Long COVID and Its Impacts: A Case–Control Study in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Assessment of Long COVID Using C19-YRS Tool
2.3. Data Collection and Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Sociodemographic Data
3.2. Health Conditions and Lifestyle Habits
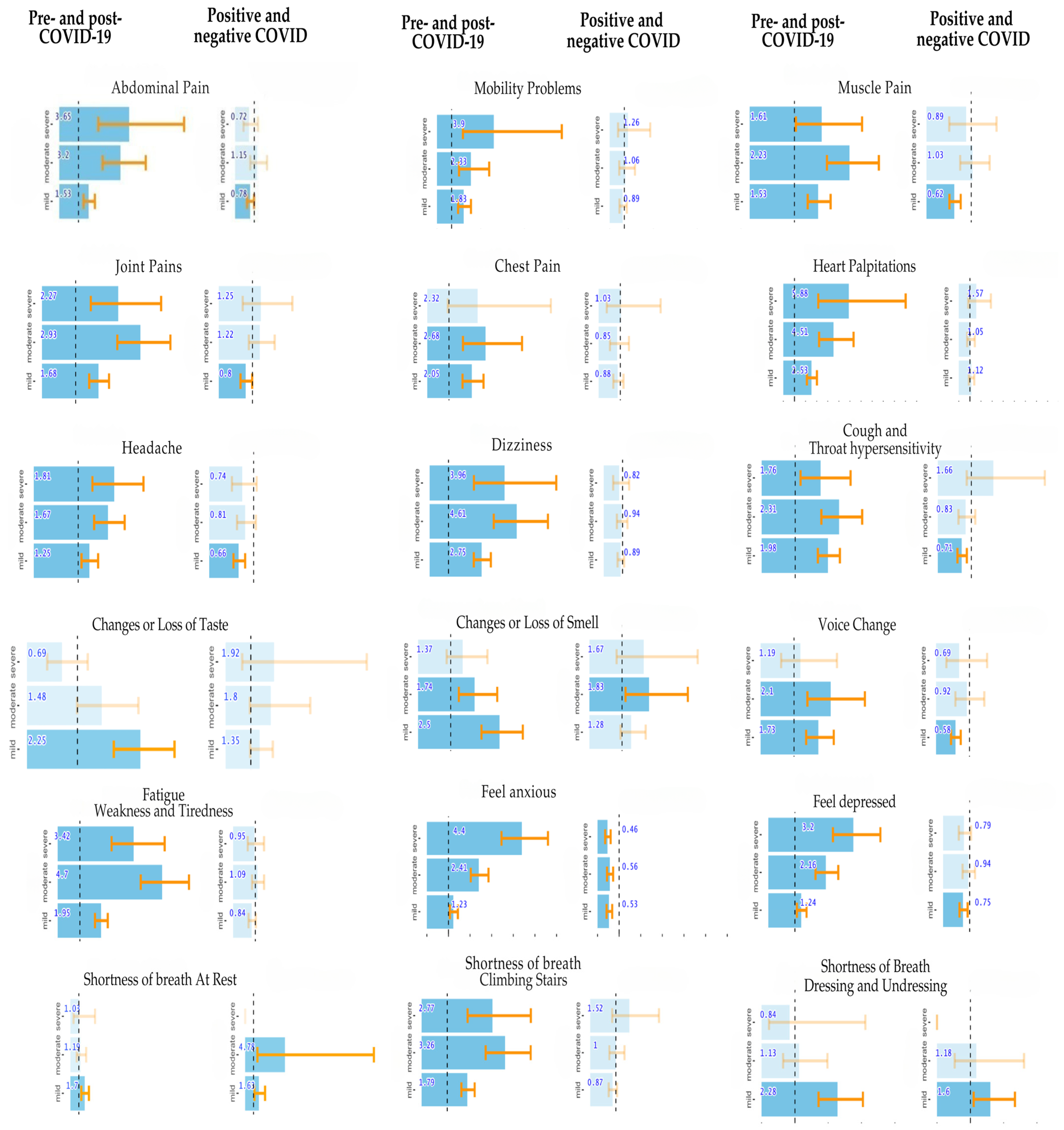
3.3. Comparison of Health Status Before and After COVID-19 Infection
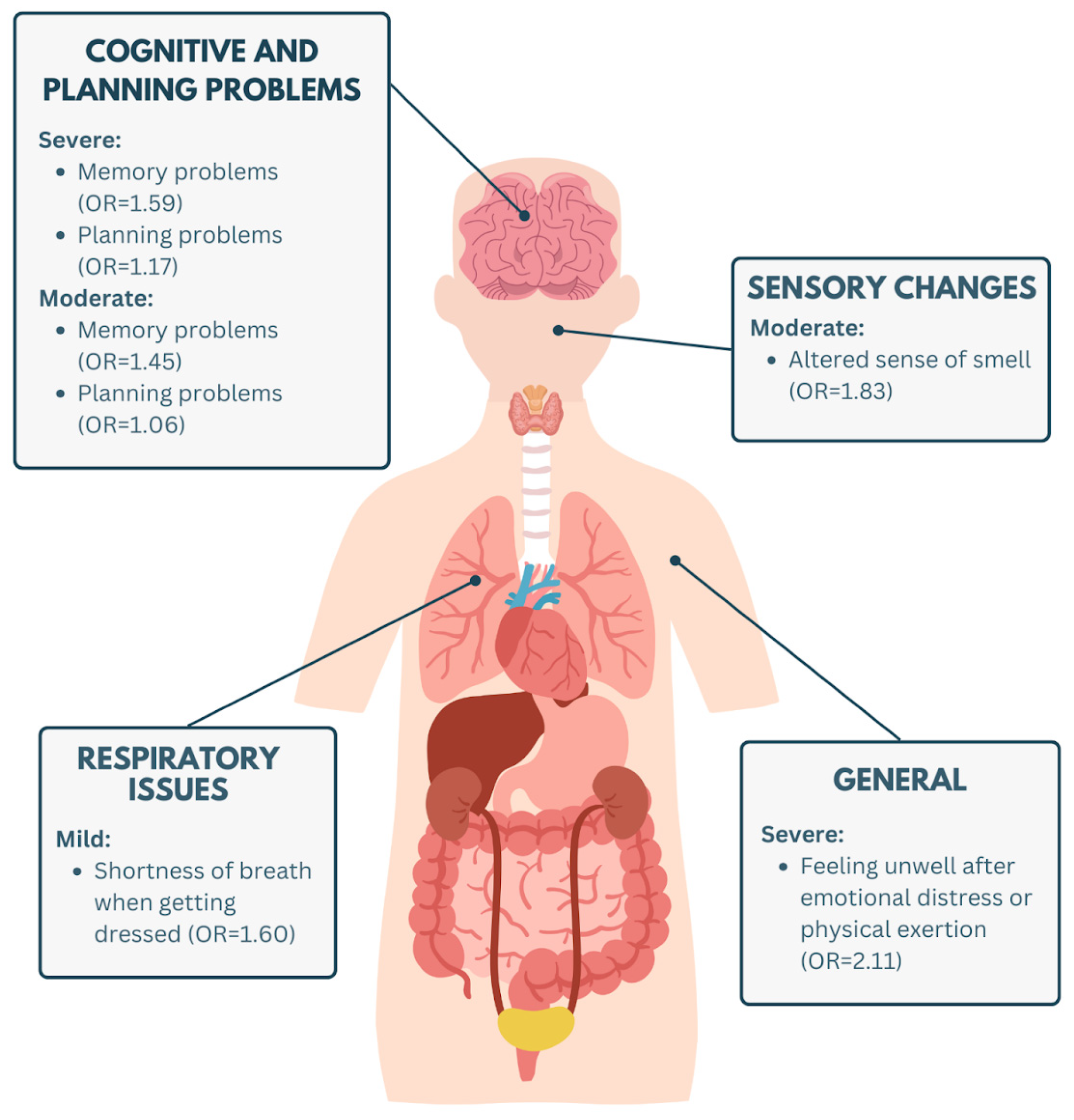
3.4. Comparison of Health Status Between Individuals Who Had and Did Not Have COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Post COVID-19 Condition (Long COVID). 2022. Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 1 January 2022).
- Kim, Y.; Bae, S.; Chang, H.-H.; Kim, S.-W. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. 2023, 13, 11207, Erratum in Sci. Rep. 2023, 13, 11960. https://doi.org/10.1038/s41598-023-39132-3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Long-Term Consequences of COVID-19 at 6 Months and Above: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6865. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Ashraf, N.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Akbarialiabad, H.; Taghrir, M.H.; Abdollahi, A.; Ghahramani, N.; Kumar, M.; Paydar, S.; Razani, B.; Mwangi, J.; Asadi-Pooya, A.A.; Malekmakan, L.; et al. Long COVID, a comprehensive systematic scoping review. Infection 2021, 49, 1163–1186. [Google Scholar] [CrossRef]
- Sreelakshmi, P.R.; Tandale, B.V.; Jadhav, A.V.; Vaidya, R.R.; Walimbhe, A.M.; Jadhav, S. A scoping review of persistent symptoms after COVID infection at different follow-up periods. Indian J. Public Health 2023, 67, 292–300. [Google Scholar] [CrossRef]
- Du, M.; Ma, Y.; Deng, J.; Liu, M.; Liu, J. Comparison of Long COVID-19 Caused by Different SARS-CoV-2 Strains: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 16010. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef] [PubMed]
- Rochmawati, E.; Iskandar, A.C.; Kamilah, F. Persistent symptoms among post-COVID -19 survivors: A systematic review and meta-analysis. J. Clin. Nurs. 2022, 33, 29–39. [Google Scholar] [CrossRef]
- Rahmati, M.; Udeh, R.; Yon, D.K.; Lee, S.W.; Dolja-Gore, X.; McEVoy, M.; Kenna, T.; Jacob, L.; Sánchez, G.F.L.; Koyanagi, A.; et al. A systematic review and meta-analysis of long-term sequelae of COVID-19 2 years after SARS-CoV-2 infection: A call to action for neurological, physical, and psychological sciences. J. Med. Virol. 2023, 95, e28852. [Google Scholar] [CrossRef]
- Abdel-Gawad, M.; Zaghloul, M.S.; Abd-Elsalam, S.; Hashem, M.; Lashen, S.A.; Mahros, A.M.; Mohammed, A.Q.; Hassan, A.M.; Bekhit, A.N.; Mohammed, W.; et al. Post-COVID-19 Syndrome Clinical Manifestations: A Systematic Review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2022, 21, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ruas, C.; Figueiredo, A.L.G.F.; Pacheco de Alencar, A.; de Souza Melo, S.; Carobin, N.V.; Cordeiro, M.A.; de Paula Sabino, A. Long COVID Syndrome: A Systematic Review of Persistent Symptoms Post-Pandemic. Qeios 2024. [Google Scholar] [CrossRef]
- Caspersen, I.H.; Magnus, P.; Trogstad, L. Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. Eur. J. Epidemiol. 2022, 37, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Universidade Federal de Minas Gerais. MonitoraCovid/UFMG e Telecovid-19 Encerram Atividades. UFMG Website. Published 7 February 2023. Available online: https://ufmg.br/comunicacao/noticias/monitoracovidufmg-e-telecovid-19-encerram-atividades (accessed on 26 March 2025).
- Sivan, M.; Preston, N.; Parkin, A.; Makower, S.; Gee, J.; Ross, D.; Tarrant, R.; Davison, J.; Halpin, S.; O’Connor, R.J.; et al. The modified COVID-19 Yorkshire Rehabilitation Scale (C19-YRSm) patient-reported outcome measure for Long Covid or Post-COVID-19 syndrome. J. Med. Virol. 2022, 94, 4253–4264. [Google Scholar] [CrossRef]
- Travassos, C.; Laguardia, J.; Marques, P.M.; da Mota, J.C.; Szwarcwald, C.L. Comparison between two race/skin color classifications in relation to health-related outcomes in Brazil. Int. J. Equity Health 2011, 10, 35. [Google Scholar] [CrossRef]
- Corbett, A.; Williams, G.; Creese, B.; Hampshire, A.; Hayman, V.; Palmer, A.; Filakovszky, A.; Mills, K.; Cummings, J.; Aarsland, D.; et al. Cognitive decline in older adults in the UK during and after the COVID-19 pandemic: A longitudinal analysis of PROTECT study data. lancet Healthy Longev. 2023, 4, e591–e599. [Google Scholar] [CrossRef]
- Panneer, S.; Kantamaneni, K.; Palaniswamy, U.; Bhat, L.; Pushparaj, R.R.B.; Nayar, K.R.; Soundari Manuel, H.; Flower, F.X.L.L.; Rice, L. Health, Economic and Social Development Challenges of the COVID-19 Pandemic: Strategies for Multiple and Interconnected Issues. Healthcare 2022, 10, 770. [Google Scholar] [CrossRef]
- Policy Response to COVID-19. Available online: https://www.imf.org/en/Topics/imf-and-covid19/Policy-Responses-to-COVID-19 (accessed on 1 January 2022).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Abrams, N.D.; Avula, L.R.; Carrick, D.M.; Chander, P.; Divi, R.L.; Dwyer, J.T.; Gannot, G.; Gordiyenko, N.; Liu, Q.; et al. Unraveling Links between Chronic Inflammation and Long COVID: Workshop Report. J. Immunol. 2024, 212, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.B.C.; Fernandez, M.V.; Barberia, L.G.; Silva, E.T.D.; Pedi, V.D.; Pontes, B.M.L.M.; Araujo, G.; Moreira, R.D.S.; Pedrosa, M.; Verotti, M.P.; et al. Panorama da COVID longa no Brasil: Análise preliminar de um inquérito para pensar políticas de saúde. Cad. Saúde Pública 2024, 40, e00094623. (In Portuguese) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szewczyk, W.; Fitzpatrick, A.L.; Fossou, H.; Gentile, N.L.; Sotoodehnia, N.; Vora, S.B.; West, T.E.; Bertolli, J.; Cope, J.R.; Lin, J.-M.S.; et al. Long COVID and recovery from Long COVID: Quality of life impairments and subjective cognitive decline at a median of 2 years after initial infection. BMC Infect. Dis. 2024, 24, 1241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stephenson, T.; Pereira, S.M.P.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’MAhoney, L.; Chalder, T.; et al. CLoCk Consortium. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): A national matched cohort study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef]
- Pan, K.-Y.; Kok, A.A.L.; Eikelenboom, M.; Horsfall, M.; Jörg, F.; Luteijn, R.A.; Rhebergen, D.; van Oppen, P.; Giltay, E.J.; Penninx, B.W.J.H. The mental health impact of the COVID-19 pandemic on people with and without depressive, anxiety, or obsessive-compulsive disorders: A longitudinal study of three Dutch case-control cohorts. Lancet Psychiatry 2020, 8, 121–129. [Google Scholar] [CrossRef]
- Østergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Antonucci, P.; Capriotti, V.; Sacchet, E.; Cragnolini, F.; D’Alessandro, A.; Zanelli, E.; et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: A one-year prospective study. Eur. Arch. Otorhinolaryngol. 2022, 279, 515–520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schambeck, S.E.; Mateyka, L.M.; Burrell, T.; Graf, N.; Brill, I.; Stark, T.; Protzer, U.; Busch, D.H.; Gerhard, M.; Riehl, H.; et al. Two-Year Follow-Up on Chemosensory Dysfunction and Adaptive Immune Response after Infection with SARS-CoV-2 in a Cohort of 44 Healthcare Workers. Life 2022, 12, 1556. [Google Scholar] [CrossRef] [PubMed]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.C.; et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Parimon, T.; Espindola, M.; Marchevsky, A.; Rampolla, R.; Chen, P.; Hogaboam, C.M. Potential mechanisms for lung fibrosis associated with COVID-19 infection. QJM 2023, 116, 487–492. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cortés-Telles, A.; López-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021, 288, 103644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cecchetto, A.; Guarnieri, G.; Torreggiani, G.; Vianello, A.; Baroni, G.; Palermo, C.; De Marchi, L.B.; Lorenzoni, G.; Bartolotta, P.; Bertaglia, E.; et al. Dyspnea in Post-Acute COVID-19: A Multi-Parametric Cardiopulmonary Evaluation. J. Clin. Med. 2023, 12, 4658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bull-Otterson, L.; Baca, S.; Saydah, S.; Boehmer, T.K.; Adjei, S.; Gray, S.; Harris, A.M. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Visconti, N.R.G.D.R.; Cailleaux-Cezar, M.; Capone, D.; Santos, M.I.V.D.; Graça, N.P.; Loivos, L.P.P.; Pinto Cardoso, A.; Queiroz Mello, F.C.D. Long-term respiratory outcomes after COVID-19: A Brazilian cohort study. Rev. Panam. Salud Pública 2023, 46, e187. [Google Scholar] [CrossRef]
- Keogh, R.H.; Cox, D. Case-Control Studies; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]


| Variable | Category | Positive for COVID-19 | Negative for COVID-19 | p-Value 1 |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Immunization of sample with respect to COVID-19 | Yes | 2087 (99.67) | 787 (99.75) | 1.000 |
| No | 7 (0.334) | 2 (0.25) | 1.000 | |
| Initial doses (COVID-19 vaccine) | 0 | 0 | 1 | |
| 1 | 354 (17.01) | 148 (81.10) | <0.001 | |
| 2 | 1727 (82.99) | 635 (0.81) | 0.2581 | |
| Booster doses (COVID-19 vaccine) | 0 | 79 (3.837) | 37 (4.75) | 0.3223 |
| 1 | 932 (45.26) | 381 (48.91) | 0.0900 | |
| 2 | 1048 (50.90) | 361 (46.34) | 0.0336 | |
| Bivalent (COVID-19 vaccine) | No | 736 (61.77) | 279 (22.96) | <0.001 |
| Yes | 1189 (38.23) | 442 (14.49) | <0.001 |
| Variable | Category | Positive for COVID-19 | Negative for COVID-19 | p-Value 1 |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Gender | Female | 1423 (68.25) | 543 (68.91) | 0.7686 1 |
| Male | 662 (31.75) | 245 (31.09) | 0.7686 1 | |
| Age | 37.5 (±12.68) | 34.45 (±12.27) | <0.0001 2 | |
| Race/Color | East Asian | 21 (1.028) | 6 (0.781) | 0.7036 1 |
| White | 1270 (62.16) | 421 (54.82) | <0.0001 1 | |
| Indigenous | 4 (0.196) | 1 (0.1302) | 1.0000 1 | |
| Brown | 571 (27.94) | 253 (32.94) | 0.01092 1 | |
| Black | 177 (8.66) | 87 (11.33) | 0.03704 1 | |
| Educational Level | Primary | 3 (0.1432) | 4 (0.508) | 0.1781 1 |
| Secondary | 489 (23.35) | 245 (31.09) | <0.0001 1 | |
| Undergraduate | 546 (26.07) | 217 (27.54) | 0.4554 1 | |
| Postgraduate | 1056 (50.43) | 322 (40.86) | <0.0001 1 | |
| Private Medical Care | Yes | 429 (20.585) | 263 (33.33) | <0.0001 1 |
| No | 1655 (79.41) | 526 (66.67) | <0.0001 1 |
| Variable | Category | Positive for COVID-19 | Negative for COVID-19 | p-Value 1 |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Common Health Conditions | Asthma | 139 (8.162) | 55 (8.593) | 0.7997 |
| Diabetes | 57 (3.347) | 18 (2.812) | 0.6008 | |
| Chronic pulmonary disease | 17 (0.998) | 6 (0.938) | 1.0000 | |
| High blood pressure (hypertension) | 192 (11.274) | 56 (8.75) | 0.0902 | |
| Immunosuppression | 37 (2.17) | 16 (2.5) | 0.7498 | |
| Heart disease | 27 (1.585) | 11 (1.719) | 0.9648 | |
| Chronic liver disease | 3 (0.176) | 1 (0.156) | 1.0000 | |
| Chronic kidney disease | 8 (0.4698) | 5 (0.781) | 0.5536 | |
| No pre-existing health conditions | 1292 (75.87) | 501 (78.28) | 0.2403 | |
| Health Conditions (in numbers) | 0 | 1377 (80.86) | 520 (81.25) | 0.8755 |
| 1 | 272 (15.97) | 100 (15.62) | 0.8877 | |
| 2 | 48 (2.818) | 19 (2.97) | 0.9559 | |
| 3 | 4 (0.235) | 1 (0.16) | 1.0000 | |
| 4 | 1 (0.00) | 0.00 | - | |
| 5 | 1 (0.00) | 0.00 | - | |
| Continuous Medication Use | Yes | 1190 (57.05) | 407 (51.78) | 0.0128 |
| No | 896 (42.95) | 379 (48.22) | 0.0128 | |
| Regular Physical Activity | Yes | 1592 (77.54) | 525 (73.43) | 0.0289 |
| No | 461 (22.45) | 190 (26.57) | 0.0289 | |
| Time Spent Engaging in Physical Activity | (<2.5 h)week | 505 (31.94) | 163 (31.59) | 0.9244 |
| (2.5 h, 5 h)/week | 697 (44.09) | 193 (37.40) | 0.0089 | |
| (5 h, 10 h)/week | 325 (20.56) | 142 (27.52) | 0.0012 | |
| (>10 h)/week | 54 (3.416) | 18 (3.488) | 1.0000 | |
| Healthy Eating (self-assessment) | Yes | 1495 (77.66) | 451 (69.07) | <0.0001 |
| No | 430 (22.33) | 202 (30.93) | <0.0001 | |
| Fresh Food Intake | Daily | 1433 (69.90) | 426 (60.0) | <0.0001 |
| Not frequently | 535 (26.10) | 248 (34.93) | <0.0001 | |
| Hardly ever | 81 (3.95) | 32 (4.51) | 0.5932 | |
| Never | 1 (0.05) | 4 (0.56) | 0.0234 | |
| Alcohol Consumption | None | 464 (22.83) | 168 (23.86) | 0.6126 |
| Once a month | 536 (26.38) | 183 (25.99) | 0.8811 | |
| Once a week | 350 (17.22) | 134 (19.03) | 0.3044 | |
| Once or twice a week | 497 (24.46) | 158 (22.44) | 0.3036 | |
| 3 or 4 days a week | 156 (7.68) | 50 (7.10) | 0.6779 | |
| 5 or 6 days a week | 21 (1.03) | 8 (1.13) | 0.9870 | |
| Everyday | 8 (0.39) | 3 (0.42) | 1.0000 | |
| Smoking | No | 1749 (85.36) | 571 (80.76) | 0.0047 |
| Yes, but not usually | 203 (9.91) | 92 (13.01) | 0.0256 | |
| Yes, daily | 97 (4.73) | 44 (6.22) | 0.1468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruas, C.M.; Silva, M.L.; Figueiredo, A.L.G.F.; Alencar, A.P.d.; Melo, S.d.S.; Castro, G.F.d.; Carobin, N.V.; Cordeiro, M.A.; Aguirre, J.F.R.; Oliveira, A.F.M.d.; et al. Long COVID and Its Impacts: A Case–Control Study in Brazil. Biomedicines 2025, 13, 1615. https://doi.org/10.3390/biomedicines13071615
Ruas CM, Silva ML, Figueiredo ALGF, Alencar APd, Melo SdS, Castro GFd, Carobin NV, Cordeiro MA, Aguirre JFR, Oliveira AFMd, et al. Long COVID and Its Impacts: A Case–Control Study in Brazil. Biomedicines. 2025; 13(7):1615. https://doi.org/10.3390/biomedicines13071615
Chicago/Turabian StyleRuas, Cristina M., Maria Laura Silva, Ana L. G. F. Figueiredo, Amanda P. de Alencar, Samuel de S. Melo, Geovani F. de Castro, Natália V. Carobin, Melina A. Cordeiro, Janete F. R. Aguirre, Amanda F. M. de Oliveira, and et al. 2025. "Long COVID and Its Impacts: A Case–Control Study in Brazil" Biomedicines 13, no. 7: 1615. https://doi.org/10.3390/biomedicines13071615
APA StyleRuas, C. M., Silva, M. L., Figueiredo, A. L. G. F., Alencar, A. P. d., Melo, S. d. S., Castro, G. F. d., Carobin, N. V., Cordeiro, M. A., Aguirre, J. F. R., Oliveira, A. F. M. d., & Sabino, A. d. P. (2025). Long COVID and Its Impacts: A Case–Control Study in Brazil. Biomedicines, 13(7), 1615. https://doi.org/10.3390/biomedicines13071615






