Differential Effects of Gynecological and Chronological Age on Low Birth Weight and Small for Gestational Age
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Procedures and Data Collection
2.2.1. Maternal Data
2.2.2. Birth Information
2.2.3. Newborn Data
2.3. Ethical Considerations
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Low Birth Weight and Small for Gestational Age
4.2. Age of Menarche
4.3. Gynecological Age
4.4. Early Motherhood in Adolescence
4.5. Anemia
4.6. Sociodemographic Factors
4.7. Strengths and Limitations
4.8. Implications
5. Conclusions
- Prioritize the treatment of maternal anemia;
- Reduce the number of adolescent pregnancies;
- Provide adequate prenatal care to young pregnant women.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| GA | gynecological age |
| GWG | gestational weight gain |
| IOM | Institute of Medicine |
| IUGR | intrauterine growth restriction |
| LBW | low birth weight |
| LGA | large for gestational age |
| OR | odds ratio |
References
- World Health Organization. The Global Health Observatory. SDG Target 3.1 Reduce the Global Maternal Mortality Ratio to Less Than 70 per 100,000 Live Births. Available online: https://www.who.int/data/gho/data/themes/topics/sdg-target-3-1-maternal-mortality (accessed on 30 May 2025).
- Pan American Health Organization; World Health Organization. Adolescent Pregnancy in Latin America and Caribbean, August 2020, PAHO/FPL/HL/20-0019. Available online: https://iris.paho.org/handle/10665.2/53133 (accessed on 30 May 2025).
- Neal, S.; Channon, A.A.; Chintsanya, J. The impact of young maternal age at birth on neonatal mortality: Evidence from 45 low and middle-income countries. PLoS ONE 2018, 13, e0195731. [Google Scholar] [CrossRef]
- Tigabu, S.; Liyew, A.M.; Geremew, B.M. Modeling spatial determinates of teenage pregnancy in Ethiopia; geographically weighted regression. BMC Womens Health 2021, 21, 254. [Google Scholar] [CrossRef]
- Emagneneh, T.; Mulugeta, C.; Susu, B.; Alamrew, A.; Ejigu, B.; Tsegaye, D. Adverse obstetrical outcomes among adolescents in North Wollo Zone Governmental hospitals, Northern Ethiopia. Sci. Rep. 2025, 15, 5696. [Google Scholar] [CrossRef]
- de Vienne, C.M.; Creveuil, C.; Dreyfus, M. Does young maternal age increase the risk of adverse obstetric, fetal and neonatal outcomes: A cohort study? Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 147, 151–156. [Google Scholar] [CrossRef]
- Kagawa, M.N.; Owori, O.A.; Nakalembe, M. Pregnancy Outcomes Among Teenagers at a National Referral Hospital in Uganda. Int. J. Reprod. Med. 2024, 2024, 6975966. [Google Scholar] [CrossRef]
- Chen, X.K.; Wen, S.W.; Fleming, N.; Demissie, K.; Rhoads, G.G.; Walker, M. Teenage pregnancy and adverse birth outcomes: A large population based retrospective cohort study. Int. J. Epidemiol. 2007, 36, 368–373. [Google Scholar] [CrossRef]
- Stevens-Simon, C.; Beach, R.K.; McGregor, J.A. Does incomplete growth and development predispose teenagers to preterm delivery? A template for research. J. Perinatol. 2002, 22, 315–323. [Google Scholar] [CrossRef]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Mendoza-Flores, M.E.; Flores-Quijano, M.E.; Gamboa, R.; Luna-Hidalgo, A.; Restrepo-Mesa, S.L.; Mier-Cabrera, J.; Peña-Camacho, G. Low Antenatal Care Number of Consultations Is Associated with Gestational Weight Gain and Birth Weight of Offspring of Teenage Mothers: A Study Based on Colombian and Mexican Cohorts. Nutrients 2024, 16, 3726. [Google Scholar] [CrossRef]
- Socolov, D.G.; Iorga, M.; Carauleanu, A.; Ilea, C.; Blidaru, I.; Boiculese, L.; Socolov, R.V. Pregnancy during Adolescence and Associated Risks: An 8-Year Hospital-Based Cohort Study (2007–2014) in Romania, the country with the Highest Rate of Teenage Pregnancy in Europe. BioMed Res. Int. 2017, 2017, 9205016. [Google Scholar] [CrossRef]
- Karai, A.; Gyurkovits, Z.; Nyári, T.A.; Sári, T.; Németh, G.; Orvos, H. Adverse perinatal outcome in teenage pregnancies: An analysis of a 5-year period in Southeastern Hungary. J. Matern. Fetal Neonatal Med. 2019, 32, 2376–2379. [Google Scholar] [CrossRef]
- Gibbs, C.M.; Wendt, A.; Peters, S.; Hogue, C.J. The impact of early age at first childbirth on maternal and infant health. Paediatr. Perinat. Epidemiol. 2012, 26, 259–284. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund (UNICEF-2004). Low Birthweight: Country, Regional and Global Estimates; World Health Organization: Geneva, Switzerland, 2004; Available online: https://iris.who.int/handle/10665/43184 (accessed on 30 May 2025).
- United Nations Children’s Fund. Child Marriage: Latest Trends and Future Prospects; UNICEF: New York, NY, USA, 2018. [Google Scholar]
- Gobierno de México. Decreto Por el Que se Reforma y Derogan Diversas Disposiciones del Código Civil Federal, en Materia de Prohibición del Matrimonio Infantil. Available online: https://www.diputados.gob.mx/sedia/biblio/prog_leg/Prog_leg_LXIV/038_DOF_03jun19.pdf (accessed on 30 May 2025).
- Socioeconomic Level Index of the Mexican Association of Market Research and Public Opinion Agencies (AMAI) September 2014 AMAI Regulation NSE 8 × 7. Available online: https://www.amai.org/descargas/NOTA_METODOLOGICA_NSE_AMAI_2024_v6.pdf (accessed on 30 May 2025).
- Phipps, M.G.; Sowers, M. Defining early adolescent childbearing. Am. J. Public Health 2002, 92, 125–128. [Google Scholar] [CrossRef]
- Kaplanoglu, M.; Bülbül, M.; Konca, C.; Kaplanoglu, D.; Tabak, M.S.; Ata, B. Gynecologic age is an important risk factor for obstetric and perinatal outcomes in adolescent pregnancies. Women Birth 2015, 28, e119–e123. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016; ISBN 9789241549912. [Google Scholar]
- World Health Organization (WHO) Multicentre Growth Reference Study Group. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. 2006, 450, 76–85. [Google Scholar]
- Institute of Medicine (IOM); National Research Council (NRC). Weight Gain During Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Sámano, R.; Martínez-Rojano, H.; Chico-Barba, G.; Gamboa, R.; Tolentino, M.; Toledo-Barrera, A.X.; Ramírez-González, C.; Mendoza-Flores, M.E.; Hernández-Trejo, M.; Godinez-Martínez, E. Serum Folate, Red Blood Cell Folate, and Zinc Serum Levels Are Related with Gestational Weight Gain and Offspring’s Birth-Weight of Adolescent Mothers. Nutrients 2024, 16, 1632. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011; Available online: https://iris.who.int/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?sequence=22 (accessed on 30 May 2025).
- Xie, L.; Liang, Z.; Wang, X.; Luo, X. The prevalence of preterm and low birth weight infants among migrant women in the Pearl River Delta region, China: A population-based birth cohort study. BMC Public Health 2024, 24, 1179. [Google Scholar] [CrossRef]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International Standards for Newborn Weight, Length, and Head Circumference by Gestational Age and Sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Lohman, T.; Roche, A.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1998; pp. 28–80. [Google Scholar]
- United Nations Children’s Fund-World Health Organization (UNICEF-WHO). Low Birthweight Estimates, 2023. Note: This Map Does Not Reflect a Position by UNICEF on the Legal Status of any Country or Territory or the Delimitation of any Frontiers. Available online: https://data.unicef.org/topic/nutrition/low-birthweight/ (accessed on 30 May 2025).
- Wong, S.P.W.; Twynstra, J.; Gilliland, J.A.; Cook, J.L.; Seabrook, J.A. Risk Factors and Birth Outcomes Associated with Teenage Pregnancy: A Canadian Sample. J. Pediatr. Adolesc. Gynecol. 2020, 33, 153–159. [Google Scholar] [CrossRef]
- Jain, L.H.; Van Eyk, N.; Woolcott, C.; Kuhle, S. Characteristics and Outcomes of Adolescent Births in Nova Scotia: A Retrospective Cohort Study. J. Obstet. Gynaecol. Can. 2018, 40, 1459–1465. [Google Scholar] [CrossRef]
- Samsury, S.F.; Tengku Ismail, T.A.; Hassan, R. Low birth weight infant among teenage pregnancy in Terengganu, Malaysia: A cross-sectional study. Malays. Fam. Physician 2022, 17, 44–51. [Google Scholar] [CrossRef]
- Okwaraji, Y.B.; Krasevec, J.; Bradley, E.; Conkle, J.; Stevens, G.A.; Gatica-Domínguez, G.; Ohuma, E.O.; Coffey, C.; Estevez Fernandez, D.G.; Blencowe, H.; et al. National, regional, and global estimates of low birthweight in 2020, with trends from 2000: A systematic analysis. Lancet 2024, 403, 1071–1080. [Google Scholar] [CrossRef]
- Adugna, D.G.; Worku, M.G. Maternal and neonatal factors associated with low birth weight among neonates delivered at the University of Gondar comprehensive specialized hospital, Northwest Ethiopia. Front. Pediatr. 2022, 10, 899922. [Google Scholar] [CrossRef]
- Lee, J.A.; Sohn, J.A.; Oh, S. Risk factors and influence on neurodevelopmental outcomes of neonatal seizures in very low birth weight infants based on nationwide cohort. Sci. Rep. 2025, 15, 10875. [Google Scholar] [CrossRef]
- Aboagye, R.G.; Ahinkorah, B.O.; Seidu, A.A.; Frimpong, J.B.; Archer, A.G.; Adu, C.; Hagan, J.E., Jr.; Amu, H.; Yaya, S. Birth weight and nutritional status of children under five in sub-Saharan Africa. PLoS ONE. 2022, 17, e0269279. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Restrepo, J.M. Low Birthweight as a Risk Factor for Non-Communicable Diseases in Adults. Front. Med. 2022, 8, 793990. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF); World Health Organization (WHO). Low Birthweight Estimates: Levels and Trends 2000–2015; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Hinojosa-Gonzalez, D.E.; Ramonfaur, D.; Morales-Palomino, K.L.; Tellez-Giron, V.C.; Latapi, X.; Insua, J.; Hernández-Escobar, C.; Apodaca-Ramos, I.; Flores-Villalba, E. Relationship of age at menarche, coitarche and first gestation: A retrospective cohort analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2023, 18, 100189. [Google Scholar] [CrossRef]
- Robellada-Zárate, C.M.; Zapata-Caballero, C.A.; Morales-Hernández, F.V.; Flores-Robles, C.M.; Roque-Sánchez, A.M.; Kably-Ambe, A. Tendencia secular de la menarquia en la población mexicana. Ginecol. Obstet. México 2024, 92, 359–363. [Google Scholar]
- Marván, M.L.; Catillo-López, R.L.; Alcalá-Herrera, V.; Callejo, D. The decreasing age at menarche in Mexico. J. Pediatr. Adolesc. Gynecol. 2016, 29, 454–457. [Google Scholar] [CrossRef]
- Jansen, E.C.; Herrán, O.F.; Villamor, E. Trends and correlates of age at menarche in Colombia: Results from a nationally representative survey. Econ. Hum. Biol. 2015, 19, 138–144. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.H.; Oh, M.; Lee, K.; Park, M. Age at menarche in Korean adolescents: Trends and influencing factors. Reprod. Health 2016, 13, 121. [Google Scholar] [CrossRef]
- Cabrera, S.M.; Bright, G.M.; Frane, J.W.; Blethen, S.L.; Lee, P.A. Age of thelarche and menarche in contemporary US females: A cross-sectional analysis. J. Pediatr. Endocrinol. Metab. 2014, 27, 47–51. [Google Scholar] [CrossRef]
- Dunbar, J.; Sheeder, J.; Lezotte, D.; Dabelea, D.; Stevens-Simon, C. Age at menarche and first pregnancy among psychosocially at-risk adolescents. Am. J. Public Health 2008, 98, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singh, M.P.; Dhillon, B.S.; Saxena, N.C. Preparing for adulthood--patterns of physical growth, sexual maturity and menarche of adolescent girls in selected urban slums and rural areas. J. Indian Med. Assoc. 2007, 105, 119–122, 126, Erratum in J. Indian Med. Assoc. 2007, 105, 230. [Google Scholar] [PubMed]
- Habiba, M.; Heyn, R.; Bianchi, P.; Brosens, I.; Benagiano, G. The development of the human uterus: Morphogenesis to menarche. Hum. Reprod. Update 2021, 27, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Marino, J.L.; Skinner, S.R.; Doherty, D.A.; Rosenthal, S.L.; Cooper Robbins, S.C.; Cannon, J.; Hickey, M. Age at menarche and age at first sexual intercourse: A prospective cohort study. Pediatrics 2013, 132, 1028–1036. [Google Scholar] [CrossRef]
- Blanquet-García, J.; Montoya-C’azarez, A.; Carranza-Lira, S. Características sociodemográficas de la adolescente embarazada en un hospital de alta especialidad. Rev. Médica Inst. Mex. Seguro Soc. 2016, 54, 238–241. [Google Scholar]
- Piras, G.N.; Bozzola, M.; Bianchin, L.; Bernasconi, S.; Bona, G.; Lorenzoni, G.; Buzi, F.; Rigon, F.; Tonini, G.; De Sanctis, V.; et al. The levelling-off of the secular trend of age at menarche among Italian girls. Heliyon 2020, 6, e04222. [Google Scholar] [CrossRef]
- Masyitah, S.; Kusharisupeni, A. Comparison of the Relationship Between Gynecological Age with Birth Weight and Chronological Age with Birth Weight in Teenage Mothers in Kota Bekasi West Java. Sociol. Anthropol. 2018, 6, 275–282. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The Sexual and Reproductive Health of Younger Adolescents: Research Issues in Developing Countries; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Demirci, O.; Yılmaz, E.; Tosun, Ö.; Kumru, P.; Arınkan, A.; Mahmutoğlu, D.; Selçuk, S.; Dolgun, Z.N.; Arısoy, R.; Erdoğdu, E.; et al. Effect of Young Maternal Age on Obstetric and Perinatal Outcomes: Results from the Tertiary Center in Turkey. Balk. Med. J. 2016, 33, 344–349. [Google Scholar] [CrossRef]
- Felice, M.E.; James, M.; Shragg, P.; Hollingsworth, D.R. Observations related to chronologic and gynecologic age in pregnant adolescents. Yale J. Biol. Med. 1984, 57, 777–785. [Google Scholar]
- Rah, J.H.; Christian, P.; Shamim, A.A.; Arju, U.T.; Labrique, A.B.; Rashid, M. Pregnancy and lactation hinder growth and nutritional status of adolescent girls in rural Bangladesh. J. Nutr. 2008, 138, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Rah, J.H.; Shamim, A.A.; Arju, U.T.; Labrique, A.B.; Klemm, R.D.; Rashid, M.; Christian, P. Difference in ponderal growth and body composition among pregnant vs. never-pregnant adolescents varies by birth outcomes. Matern. Child Nutr. 2010, 6, 27–37. [Google Scholar] [CrossRef]
- Grobeisen-Duque, O.; Villavicencio-Carrisoza, O.; Mora-Vargas, C.D.; Arteaga-Lopez, C.P.; Martinez-Salazar, M.G.; Rosas-Balan, A.; León-Juárez, M.; Villegas-Mota, M.I.; Zaga-Clavellina, V.; Aguilera-Arreola, M.G.; et al. Impact of Pre-Gestational BMI and Gestational Weight Gain on Fetal Development Outcomes in Adolescent Pregnant Women. J. Clin. Med. 2024, 13, 1839. [Google Scholar] [CrossRef]
- Santos, S.F.M.D.; Costa, A.C.C.D.; Araújo, R.G.P.D.S.; Silva, L.A.T.; Gama, S.G.N.D.; Fonseca, V.M. Factors associated with the adequacy of gestational weight gain among Brazilian teenagers. Ciência Saúde Coletiva 2022, 27, 2629–2642, (In Portuguese, English). [Google Scholar] [CrossRef]
- Scholl, T.O.; Hediger, M.L.; Salmon, R.W.; Belsky, D.H.; Ances, I.G. Association between low gynaecological age and preterm birth. Paediatr. Perinat. Epidemiol. 1989, 3, 357–366. [Google Scholar] [CrossRef]
- Sharma, V.; Katz, J.; Mullany, L.C.; Khatry, S.K.; LeClerq, S.C.; Shrestha, S.R.; Darmstadt, G.L.; Tielsch, J.M. Young maternal age and the risk of neonatal mortality in rural Nepal. Arch. Pediatr. Adolesc. Med. 2008, 162, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.; Fuentes-Rivera, E.; Schiavon, R.; Darney, B.G. Prenatal Care Utilization and Perinatal Outcomes Among Pregnant Adolescents in Mexico, 2008–2019. Int. J. Gynaecol. Obstet. 2024, 165, 1047–1055. [Google Scholar] [CrossRef]
- Markovi’c, S.; Cerovac, A.; Cerovac, E.; Markovi’c, D.; Bogdanovi’c, G.; Kunosi’c, S. Antenatal Care and Weight Gain in Adolescent Compared to Adult Pregnancy. Int. J. Prev. Med. 2020, 11, 115. [Google Scholar]
- Amoadu, M.; Ansah, E.W.; Assopiah, P.; Acquah, P.; Ansah, J.E.; Berchie, E.; Hagan, D.; Amoah, E. Socio-Cultural Factors Influencing Adolescent Pregnancy in Ghana: A Scoping Review. BMC Pregnancy Childbirth 2022, 22, 834. [Google Scholar] [CrossRef]
- Banke-Thomas, O.E.; Banke-Thomas, A.O.; Ameh, C.A. Factors Influencing Utilisation of Maternal Health Services by Adolescent Mothers in Low-and Middle-Income Countries: A Systematic Review. BMC Pregnancy Childbirth 2017, 17, 65. [Google Scholar] [CrossRef]
- Uzunov, A.V.; Cîrstoiu, M.M.; Secară, D.C.; Crîngu-Ionescu, A.; Matei, A.; Mehedințu, C.; Varlas, V.N. Mode of Delivery and Neonatal Outcome in Adolescent Pregnancy (13–16 Years Old) Associated with Anemia. Medicina 2022, 58, 1796. [Google Scholar] [CrossRef]
- Rammohan, A.; Chu, H.; Awofeso, N.; Goli, S. Adolescent pregnancy, maternal and child anaemia: Empirical analysis from India, Bangladesh, and Nigeria. Matern. Child Nutr. 2025, 21, e13723. [Google Scholar] [CrossRef]
- Brosens, I.; Muter, J.; Gargett, C.E.; Puttemans, P.; Benagiano, G.; Brosens, J.J. The impact of uterine immaturity on obstetrical syndromes during adolescence. Am. J. Obstet. Gynecol. 2017, 217, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Delpishe, A.; Azami, M.; Hafezi Ahmadi, M.R.; Sayehmiri, K. Maternal Anemia during pregnancy and infant low birth weight: A systematic review and Meta-analysis. Int. J. Reprod. Biomed. 2017, 15, 125–134. [Google Scholar] [CrossRef]
- Rahmati, S.; Delpisheh, A.; Parizad, N.; Sayehmiri, K. Maternal anemia and pregnancy outcomes: A systematic review and meta-analysis. Int. J. Pediatr. 2016, 4, 3323–3342. [Google Scholar]
- Jung, J.; Rahman, M.M.; Rahman, M.S.; Swe, K.T.; Islam, M.R.; Rahman, M.O.; Akter, S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Sukrat, B.; Wilasrusmee, C.; Siribumrungwong, B.; McEvoy, M.; Okascharoen, C.; Attia, J.; Thakkinstian, A. Hemoglobin concentration and pregnancy outcomes: A systematic review and meta-analysis. Biomed. Res. Int. 2013, 2013, 769057. [Google Scholar] [CrossRef]
- Dorsamy, V.; Bagwandeen, C.; Moodley, J. The prevalence, risk factors and outcomes of anaemia in South African pregnant women: A systematic review and meta-analysis. Syst. Rev. 2022, 11, 16. [Google Scholar] [CrossRef]
- Figueiredo, A.C.M.G.; Gomes-Filho, I.S.; Silva, R.B.; Pereira, P.P.S.; Mata, F.A.F.D.; Lyrio, A.O.; Souza, E.S.; Cruz, S.S.; Pereira, M.G. Maternal Anemia and Low Birth Weight: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 601. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abe, S.K.; Rahman, M.S.; Kanda, M.; Narita, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 495–504. [Google Scholar] [CrossRef]
- Xiong, X.; Buekens, P.; Alexander, S.; Demianczuk, N.; Wollast, E. Anemia during pregnancy and birth outcome: A meta-analysis. Am. J. Perinatol. 2000, 17, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Oaks, B.M.; Tandon, S.; Martorell, R.; Dewey, K.G.; Wendt, A.S. Maternal hemoglobin concentrations across pregnancy and maternal and child health: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Deressa, A.T.; Cherie, A.; Belihu, T.M.; Tasisa, G.G. Factors associated with spontaneous preterm birth in Addis Ababa public hospitals, Ethiopia: Cross sectional study. BMC Pregnancy Childbirth 2018, 18, 332. [Google Scholar] [CrossRef] [PubMed]
- Adane, A.A.; Ayele, T.A.; Ararsa, L.G.; Bitew, B.D.; Zeleke, B.M. Adverse birth outcomes among deliveries at Gondar University Hospital, Northwest Ethiopia. BMC Pregnancy Childbirth 2014, 14, 90. [Google Scholar] [CrossRef]
- Moran, V.H. Nutritional status in pregnant adolescents: A systematic review of biochemical markers. Matern. Child Nutr. 2007, 3, 74–93. [Google Scholar] [CrossRef]
- Nshutiyukuri, C.; Uwingabire, F.; Musabwasoni, M.G.S.; Rutayisire, J.B.; Rutayisire, R.; Benimana, I.; Kaberuka, G.; Bazakare Ishimwe, L.; Kyame, K.B.; Mutabazi, L.; et al. Perceived factors contributing to teenage pregnancy and their perceived effects on teenage females health in eastern province of Rwanda. Womens Health 2025, 21, 17455057251325044. [Google Scholar] [CrossRef]
- Faith, E.G.; Ikwara, E.A.; Marvin, M.; Isiko, I. Teenage Pregnancy and Its Associated Factors Among Girls Aged 13–19 Years in Apac District, Uganda: A Community-Based Cross-Sectional Study. Health Sci. Rep. 2025, 8, e70471. [Google Scholar] [CrossRef]
- Diabelková, J.; Rimárová, K.; Dorko, E.; Urdzík, P.; Houžvičková, A.; Argalášová, Ľ. Adolescent Pregnancy Outcomes and Risk Factors. Int. J. Environ. Res. Public Health 2023, 20, 4113. [Google Scholar] [CrossRef]
- Dorn, L.D.; Sontag-Padilla, L.M.; Pabst, S.; Tissot, A.; Susman, E.J. Longitudinal reliability of self-reported age at menarche in adolescent girls: Variability across time and setting. Dev. Psychol. 2013, 49, 1187–1193. [Google Scholar] [CrossRef]
- Mao, Y.; Lian, Q.; Zuo, X.; Zhang, Y.; Luo, S.; Zhang, S.; Tu, X.; Lou, C.; Zhou, W. Validity of self-reported age at menarche in computer-assisted interview among Chinese schoolgirls: A cross-sectional study. BMJ Open 2018, 8, e016799. [Google Scholar] [CrossRef]
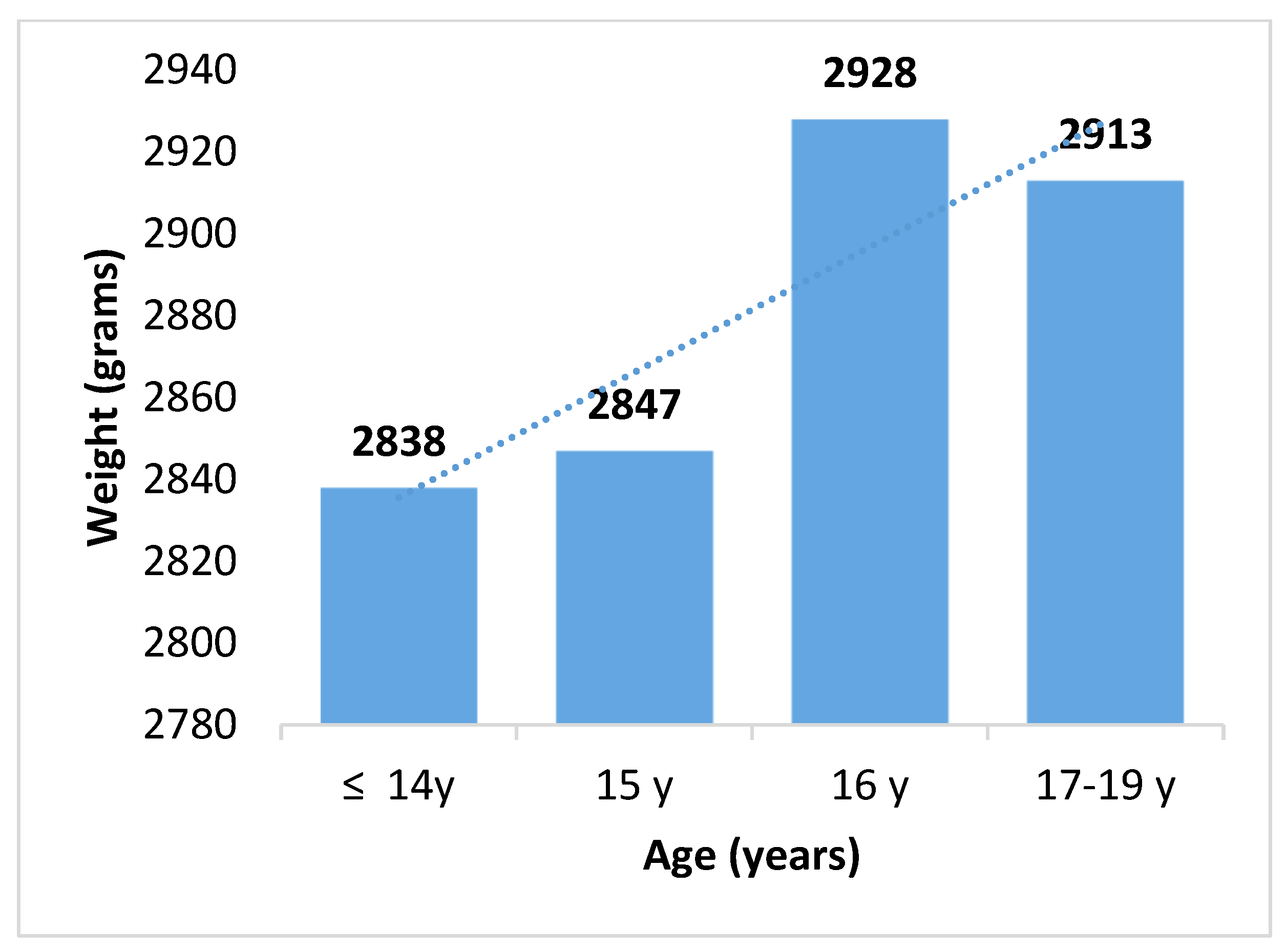
| Variable | Chronological Age, n (%) | p-Value * | |
|---|---|---|---|
| <15 y, n = 491 (40) | ≥15 y, n = 751 (60) | ||
| Menarche age (y) a | 11.3 ± 1 | 11.7 ± 1 | 0.001 |
| Gynecological age (y) a | 3.1 ± 1 | 4.8 ± 1 | 0.001 |
| Pre-pregnancy weight (kg) a | 51 (46–58) | 51 (47–58) | 0.359 |
| Height (cm) a | 155.5 ± 5 | 155.7 ± 5 | 0.657 |
| Height | |||
| Less than 150 cm | 79 (16) | 122 (16) | 0.942 |
| pBMI (kg/m2) a | 21.5 ± 3 | 21.7 ± 3 | 0.262 |
| Low weight | 98 (20) | 113 (15) | 0.039 |
| Normal weight | 332 (68) | 530 (70) | |
| Overweight | 51(10) | 89 (12) | |
| Obesity | 10 (2) | 19 (3) | |
| Final gestational weight (kg) b | 64 (58–72) | 63 (58–71) | 0.111 |
| Gestational weight gain (kg) b | 13 (9–16) | 12 (8–16) | 0.551 |
| Adequacy of GWG (%) b | 110 (79–158) | 98 (71–140) | 0.018 |
| Hemoglobin (g/dL) a | 12.4 ± 1 | 12.6 ± 1 | 0.009 |
| Start of prenatal care (weeks) | 24 (20–30) | 25 (20–29) | 0.097 |
| 1st trimester | 10 (2) | 9 (1) | 0.505 |
| 2nd trimester | 368 (75) | 570 (76) | |
| 3rd trimester | 113 (23) | 172 (23) | |
| Less than 8 antenatal visit | 478 (97) | 741 (99) | 0.073 |
| 8 and more antenatal visit | 13 (3) | 10 (1) | |
| Mode of delivery | |||
| Cesarean section | 273 (56) | 385 (51) | 0.075 |
| Gestational age at delivery (weeks) | 38.2 ± 2 | 39 ± 2 | 0.001 |
| Birth weight (g) | 2877 (2688–3070) | 2943 (2670–3206) | 0.015 |
| Small for gestational age (<10 percentile) | 92 (19) | 162 (22) | 0.260 |
| Adequate for gestational age (10–90 percentile) | 392 (80) | 578 (77) | |
| Large for gestational age (>90 percentile) | 7 (1) | 11 (1) | |
| Low birth weight (≤2500 g) | 74 (15) | 116 (15) | 0.016 |
| Adequate birth weight (2501–3999 g) | 401 (82) | 583 (78) | |
| Macrosomic (≥4000 g) | 16 (3) | 52 (7) | |
| Sociodemographic variables | |||
| Occupation | 0.358 | ||
| Homemaker/housekeeper | 434 (88) | 670 (89) | |
| Works outside the home or student | 26 (14) | 31 (10) | |
| Educational level | 0.001 | ||
| Elementary | 277 (56) | 153 (20) | |
| Secondary | 214 (44) | 560 (75) | |
| High school | 0 (0) | 38 (5) | |
| Educational lag | 251 (51) | 363 (48) | 0.184 |
| Socioeconomic level | 0.163 | ||
| Middle low | 357 (73) | 566 (75) | |
| Very low | 134 (27) | 185 (25) | |
| Family structure | 0.320 | ||
| Nuclear | 263 (56) | 389 (55) | |
| Extended/blended | 205 (44) | 323 (45) | |
| Marital status | 0.001 | ||
| Cohabiting | 170 (35) | 335 (45) | |
| Single | 321 (65) | 416 (55) | |
| Residence | 0.440 | ||
| Urban (<50 km) | 481 (64) | 319 (65) | |
| Suburban or rural (>50 km) | 270 (36) | 172 (35) | |
| Variable | Gynecological Age, n (%) | p-Value * | |
|---|---|---|---|
| <3 y, n = 175 (14) | ≥3 y, n = 1067 (86) | ||
| Menarche age (y) a | 12.6 ± 1 | 11.3 ± 1 | 0.001 |
| Pre-pregnancy weight (kg) a | 51 (46–58) | 51 (47–58) | 0.359 |
| Height (cm) a | 155.6 ± 5 | 155.7 ± 5 | 0.657 |
| Less than 150 cm | 26 (15) | 175 (16) | 0.349 |
| pBMI (kg/m2) a | 21.6 ± 3 | 21.6 ± 3 | 0.097 |
| Low weight | 36 (21) | 175 (16) | 0.218 |
| Normal weight | 110 (63) | 752 (71) | |
| Overweight | 25 (14 | 115 (11) | |
| Obesity | 4 (2) | 25 (2) | |
| Final gestational weight (kg) | 64 (56–74) | 63 (58–71) | 0.579 |
| Gestational weight gain (kg) | 13 (10–16) | 12 (8–16) | 0.551 |
| Adequacy of GWG (%) | 110 (79–167) | 103 (72–143) | 0.018 |
| Hemoglobin (g/dL) a | 12.4 ± 1 | 12.6 ± 1 | 0.008 |
| Start of prenatal care (weeks) b | 25 (20–31) | 25 (20–29) | 0.538 |
| 1st trimester | 4 (2) | 15 (1) | 0.679 |
| 2nd trimester | 131 (75) | 807 (76) | |
| 3rd trimester | 40 (23) | 245 (23) | |
| Less than 8 antenatal visit | 171 (98) | 1048 (98) | 0.411 |
| 8 and more antenatal visit | 4 (2) | 19 (2) | |
| Mode of delivery | |||
| Cesarean- section | 81 (46) | 503 (47) | 0.449 |
| Gestational age at delivery (weeks) a | 38 ± 1 | 39 ± 1 | 0.001 |
| Bith weight (g) b | 2826 (2648–3022) | 2952 (2682–3214) | 0.001 |
| Small for gestational age (<10 percentile) | 38 (21.5) | 216 (20) | 0.475 |
| Adequate for gestational age (10–90 percentile) | 136 (78) | 834 (78) | |
| Large for gestational age (>90 percentile) | 1 (0.5) | 17 (2) | |
| Low birth weight (≤2500 g) | 30 (17.2) | 160 (15) | 0.008 |
| Adequate birth weight (2501–3999 g) | 144 (82.3) | 840 (79) | |
| Macrosomic (≥4000 g) | 1 (0.5) | 67 (6) | |
| Sociodemographic variables | |||
| Occupation | 0.153 | ||
| Homemaker/housekeeper | 160 (91) | 944 (89) | |
| Work out at home or student | 15 (9) | 123 (11) | |
| Educational level | |||
| Elementary | 97 (55) | 333 (31) | 0.001 |
| Secondary | 77 (44) | 697 (65) | |
| High school | 1 (0.5) | 37 (4) | |
| Educational lag | 87 (50) | 527 (49) | 0.501 |
| Economic level | |||
| Middle low | 128 (73) | 795 (74) | 0.382 |
| Very low | 47 (27) | 272 (26) | |
| Family structure | |||
| Nuclear | 109 (62.3) | 576 (54) | 0.036 |
| Extended/blended | 66 (37.7) | 491 (46) | |
| Marital status | |||
| Cohabiting | 56 (32) | 449 (42) | 0.007 |
| Single | 119 (68) | 618 (58) | |
| Residence | 0.320 | ||
| Urban (<50 km) | 105 (60) | 693 (65) | |
| Suburban or rural (>50 km) | 70 (40) | 374 (35) | |
| Variable | Fetal Growth, n (%) | p-Value | |
|---|---|---|---|
| SGA, n = 254 (20) | Adequate + LGA, n = 988 (80) | ||
| Maternal age | 0.679 | ||
| <14 y | 34 (13) | 152 (15) | |
| 15 y | 58 (23) | 247 (25) | |
| 16 y | 100 (40) | 366 (38) | |
| 17–19 y | 62 (24) | 223 (22) | |
| Height | 0.152 | ||
| <150 cm | 47 (18) | 154 (16) | |
| ≥150 cm | 207 (82) | 834 (84) | |
| pBMI | 0.056 | ||
| Low weight | 52 (20) | 159 (16) | |
| Normal weight | 166 (66) | 696 (70) | |
| Overweight | 34 (13) | 106 (11) | |
| Obesity | 2 (1) | 27 (3) | |
| Gestational weight gain | 0.020 | ||
| Insufficient | 118 (46) | 365 (37) | |
| Adequate | 58 (23) | 278 (28) | |
| Excessive | 78 (31) | 345 (35) | |
| Hemoglobin (g/dL) | 0.001 | ||
| <11.5 | 100 (34) | 101 (10) | |
| ≥11.5 | 154 (61) | 887 (90) | |
| Gynecological age (y) | 0.360 | ||
| <3 | 38 (15) | 137 (14) | |
| ≥3 | 216 (85) | 851 (86) | |
| Menarcheal age (y) | 0.001 | ||
| ≤11 | 88 (35) | 466 (47) | |
| >12 | 166 (65) | 522 (53) | |
| Gestational age at birth | 0.001 | ||
| Term | 213 (84) | 878 (89) | |
| Preterm | 41 (16) | 110 (11) | |
| Mode of delivery | 0.033 | ||
| Vaginal | 121 (48) | 537 (54) | |
| Cesarean-section | 133 (52) | 451 (46) | |
| Start of prenatal care | |||
| 1st trimester | 1 (0.4) | 18 (2) | 0.202 |
| 2nd trimester | 190 (74.8) | 748 (76) | |
| 3rd trimester | 63 (24.8) | 222 (22) | |
| Less than 8 antenatal visits | 251 (99) | 968 (98) | 0.276 |
| 8 and more antenatal visits | 3 (1) | 20 (2) | |
| Socioeconomic level | 0.001 | ||
| Middle low | 159 (63) | 764 (77) | |
| Very low | 95 (37) | 224 (23) | |
| Marital status | 0.295 | ||
| Cohabiting | 99 (39) | 406 (41) | |
| Single | 155 (61) | 582 (59) | |
| Residence | 0.001 | ||
| Urban (<50 km) | 124 (49) | 692 (70) | |
| Suburban or rural (>50 km) | 130 (51) | 296 (30) | |
| Variable | Birth Weight by 2500 g as Reference, n (%) | p-Value * | |
|---|---|---|---|
| LBW, n = 190 (15.3) | NLBW, n = 1052 (84.7) | ||
| Maternal age | 0.010 | ||
| <14 y | 26 (14) | 160 (15) | |
| 15 y | 48 (25) | 257 (24) | |
| 16 y | 56 (29) | 410 (39) | |
| 17–19 y | 60 (32) | 225 (21) | |
| Height | 0.021 | ||
| <150 cm | 41 (22) | 160 (15) | |
| ≥150 cm | 149 (78) | 892 (85) | |
| pBMI | 0.184 | ||
| Low weight | 12 (6) | 37 (4) | |
| Normal weight | 150 (79) | 815 (78) | |
| Overweight | 20 (11) | 142 (14) | |
| Obesity | 8 (4) | 58 (6) | |
| Gestational weight gain | 0.796 | ||
| Insufficient | 78 (41) | 405 (39) | |
| Adequate | 49 (26) | 287 (27) | |
| Excessive | 63 (33) | 360 (34) | |
| Hemoglobin (g/dL) | 0.001 | ||
| <11.5 | 56 (30) | 145 (14) | |
| ≥11.5 | 134 (70) | 907 (86) | |
| Gynecological age (y) | 0.264 | ||
| <3 y | 30 (16) | 145 (14) | |
| ≥3 y | 160 (84) | 907 (86) | |
| Menarcheal age (y) | 0.161 | ||
| <11 | 78 (41) | 476 (45) | |
| ≥11 | 112 (59) | 579 (55) | |
| Gestational age at birth | 0.001 | ||
| Term | 72 (38) | 1019 (97) | |
| Preterm | 118 (62) | 33 (3) | |
| Mode of delivery | 0.006 | ||
| Vaginal | 82 (43) | 576 (55) | |
| Cesarean section | 108 (57) | 476 (45) | |
| Start of prenatal care | |||
| 1st trimester | 3 (2) | 16 (2) | 0.139 |
| 2nd trimester | 154 (81) | 784 (74) | |
| 3rd trimester | 33 (17) | 252 (24) | |
| Less than 8 antenatal visits | 187 (98) | 1032 (98) | 0.523 |
| 8 and more antenatal visits | 3 (2) | 20 (2) | |
| Marital status | 0.139 | ||
| Cohabiting | 70 (37) | 435 (41) | |
| Single | 120 (63) | 617 (59) | |
| Socioeconomic level | 0.028 | ||
| Middle low | 130 (68) | 793 (75) | |
| Very low | 60 (32) | 259 (25) | |
| Residence | 0.002 | ||
| Urban (<50 km) | 93 (49) | 726 (69) | |
| Suburban (>50 km) | 97 (51) | 326 (31) | |
| Variable | aOR * | CI 95% | p-Value |
|---|---|---|---|
| Small for Gestational Age | |||
| Gynecological age < 3 y | 2.462 | 1.081–5.605 | 0.032 |
| Age < 15 y | 1.149 | 0.404–3.264 | 0.794 |
| Menarcheal age < 11 y | 0.367 | 0.182–0.744 | 0.005 |
| Hemoglobin < 11.5 g/dL | 2.164 | 1.081–5.605 | 0.019 |
| Insufficient GWG | 1.858 | 1.059–3.260 | 0.031 |
| Preterm birth | 1.689 | 1.133–2.519 | 0.010 |
| Start antenatal care in 2nd or 3rd trimester | 4.695 | 0.624–35.335 | 0.133 |
| Suburban or rural residence | 2.256 | 1.263–4.031 | 0.006 |
| Height < 150 cm | 0.907 | 0.448–1.837 | 0.786 |
| Low Birth Weight | |||
| Gynecological age < 3 y | 3.799 | 1.458–9.725 | 0.006 |
| Age < 15 y | 5.740 | 1.343–26.369 | 0.019 |
| Menarche age < 11 y | 0.382 | 0.173–0.841 | 0.017 |
| Hemoglobin < 11.5 g/dL | 1.965 | 0.992–3.892 | 0.053 |
| Insufficient GWG | 1.633 | 0.902–2.956 | 0.105 |
| Preterm birth | 54.401 | 33.887–87.335 | 0.001 |
| Start antenatal care in 2nd or 3rd trimester | 4.221 | 0.325–54.776 | 0.271 |
| Suburban or rural residence | 1.930 | 1.053–3.536 | 0.033 |
| Height < 150 cm | 1.794 | 0.903–3.566 | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sámano, R.; Chico-Barba, G.; Godínez-Martínez, E.; Martínez-Rojano, H.; Díaz-Medina, A.; Hernández-Trejo, M.; Navarro-Vargas, P.C.; Flores-Quijano, M.E.; Mendoza-Flores, M.E.; Luna-Espinosa, V.S. Differential Effects of Gynecological and Chronological Age on Low Birth Weight and Small for Gestational Age. Biomedicines 2025, 13, 1639. https://doi.org/10.3390/biomedicines13071639
Sámano R, Chico-Barba G, Godínez-Martínez E, Martínez-Rojano H, Díaz-Medina A, Hernández-Trejo M, Navarro-Vargas PC, Flores-Quijano ME, Mendoza-Flores ME, Luna-Espinosa VS. Differential Effects of Gynecological and Chronological Age on Low Birth Weight and Small for Gestational Age. Biomedicines. 2025; 13(7):1639. https://doi.org/10.3390/biomedicines13071639
Chicago/Turabian StyleSámano, Reyna, Gabriela Chico-Barba, Estela Godínez-Martínez, Hugo Martínez-Rojano, Ashley Díaz-Medina, María Hernández-Trejo, Pablo César Navarro-Vargas, María Eugenia Flores-Quijano, María Eugenia Mendoza-Flores, and Valeria Sujey Luna-Espinosa. 2025. "Differential Effects of Gynecological and Chronological Age on Low Birth Weight and Small for Gestational Age" Biomedicines 13, no. 7: 1639. https://doi.org/10.3390/biomedicines13071639
APA StyleSámano, R., Chico-Barba, G., Godínez-Martínez, E., Martínez-Rojano, H., Díaz-Medina, A., Hernández-Trejo, M., Navarro-Vargas, P. C., Flores-Quijano, M. E., Mendoza-Flores, M. E., & Luna-Espinosa, V. S. (2025). Differential Effects of Gynecological and Chronological Age on Low Birth Weight and Small for Gestational Age. Biomedicines, 13(7), 1639. https://doi.org/10.3390/biomedicines13071639






