colorectal cancer heterogeneity and the impact on precision medicine and therapy efficacy. Biomedicines 2022, 10, 1035. [Google Scholar] [CrossRef]
- Falcone, A.; Masi, G.; Allegrini, G.; Danesi, R.; Pfanner, E.; Brunetti, I.M.; Di Paolo, A.; Cupini, S.; Del Tacca, M.; Conte, P. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional fluorouracil, and leucovorin: A pilot study in patients with metastatic colorectal cancer. J. Clin. Oncol. 2002, 20, 4006–4014. [Google Scholar] [CrossRef]
- Souglakos, J.; Ziras, N.; Kakolyris, S.; Boukovinas, I.; Kentepozidis, N.; Makrantonakis, P.; Xynogalos, S.; Christophyllakis, C.; Kouroussis, C.; Vamvakas, L.; et al. Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs. FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br. J. Cancer 2012, 106, 453–459. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, R.; Hu, H.; Zhang, J.; Ling, J.; Wu, Z.; Yang, L.; Li, J.; Deng, Y. Analysis on safety and preliminary efficacy of dose-modified regimen of 5-fluorouracil plus oxaliplatin and irinotecan (FOLFOXIRI) in advanced colorectal cancer. Zhonghua Wei Chang. Wai Ke Za Zhi Chin. J. Gastrointest. Surg. 2018, 21, 1045–1050. [Google Scholar]
- Le Huy, T.; Bui, M.H.; Dinh, T.C.; Xuyen, H.T.H. Efficacy and toxicity of folfoxiri for patients with metastatic colorectal cancer. Open Access Maced. J. Med. Sci. 2019, 7, 4244. [Google Scholar] [CrossRef]
- García-Alfonso, P.; Martín, A.J.M.; Morán, L.O.; Alsar, J.S.; Pérez-Solero, G.T.; Codesido, M.B.; Ferrandiz, P.A.C.; Cicala, S.G. Oral drugs in the treatment of metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211009001. [Google Scholar] [CrossRef]
- Walko, C.M.; Lindley, C. Capecitabine: A review. Clin. Ther. 2005, 27, 23–44. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B.; Pushparaj, P.N. 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer. Oncol. Rep. 2023, 50, 175. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, M.; Shen, X.; Yang, C.; Yang, L.; Zhang, J. Capecitabine plus irinotecan versus 5-FU/leucovorin plus irinotecan in the treatment of colorectal cancer: A meta-analysis. Clin. Color. Cancer 2014, 13, 110–118. [Google Scholar] [CrossRef]
- Montagnani, F.; Chiriatti, A.; Licitra, S.; Aliberti, C.; Fiorentini, G. Differences in efficacy and safety between capecitabine and infusional 5-fluorouracil when combined with irinotecan for the treatment of metastatic colorectal cancer. Clin. Color. Cancer 2010, 9, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Haller, D.G.; Cassidy, J.; Clarke, S.; Cunningham, D.; Van Cutsem, E.; Hoff, P.; Rothenberg, M.; Saltz, L.; Schmoll, H.J.; Twelves, C. Tolerability of fluoropyrimidines appears to differ by region. J. Clin. Oncol. 2006, 24, 3514. [Google Scholar] [CrossRef]
- Xu, R.-H.; Muro, K.; Morita, S.; Iwasa, S.; Han, S.W.; Wang, W.; Kotaka, M.; Nakamura, M.; Ahn, J.B.; Deng, Y.-H.; et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018, 19, 660–671. [Google Scholar] [PubMed]
- Wu, Q.; Zhang, P.; Wang, X.; Zhang, M.; Liao, W.; Li, Q. Cost-effectiveness of capecitabine+ irinotecan versus leucovorin+ fluorouracil+ irinotecan in the second-line treatment of metastatic colorectal cancer in China. Clin. Ther. 2020, 42, 2148–2158. [Google Scholar] [CrossRef]
- Formica, V.; Sera, F.; Cremolini, C.; Riondino, S.; Morelli, C.; Arkenau, H.T.; Roselli, M. KRAS and BRAF Mutations in Stage II and III Colon Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2022, 114, 517–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Midthun, L.; Shaheen, S.; Deisch, J.; Senthil, M.; Tsai, J.; Hsueh, C.T. Concomitant KRAS and BRAF mutations in colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 577–581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Z.N.; Zhao, L.; Yu, L.F.; Wei, M.J. BRAF and KRAS mutations in metastatic colorectal cancer: Future perspectives for personalized therapy. Gastroenterol. Rep. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Ros, J.; Saoudi, N.; Baraibar, I.; Salva, F.; Tabernero, J.; Elez, E. Encorafenib plus cetuximab for the treatment of BRAF-V600E-mutated metastatic colorectal cancer. Ther. Adv. Gastroenterol. 2022, 15, 17562848221110644. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Murphy, D.A.; Pu, J.; Ciardiello, F.; Desai, J.; Van Cutsem, E.; Wasan, H.S.; Yoshino, T.; Saffari, H.; Zhang, X.; et al. Molecular profiling of BRAF-V600E-mutant metastatic colorectal cancer in the phase 3 BEACON CRC trial. Nat. Med. 2024, 30, 3261–3271. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.M.; Burge, M.E.; Whitehall, V.L. The therapeutic landscape for KRAS-mutated colorectal cancers. Cancers 2023, 15, 2375. [Google Scholar] [CrossRef]
- Goebel, L.; Müller, M.P.; Goody, R.S.; Rauh, D. KRasG12C inhibitors in clinical trials: A short historical perspective. RSC Med. Chem. 2020, 11, 760–770. [Google Scholar] [CrossRef]
- Pak, H.; Maghsoudi, L.H.; Soltanian, A.; Gholami, F. Surgical complications in colorectal cancer patients. Ann. Med. Surgery 2020, 55, 13–18. [Google Scholar] [CrossRef]
- Gachabayov, M.; Senagore, A.J.; Abbas, S.K.; Yelika, S.B.; You, K.; Bergamaschi, R. Perioperative hyperglycemia: An unmet need within a surgical site infection bundle. Tech. Coloproctol. 2018, 22, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Van der Kaaij, M.; Van Dorst, E.; Creutzberg, C.; Huyghe, E.; Kiserud, C.E. Sexual dysfunction and infertility as late effects of cancer treatment. Eur. J. Cancer Suppl. 2014, 12, 41–53. [Google Scholar] [CrossRef]
- Lee, W.K.; Park, Y.H.; Choi, S.; Lee, W.S. Is liquid-based hyaluronic acid equivalent to sodium hyaluronate-based bioresorbable membrane to reduce small bowel obstruction in patients undergoing colorectal surgery. Asian J. Surg. 2019, 42, 443–449. [Google Scholar] [CrossRef]
- Fesharakizadeh, M.; Taheri, D.; Dolatkhah, S.; Wexner, S.D. Postoperative ileus in colorectal surgery: Is there any difference between laparoscopic and open surgery? Gastroenterol. Rep. 2013, 1, 138–143. [Google Scholar] [CrossRef]
- Smolarek, S.; Shalaby, M.; Angelucci, G.P.; Missori, G.; Capuano, I.; Franceschilli, L.; Quaresima, S.; Di Lorenzo, N.; Sileri, P. Small-bowel obstruction secondary to adhesions after open or laparoscopic colorectal surgery. J. Soc. Laparoendosc. Surg. J. Soc. Laparoendosc. Surg. 2016, 20, e201600073. [Google Scholar] [CrossRef]
- Yang, K.M.; Yu, C.S.; Lee, J.L.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Lim, S.B.; Kim, J.C. The long-term outcomes of recurrent adhesive small bowel obstruction after colorectal cancer surgery favor surgical management. Medicine 2017, 96, e8316. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Di Saverio, S.; Haukijärvi, E.; Juusela, R.; Wikström, H.; Koivukangas, V.; Catena, F.; Enholm, B.; Birindelli, A.; Leppäniemi, A.; et al. Laparoscopic versus open adhesiolysis for adhesive small bowel obstruction (LASSO): An international, multicentre, randomized, open-label trial. Lancet Gastroenterol. Hepatol. 2019, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Tanaka, E.; Matsui, Y.; Ikeda, A.; Murakami, T.; Okumoto, T.; Harada, T. Does laparoscopic adhesiolysis decrease the risk of recurrent symptoms in small bowel obstruction? A propensity score-matched analysis. Surg. Endosc. 2017, 31, 5348–5355. [Google Scholar] [CrossRef]
- Fujii, S.; Tsukamoto, M.; Shimada, R.; Okamoto, K.; Hayama, T.; Tsuchiya, T.; Nozawa, K.; Matsuda, K.; Ishibe, A.; Ota, M.; et al. Absorptive anti-adhesion barrier for the prevention of bowel obstruction after laparoscopic colorectal cancer surgery. J. Anus Rectum Colon. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Elia-Guedea, M.; de Laspra, E.C.-D.; Echazarreta-Gallego, E.; Lazaro, M.I.V.; Ramirez-Rodriguez, J.M.; Aguilella-Diago, V. Colorectal surgery and surgical site infection: Is a change of attitude necessary? Int. J. Color. Dis. 2017, 32, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Z.; Fang, K.; Ma, W.L.; Shi, Z.H.; Ren, X.Q. Risk of postoperative deep venous thrombosis in patients with colorectal cancer treated with open or laparoscopic colorectal surgery: A meta-analysis. Indian J. Canc. 2015, 51 (Suppl. 2), e42–e44. [Google Scholar]
- Sebastian, E.; Courtier, R.; Macia, F.; Grande, L.; Pera, M. The impact of screening on short-term outcome after surgery for colorectal cancer. Rev. Esp. Enferm. Dig. 2017, 109, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Zaghiyan, K.N.; Sax, H.C.; Miraflor, E.; Cossman, D.; Wagner, W.; Mirocha, J.; Gewertz, B.; Fleshner, P.; Cedars-Sinai, D.V.T. Timing of chemical thromboprophylaxis and deep vein thrombosis in major colorectal surgery: A randomized clinical trial. Ann. Surg. 2016, 264, 632–639. [Google Scholar] [CrossRef]
- Holwell, A.; McKenzie, J.L.; Holmes, M.; Woods, R.; Nandurkar, H.; Tam, C.S.; Bazargan, A. Venous thromboembolism prevention in patients undergoing colorectal surgery for cancer. ANZ J. Surg. 2014, 84, 284–288. [Google Scholar] [CrossRef]
- Carrier, M.; Altman, A.D.; Blais, N.; Diamantouros, A.; McLeod, D.; Moodley, U.; Nguyen, C.; Young, S.; Schwenter, F. Extended thromboprophylaxis with low-molecular weight heparin (LMWH) following abdominopelvic cancer surgery. Am. J. Surg. 2018, 218, 537–550. [Google Scholar] [CrossRef]
- Saha, A.K.; Chowdhury, F.; Jha, A.K.; Chatterjee, S.; Das, A.; Banu, P. Mechanical bowel preparation versus no preparation before colorectal surgery: A randomized prospective trial in a tertiary care institute. J. Nat. Sci. Biol. Med. 2014, 5, 421–424. [Google Scholar] [PubMed]
- Lange, M.M.; van de Velde, C.J. Urinary and sexual dysfunction after rectal cancer treatment. Nat. Rev. Urol. 2011, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.; Nelson, C.J.; Shuk, E.; Starr, T.D.; Temple, L.; Jandorf, L.; Schover, L.; Mulhall, J.P.; Woo, H.; Jennings, S.; et al. Men’s Experience with Sexual Dysfunction Post-rectal Cancer Treatment: A Qualitative Study. J. Cancer Educ. 2013, 28, 494–502. [Google Scholar] [CrossRef]
- Hendren, S.K.; O’Connor, B.I.; Liu, M.; Asano, T.; Cohen, Z.; Swallow, C.J.; MacRae, H.M.; Gryfe, R.; McLeod, R.S. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann. Surg. 2005, 242, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, G.; Schijven, M.P.; Hendrikx, A.; Martijn, H.; Rutten, H.J.; Wiggers, T. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur. J. Surg. Oncol. 2001, 27, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.P.; Lee, Y.; Stein, S.L.; Temple, L.K. Sexual function after treatment for rectal cancer: A review. Dis. Colon Rectum 2011, 54, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.R.G.; Abd El Aziz, M.A.; Duchalais, E.; Grass, F.; Behm, K.T.; Mathis, K.L.; Kelley, S.R. Sexual dysfunction following surgery for rectal cancer: A single-institution experience. Updates Surg. 2021, 73, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Cyranowski, J.M.; Espindle, D. Men’s sexual self-schema. J. Pers. Soc. Psychol. 1999, 76, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.J.; Deveci, S.; Stasi, J.; Scardino, P.T.; Mulhall, J.P. Sexual bother following radical prostatectomy. J. Sex. Med. 2010, 7, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ghomeshi, A.; Zizzo, J.; Reddy, R.; White, J.; Swayze, A.; Swain, S.; Ramasamy, R. The erectile and ejaculatory implications of the surgical management of rectal cancer. Int. J. Urol. 2023, 30, 827–837. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Gupta, M. PDE5 Inhibitors in StatPearls [Internet]; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hor, M.; Baradeiya, A.M.; Qasim, H.; Nasr, M.; Mohammad, A. Non-Arteritic anterior ischemic optic neuropathy associated with the use of phosphodiesterase type 5 inhibitors: A literature review. Cureus 2022, 14, e27642. [Google Scholar] [CrossRef] [PubMed]
- Değer, M.D.; Madendere, S. Erectile dysfunction treatment with Phosphodiesterase-5 inhibitors: Google trends analysis of last 10 years and COVID-19 pandemic. Arch. Ital. Urol. Androl. 2021, 93, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, H.C.; Huang, W.J.; Wang, C.J.; Hwang, T.I.S.; Liao, C.H.; Liu, C.C.; Pang, S.T.; Huang, E.Y.H.; Tsao, C.W.; et al. Consensus of experts on the treatment of sexual dysfunction after surgery for prostate cancer in Taiwan. J. Clin. Med. 2023, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Cuzin, B. Alprostadil cream in the treatment of erectile dysfunction: Clinical evidence and experience. Ther. Adv. Urol. 2016, 8, 249–256. [Google Scholar] [CrossRef]
- Hatzimouratidis, K.; Salonia, A.; Adaikan, G.; Buvat, J.; Carrier, S.; el-Meliegy, A.; McCullough, A.; Torres, L.O.; Khera, M. Pharmacotherapy for erectile dysfunction: Recommendations from the fourth international consultation for sexual medicine (ICSM 2015). J. Sex. Med. 2016, 13, 465–488. [Google Scholar] [CrossRef]
- Das, S.; Dodd, S.; Soni, B.M.; Sharma, S.D.; Gazvani, R.; Lewis-Jones, D.I. Does repeated electro-ejaculation improve sperm quality in spinal cord injured men? Spinal Cord 2006, 44, 753–756. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, R.; Agarwal, A.; Parekh, N.; Finelli, R.; Shah, R.; Kandil, H.; Saleh, R.; Arafa, M.; Ko, E.; et al. A comprehensive guide to sperm recovery in infertile men with retrograde ejaculation. World J. Mens. Health 2022, 40, 208–216. [Google Scholar] [CrossRef]
- Cong, R.; Zhang, Q.; Wang, Y.; Meng, X.; Wang, Z.; Song, N. Two cases of psychogenic anejaculation patients got normal ejaculation ability after penile vibratory stimulation or electroejaculation. Transl. Androl. Urol. 2019, 8, 758–761. [Google Scholar] [CrossRef]
- Surbone, A.; Vaucher, L.; Primi, M.P.; Leyvraz, C.; Pitteloud, N.; Ballabeni, P.; Mathevet, P.; Vulliemoz, N. Clomiphene citrate effect on testosterone level and semen parameters in 18 infertile men with low testosterone level and normal/low gonadotropines level. Eur. J. Obs. Gynecol. Reprod. Biol. 2019, 238, 104–109. [Google Scholar] [CrossRef]
- Canada, A.L.; Neese, L.E.; Sui, D.; Schover, L.R. Pilot Intervention to Enhance Sexual Rehabilitation for Couples after Treatment for Localized Prostate Carcinoma. Cancer 2006, 104, 2689–2700. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136. [Google Scholar] [CrossRef] [PubMed]
- Horesh, N.; Emile, S.H.; Garoufalia, Z.; Gefen, R.; Zhou, P.; Wexner, S.D. Trends in management and outcomes of colon cancer in the United States over 15 years: Analysis of the National Cancer Database. Int. J. Cancer 2024, 155, 139–148. [Google Scholar] [CrossRef]
- Jairam, V.; Lee, V.; Park, H.S.; Thomas, C.R.; Melnick, E.R.; Gross, C.P.; Presley, C.J.; Adelson, K.B.; Yu, J.B. Treatment-related complications of systemic therapy and radiotherapy. JAMA Oncol. 2019, 5, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.R.; Sanford, N.N. Toxicity Management in the Era of Changing Treatment Paradigms for Locally Advanced Rectal Cancer. Curr. Color. Cancer Rep. 2022, 18, 55–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.X. The impact of gut microbiota on radiation-induced enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, Y.; Zang, J.; Liu, P.; Liu, W.; Sun, C.; Tian, D.; Li, P.; Tian, J.; Xiao, J. Investigating the relationship between postoperative radiotherapy and intestinal flora in rectal cancer patients: A study on efficacy and radiation enteritis. Front. Oncol. 2024, 14, 1408436. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Li, S.T.; Shu, Y.; Zhan, H.Q. Probiotics for prevention of radiation-induced diarrhea: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0178870. [Google Scholar] [CrossRef]
- Garczyk, A.; Kaliciak, I.; Drogowski, K.; Horwat, P.; Kopeć, S.; Staręga, Z.; Mardas, M. Influence of probiotics in prevention and treatment of patients who undergo chemotherapy or/and radiotherapy and suffer from mucositis, diarrhoea, constipation, nausea and vomiting. J. Clin. Med. 2022, 11, 3412. [Google Scholar] [CrossRef]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H.B. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef]
- Skrzypek, M.; Wdowiak, A.; Panasiuk, L.; Stec, M.; Szczygieł, K.; Zybała, M.; Filip, M. Effect of ionizing radiation on the female reproductive system. Ann. Agric. Environ. Med. 2019, 26, 606–616. [Google Scholar] [CrossRef]
- Aldoury, R.S.M. A Review Article: Effect of Radiation on Infertility. Int. J. Res. Appl. Sci. Biotechnol. 2022, 9, 45–65. [Google Scholar] [CrossRef]
- Thyø, A.; Elfeki, H.; Laurberg, S.; Emmertsen, K.J. Female sexual problems after treatment for colorectal cancer—A population-based study. Color. Dis. 2019, 21, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Kesari, K.K.; Agarwal, A.; Henkel, R. Radiations and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, H.; Yokoya, A.; Prise, K.M. A brief overview of radiation-induced effects on spermatogenesis and oncofertility. Cancers 2022, 14, 805. [Google Scholar] [CrossRef]
- Aral, İ.P.; Beyaz, H.; Arslan, S.A.; Açıkgöz, S.G.; Tezcan, Y. Radiotherapy, Female Fertility and Ootoxicity. Jinekoloji-Obstet. Neonatoloji Tıp Derg. 2023, 20, 2048–2054. [Google Scholar] [CrossRef]
- Beyer, S.; Sandu, A.; White, J. Impact and timing of breast cancer radiation therapy and fertility preservation. Curr. Breast Cancer Rep. 2020, 12, 375–380. [Google Scholar] [CrossRef]
- Bolus, N.E. Basic review of radiation biology and terminology. J. Nucl. Med. Technol. 2001, 29, 67–73. [Google Scholar] [CrossRef]
- Marci, R.; Mallozzi, M.; Di Benedetto, L.; Schimberni, M.; Mossa, S.; Soave, I.; Palomba, S.; Caserta, D. Radiations and female fertility. Reprod. Biol. Endocrinol. 2018, 16, 112. [Google Scholar] [CrossRef]
- McQuade, R.; Bornstein, J.; Nurgali, K. Anti-Colorectal Cancer Chemotherapy-Induced Diarrhoea: Current Treatments and Side-Effects. Int. J. Clin. Med. 2014, 5, 393–406. [Google Scholar] [CrossRef]
- Baek, K.L.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kiang, W.K.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; et al. Oxaliplatin-Induced Chronic Peripheral Neurotocixity: A Prospective Analysis in Patients with Colorectal Cancer. Cancer Res. Treat. 2010, 45, 185–190. [Google Scholar] [CrossRef]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S. Chemotherapy-Induced Diarrhoea Is Associated with Changes in the Luminal Environment in the DA Rat. Exp. Biol. Med. 2007, 232, 96–106. [Google Scholar]
- Verstappen, C.C.P.; Heimans, J.J.; Hoekman, K.; Postma, T.J. Nuerotoxic Complocations of Chemotherapy in Patients with Cancer, Clinical Signs and Optimal Management. Ther. Pract. 2003, 63, 1549–1563. [Google Scholar]
- Benson, A.B.; Ajani, J.A.; Catalano, R.B.; Engelking, C.; Kornblau, S.M.; Martenson, J.A.; McCallum, R.; Mitchell, E.P.; O’DOrisio, T.M.; Vokes, E.E.; et al. Recommended Guidelines for the Treatment of Cancer Treatment-Induced Diarrhea. J. Clin. Oncol. 2004, 22, 2918–2926. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Review: Chemotherapy-Induced Diarrhea: Pathophysiology, Frequency and Guideline Based Management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef]
- Keefe, D.M.; Schubert, M.M.; Elting, L.S.; Sonis, S.T.; Epstein, J.B.; Raber-Durlacher, J.E.; Migliorati, C.A.; McQuire, D.B.; Hutchins, R.D.; Peterson, D.E. Updates Clinical Practice Guidelines for the Prevention and Treatment of Mucositis. Cancer 2007, 109, 820–832. [Google Scholar] [CrossRef]
- Richardson, G.G.; Dobish, R.R. Chemotherapy-Induced Diarrhea. J. Oncol. Pharm. Pract. 2007, 13, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.P. Gastrointestinal Toxicity of Chemotherapeutic Agents. Semin. Oncol. 2006, 33, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.A.; Bresalier, R.S. The Gastrointestinal Complications of Oncologic Therapy. Gastroenterol. Clin. N. Am. 2010, 39, 629–647. [Google Scholar] [CrossRef]
- Kuebler, J.P.; Colengalo, L.; O’Connell, M.J.; Smith, R.E.; Yothers, G.; Begovic, M.; Robinson, B.; Seay, T.E.; Wol-Mark, N. Severe Enteropathy among Patients with Stage II/III Colon Cancer Treated on a Randomized Trial of Bolus 5-FU/Leucovorin Plus or Minus Oxaliplatin. Cancer 2007, 110, 1945–1950. [Google Scholar] [CrossRef]
- Dranitsaris, G.; Maroun, J.; Shah, A. Estimating the Cost of Illness in Colorectal Cancer Patients Who Were Hospitalized for Severe Chemotherapy-Induced Diarrhea. Can. J. Gastroenterol. 2005, 19, 83–87. [Google Scholar] [CrossRef]
- Arbuckle, R.B.; Huber, S.L.; Zacker, C. The Consequences of Diarrhea Occurring during Chemotherapy for Colorectal Cancer: A Retrospective Study. Oncologist 2000, 5, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Ashton, K.; Yeoh, A.S.J.; Al-Dasooqi, N.; Keefe, D.M.K. Irinotecan-Induced Mucositis Manifesting as Diarrhoea Corresponds with Amended Intestinal Flora and Mucin Profile. Int. J. Exp. Pathol. 2009, 90, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Salazar-Lindo, E.; Leon-Barua, R. Differentiation of Osmotic and Secretory Diar- rhoea by Stool Carbohydrate and Osmolar Gap Measurements. Arch. Dis. Child. 1997, 77, 201–205. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Coyle, N. Textbook of Palliative Nursing; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Vera, G.; Castillo, M.; Cabezos, P.A.; Chairlone, A.; Martin, M.I.; Gori, A.; Paquinelli, G.; Barbara, G.; Staghelleni, V.; Corinaldesi, R.; et al. Enteric Neuropathy Evoked by Repeated Cisplatin in the Rat. Neurogastroenterol. Motil. 2011, 23, 370–378. [Google Scholar] [CrossRef]
- Wafai, L.; Taher, M.; Jovanovska, V.; Bornstein, J.C.; Dass, C.R.; Nurgali, K. Effects of Oxaliplatin on Mouse Myenteric Neurons and Colonic Motility. Front. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef]
- Keramida, K.; Charalampopoulos, G.; Filippiadis, D.; Tsougos, E.; Farmakis, D. Cardiovascular complications of metastatic colorectal cancer treatment. J. Gastrointest. Oncol. 2019, 10, 797–806. [Google Scholar] [CrossRef]
- Becker, K.; Erckenbrecht, J.F.; Haussinger, D.; Fueling, T. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999, 57, 475–484. [Google Scholar] [CrossRef]
- Georgiyeva, K.; Blake, P. Cardiovascular Side Effects of Colon Cancer Therapy. Sci. Eur. 2022, 99, 20–24. [Google Scholar]
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, J.; Coutsouvelis, J. Capecitabine: Effective oral fluoropyrimidine chemotherapy. Expert. Opin. Pharmacother. 2005, 6, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Leicher, L.W.; de Graaf, J.C.; Coers, W.; Tascilar, M.; de Groot, J.W. Tolerability of Capecitabine Monotherapy in Metastatic Colorectal Cancer: A Real-World Study. Drugs R. D 2017, 17, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.R.; Shah, A.; Rather, A. Ventricular fibrillation as a likely consequence of capecitabine-induced coronary vasospasm. J. Oncol. Pharm. Pr. 2012, 18, 132–135. [Google Scholar] [CrossRef]
- Kwakman, J.J.; Simkens, L.H.; Mol, L.; Kok, W.E.; Koopman, M.; Punt, C.J. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: A retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur. J. Cancer 2017, 76, 93–99. [Google Scholar] [CrossRef]
- Saif, M.W.; Shah, M.M.; Shah, A.R. Fluoropyrimidine associated cardiotoxicity: Revisited. Expert Opin. Drug Saf. 2009, 8, 191–202. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Autorino, R.; Bruni, G.; Carteni, G.; Ricevuto, E.; Tudini, M.; Ficorella, C.; Romano, C.; Aieta, M.; Giordano, A.; et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: A multicenter analysis. Ann. Oncol. 2009, 20, 1535–1542. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, S.; Dahut, W.L.; Parikh, C.R. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am. J. Kidney Dis. 2007, 49, 186–193. [Google Scholar] [CrossRef]
- Brinda, B.J.; Viganego, F.; Vo, T.; Dolan, D.; Fradley, M.G. Anti-VEGF-Induced Hypertension: A Review of Pathophysiology and Treatment Options. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 33. [Google Scholar] [CrossRef]
- Cai, J.; Ma, H.; Huang, F.; Zhu, D.; Bi, J.; Ke, Y.; Zhang, T. Correlation of bevacizumab induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: A systematic review and meta-analysis. World J. Surg. Oncol. 2013, 11, 306. [Google Scholar] [CrossRef]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients With Cancer Treated with Bevacizumab: A Meta-Analysis of More Than 20,000 Patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Saltz, L.B.; Van Cutsem, E.; Cassidy, J.; Wiedemann, J.; Sirzén, F.; Lyman, G.H.; Rohr, U.P. Venous thromboembolic events with chemotherapy plus bevacizumab: A pooled analysis of patients in randomized phase II and III studies. J. Clin. Oncol. 2011, 29, 1757–1764. [Google Scholar] [CrossRef]
- Baron Esquivias, G.; Asteggiano, R. Cardiac Management of Oncology Patients: Clinical Handbook for Cardio-Oncology; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar]
- Petrelli, F.; Cabiddu, M.; Borgonovo, K.; Barni, S. Risk of venous and arterial thromboembolic events associated with anti EGFR agents: A meta-analysis of randomized clinical trials. Ann. Oncol. 2012, 23, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Chen, H.; Liu, Y.; Huang, B.L.; Zhang, X.Q.; Yuan, J.M.; He, X. The cardiotoxicity of cetuximab as single therapy in Chinese chemotherapy-refractory metastatic colorectal cancer patients. Medicine 2017, 96, e5946. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Chen, H.; Li, Q.; Song, Y.; Zhang, S.; Xu, X.S.; Xu, Y.; Chen, S. Assessment of the cardiac safety between cetuximab and panitumumab as single therapy in Chinese chemotherapy-refractory mCRC. Onco Targets Ther. 2017, 11, 123–129. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, F.; Zhang, R.; Sun, C.; Ran, Q.; Zhang, C.; Shen, C.; Yao, Z.; Wang, M.; Song, L.; Peng, C. Oxaliplatin-induced peripheral neurotoxicity in colorectal cancer patients: Mechanisms, pharmacokinetics and strategies. Front. Pharmacol. 2023, 14, 1231401. [Google Scholar] [CrossRef]
- Krarup-Hansen, A.; Rietz, B.; Krarup, C.; Heydorn, K.; Rorth, M.; Schmalbruch, H. Histology and platinum content of sensory ganglia and sural nerves in patients treated with cisplatin and carboplatin: An autopsy study. Neuropathol. Appl. Neurobiol. 1999, 25, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, A.G.; Balteskard, L.; Wanderas, E.H.; Frykholm, G.; Wilsgaard, T.; Dahl, O.; Guren, M.G. Impaired health-related quality of life after chemoradiotherapy for anal cancer: Late effects in a national cohort of 128 survivors. Acta Oncol. 2013, 52, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Pankratz, V.S.; Velazquez, A.I.; Aakre, J.A.; Loprinzi, C.L.; Staff, N.P.; Windebank, A.J.; Yang, P. Candidate pathway-based genetic association study of platinum and platinum-taxane related toxicity in a cohort of primary lung cancer patients. J. Neurol. Sci. 2015, 349, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Qi, Y.; Mica, Y.; Lee, G.; Zhang, X.J.; Niu, L.; Bilsland, J.; Cao, L.; Stevens, E.; Whiting, P.; et al. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012, 30, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, J.N.; Grimm, M.; Shinde, N.V.; Nolan, T.; Worthen-Chaudhari, L.; Williams, N.O.; Lustberg, M.B. Updates in the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Curr. Treat. Options Oncol. 2022, 23, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Anderson, E.M.; Bokrand-Donatelli, Y.; Neubert, J.K.; Caudle, R.M. Anti-nociceptive effect of a conjugate of substance P and light chain of botulinum neurotoxin type A. Pain 2013, 154, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, J.; Jiao, S.; Ji, T. Ganglioside-monosialic acid (GM1) prevents oxaliplatin-induced peripheral neurotoxicity in patients with gastrointestinal tumors. World J. Surg. Oncol. 2013, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Glimelius, B.; Manojlovic, N.; Pfeiffer, P.; Mosidze, B.; Kurteva, G.; Karlberg, M.; Mahalingam, D.; Buhl Jensen, P.; Kowalski, J.; Bengtson, M.; et al. Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx®): A placebo-controlled randomised phase II study (PLIANT). Acta Oncol. 2018, 57, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Szepanowski, F.; Derksen, A.; Steiner, I.; Meyer zu Hörste, G.; Daldrup, T.; Hartung, H.P.; Kieseier, B.C. Fingolimod promotes peripheral nerve regeneration via modulation of lysophospholipid signaling. J. Neuroinflamm. 2016, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, L.; Bernards, R. Rational combinations of targeted cancer therapies: Background, advances and challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef]
- Mohapatra, L.; Tripathi, A.S.; Mishra, D.; Parida, S.K.; Yasir, M.; Maurya, R.K.; Prajapati, B.G. Current drug therapy for colorectal cancer. In Colorectal Cancer 2024; Academic Press: Cambridge, MA, USA, 2024; pp. 115–148. [Google Scholar]
- Wu, J.; Wang, Z.; Jin, C.; Ren, H.; Hu, Y.; Yang, B.; Hu, Y. Effect of cetuximab combined with chemotherapy in treating metastatic colorectal cancer and its prognostic analysis. J. BUON 2021, 26, 101–108. [Google Scholar]
- Kasi, P.M.; Afable, M.G.; Herting, C.; Lukanowski, M.; Jin, Z. Anti-EGFR antibodies in the management of advanced colorectal cancer. Oncologist 2023, 28, 1034–1048. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, S.; Teng, X.; Zhong, L.; Liu, M.; Jin, Y.; Dong, M. A comparison of panitumumab and cetuximab in the treatment of KRAS wild-type metastatic colorectal cancer: A systematic review and meta-analysis. Immunopharmacol. Immunotoxicol. 2023, 45, 1–9. [Google Scholar] [CrossRef]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Panitumumab vs. bevacizumab added to standard first-line chemotherapy and overall survival among patients with RAS wild-type, left-sided metastatic colorectal cancer: A randomized clinical trial. JAMA 2023, 329, 1271–1282. [Google Scholar] [CrossRef]
- Chibani, H.; El Bairi, K.; Al Jarroudi, O.; Afqir, S. Bevacizumab in metastatic colorectal cancer in a real-life setting–toxicity profile, survival outcomes, and impact of tumor sidedness. Contemp. Oncol. Współczesna Onkol. 2022, 26, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Moisuc, D.C.; Marinca, M.V.; Matei, A.M.; Popovici, L.; Cianga, P. The Impact of Bevacizumab and Chemotherapy on Quality of Life in Metastatic Colorectal Cancer Patients. Healthcare 2023, 11, 591. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, R.; Xue, H.; Zhou, R.; Chen, Z. Clinical Efficacy and Adverse Effects of Bevacizumab in Combination with Chemotherapy for Metastatic Colorectal Cancer. Altern. Ther. Health Med. 2024, 31, 150–155. [Google Scholar]
- Manz, S.M.; Losa, M.; Fritsch, R.; Scharl, M. Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Ther. Adv. Gastroenterol. 2021, 14, 17562848211002018. [Google Scholar] [CrossRef]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Nasca, V.; Barretta, F.; Corti, F.; Lonardi, S.; Niger, M.; Elez, M.E.; Fakih, M.; Jayachandran, P.; Shah, A.T.; Salati, M.; et al. Association of immune-related adverse events with the outcomes of immune checkpoint inhibitors in patients with dMMR/MSI-H metastatic colorectal cancer. J. Immunother. Cancer 2023, 11, e005493. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef]
- Collins, M.; Soularue, E.; Marthey, L.; Carbonnel, F. Management of patients with immune checkpoint inhibitor-induced enterocolitis: A systematic review. Clin. Gastroenterol. Hepatol. 2020, 18, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Wang, X.; Mallepally, N.; Chen, E.; Altan, M.; Bresalier, R.S.; Charabaty, A.; Dadu, R.; Jazaeri, A.; et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2019, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleke, A.; Adebayo, A.S.; Agbaje, K.; Olajubutu, O.; Adesina, S.K. Colorectal Cancer: Therapeutic Approaches and Their Complications. Biomedicines 2025, 13, 1646. https://doi.org/10.3390/biomedicines13071646
Adeleke A, Adebayo AS, Agbaje K, Olajubutu O, Adesina SK. Colorectal Cancer: Therapeutic Approaches and Their Complications. Biomedicines. 2025; 13(7):1646. https://doi.org/10.3390/biomedicines13071646
Chicago/Turabian StyleAdeleke, Adebisi, Amusa S. Adebayo, Kafilat Agbaje, Oluwabukunmi Olajubutu, and Simeon K. Adesina. 2025. "Colorectal Cancer: Therapeutic Approaches and Their Complications" Biomedicines 13, no. 7: 1646. https://doi.org/10.3390/biomedicines13071646
APA StyleAdeleke, A., Adebayo, A. S., Agbaje, K., Olajubutu, O., & Adesina, S. K. (2025). Colorectal Cancer: Therapeutic Approaches and Their Complications. Biomedicines, 13(7), 1646. https://doi.org/10.3390/biomedicines13071646





