Myocardial Damage Patterns in Patients with Left Ventricular Systolic Dysfunction with and Without Coronary Artery Disease Referred for Cardiac Magnetic Resonance †
Abstract
1. Introduction
2. Materials and Methods
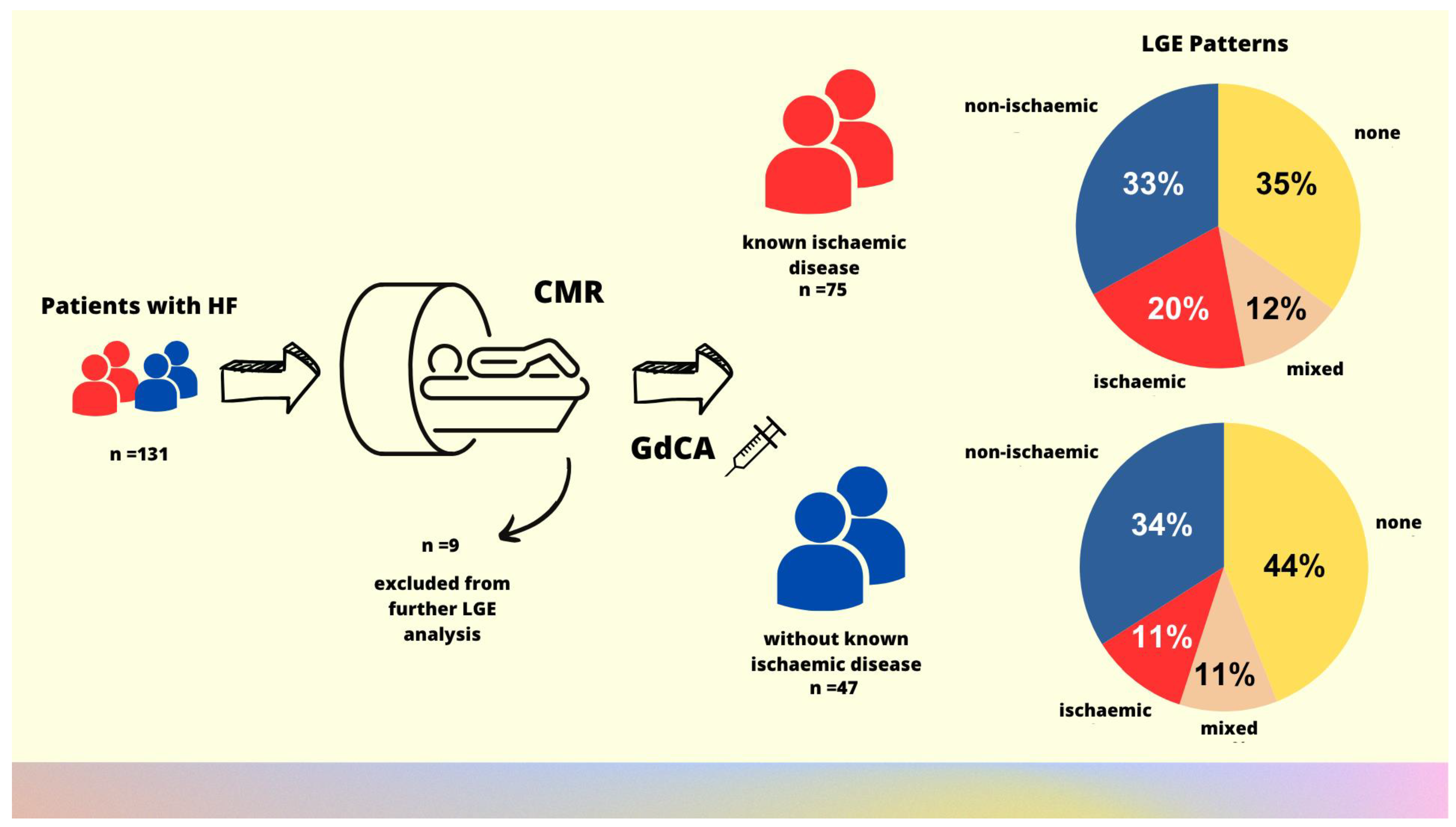
2.1. Study Population
2.2. Laboratory Measurements
2.3. Cardiac Magnetic Resonance Imaging
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Studied Subgroups
3.3. CMR Findings
4. Discussion
4.1. Clinical Implications
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMR | Cardiac magnetic resonance |
| LV | Left ventricle |
| CAD | Coronary artery disease |
| LVEF | Left ventricular ejection fraction |
| LGE | Late gadolinium enhancement |
| HF | Heart failure |
| PSIR | Phase-sensitive inversion recovery |
Appendix A

References
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.A.; Inamdar, A.C. Heart Failure: Diagnosis, Management and Utilization. J. Clin. Med. 2016, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Francis, J.M.; Myerson, S.; Selvanayagam, J.B.; Neubauer, S. The Role of Cardiovascular Magnetic Resonance Imaging in Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Eisenblätter, M.; Gielen, S. Myocardial Late Gadolinium Enhancement (LGE) in Cardiac Magnetic Resonance Imaging (CMR)-An Important Risk Marker for Cardiac Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.Y.; Su, M.Y.; Tseng, Y.H. Introduction to Cardiovascular Magnetic Resonance: Technical Principles and Clinical Applications. Acta Cardiol. Sin. 2016, 32, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.S.; François, C.J.; Leiner, T. Cardiac MRI: State of the Art. Radiology 2023, 307, e223008. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, M.T.; Capodarco, M.D.; Anamika, F.; Gupta, V.; Jain, R. Cardiac MRI: An Overview of Physical Principles With Highlights of Clinical Applications and Technological Advancements. Cureus 2024, 16, e55519. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Arpinar, V.E.; Muftuler, L.T.; Stojanovska, J.; Nencka, A.S.; Koch, K.M. Cardiac functional magnetic resonance imaging at 7T: Image quality optimization and ultra-high field capabilities. World J. Radiol. 2020, 12, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Sokolska, J.M.; Logoń, K.; Pszczołowska, M.; Kosmala, W. The aetiology of myocardial damage in patients with left ventricular systolic dysfunction with and without coronary artery disease referred for cardiac magnetic resonance. In Proceedings of the Heart Failure 2023 and the World Congress on Acute Heart Failure, Prague, Czechia, 20–23 May 2023, ISSN 1388-9842. [Google Scholar]
- Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Klem, I.; Judd, R.M.; Kim, R.J.; Kim, H.W. Revisiting how we perform late gadolinium enhancement CMR: Insights gleaned over 25 years of clinical practice. J. Cardiovasc. Magn. Reson. 2023, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Khatibzadeh, S.; Farzadfar, F.; Oliver, J.; Ezzati, M.; Moran, A. Worldwide risk factors for heart failure: A systematic review and pooled analysis. Int. J. Cardiol. 2013, 168, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, L.; Cautela, J.; Resseguier, N.; Laine, M.; Arques, S.; Pinto, J.; Orabona, M.; Barraud, J.; Peyrol, M.; Paganelli, F.; et al. Prevalence and characteristics of coronary artery disease in heart failure with preserved and mid-range ejection fractions: A systematic angiography approach. Arch. Cardiovasc. Dis. 2018, 111, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Deney, A.; Nader, V.; Matta, A.; Itier, R.; Fournier, P.; Lairez, O.; Pizzinat, N.; Carrié, D.; Boal, F.; Galinier, M.; et al. Retrospective Study of 573 Patients with Heart Failure Evaluated for Coronary Artery Disease at Toulouse University Center, France. Med. Sci. Monit. 2022, 28, e934804. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.M.; Greve, A.M.; Aspelund, T.; Schelbert, E.B.; Cao, J.J.; Danielsen, R.; Þorgeirsson, G.; Sigurðsson, S.; Eiríksdóttir, G.; Harris, T.B.; et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur. Heart J. 2019, 40, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ojrzyńska-Witek, N.; Marczak, M.; Mazurkiewicz, Ł.; Petryka-Mazurkiewicz, J.; Miłosz-Wieczorek, B.; Grzybowski, J.; Śpiewak, M. Role of cardiac magnetic resonance in heart failure of initially unknown etiology: A 10-year observational study. Kardiol. Pol. 2022, 80, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Japp, A.G.; Raza, S.; Halliday, B.P.; Jones, D.A.; Newsome, S.; Ismail, N.A.; Morarji, K.; Khwaja, J.; Spath, N.; et al. Absence of Myocardial Fibrosis Predicts Favorable Long-Term Survival in New-Onset Heart Failure. Circ. Cardiovasc. Imaging 2018, 11, e007722. [Google Scholar] [CrossRef] [PubMed]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Cheong, B.Y.; Muthupillai, R.; Wilson, J.M.; Sung, A.; Huber, S.; Amin, S.; Elayda, M.A.; Lee, V.V.; Flamm, S.D. Prognostic significance of delayed-enhancement magnetic resonance imaging: Survival of 857 patients with and without left ventricular dysfunction. Circulation 2009, 120, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.M.; Hammar, P.; Ahlström, H.; Bjerner, T.; Duvernoy, O.; Lindahl, B. Unrecognized myocardial infarction assessed by cardiac magnetic resonance imaging is associated with adverse long-term prognosis. PLoS ONE 2018, 13, e0200381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Zhu, H.; Pan, X.F.; Hu, Y.; Arnott, C.; Mai, W.; Cai, X.; Huang, Y. Prognosis of unrecognised myocardial infarction determined by electrocardiography or cardiac magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2020, 369, m1184. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Studied Population n = 131 | Patients Without CAD n = 50 (38%) | Patients with CAD n = 81 (62%) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 55 ± 15 | 55 ± 16 | 55 ± 15 | 0.90 |
| Male sex, n (%) | 100 (76) | 38 (76) | 62 (77) | 0.94 |
| LVEF, % (echocardiography) | 35 ± 10 | 39 ± 9 | 33 ± 10 | 0.006 * |
| Clinical data | ||||
| Known coronary artery disease (CAD), n (%); including previous acute coronary syndromes (UA and/or MI), n (n%) | 81 (62); 18 (14) | 0 0 | 81 (100) 18 (22) | <0.001 * |
| Hypertension, n (%) | 66 (54) | 21 (48) | 45 (57) | 0.32 |
| Atrial fibrillation, n (%) | 42 (34) | 17 (39) | 25 (32) | 0.43 |
| Significant (moderate or severe) valve disease, n (%) | 28 (23) | 9 (20) | 19 (24) | 0.58 |
| Chronic kidney disease, n (%) | 23 (19) | 8 (18) | 15 (19) | 0.91 |
| Liver diseases, n (%) | 13 (11) | 6 (14) | 7 (9) | 0.04 * |
| Chronic obstructive pulmonary disease/asthma, n (%) | 4 (3) | 4 (9) | 0 | 0.02 * |
| Number of comorbidities, n (%) | 2.9 ± 1.8 | 1.9 ± 1.3 | 3.4 ± 1.8 | <0.001 * |
| Basic laboratory parameters | ||||
| Hemoglobin (g/dL) | 14.4 ± 2.0 | 14.6 ± 1.9 | 14.2 ± 2.0 | 0.69 |
| Platelets (103/μL) | 225 ± 69 | 203 ± 53 | 237 ± 74 | 0.01 * |
| Creatinine (mg/dL) | 1.24 ± 1.22 | 1.15 ± 1 | 1 ± 1 | 0.55 |
| NTproBNP (pg/L) | 3433 ± 8131 | 2639 ± 5683 | 3782 ± 9011 | 0.51 |
| Treatment | ||||
| Loop diuretics, n (%) | 66 (57) | 19 (45) | 47 (64) | 0.06 |
| ACEI/ARB, n (%) | 43 (37) | 17 (41) | 26 (35) | 0.53 |
| Sacubitril + valsartan, n (%) | 44 (38) | 17 (41) | 27 (36) | 0.63 |
| Beta-blockers, n (%) | 100 (86) | 38 (90) | 62 (83) | 0.25 |
| Aldosterone antagonists, n (%) | 81 (69) | 28 (67) | 53 (71) | 0.65 |
| SGLT-2 inhibitors, n (%) | 78 (67) | 27 (64) | 51 (68) | 0.68 |
| Statins, n (%) | 69 (59) | 25 (60) | 44 (59) | 0.93 |
| Acetylsalicylic acid, n (%) | 27 (23) | 6 (14) | 21 (28) | 0.09 |
| P2Y12 inhibitors (clopidogrel/prasugrel/ticagrelor), n (%) | 12 (10) | 0 | 12 (16) | 0.006 * |
| Dual antiplatelet therapy, n (%) | 9 (7) | 0 | 9 (11) | 0.003 * |
| Parameters | Studied Population n = 131 | Patients Without CAD n = 50 | Patients with CAD n = 81 | p |
|---|---|---|---|---|
| Left ventricle and atrium | ||||
| IVSd (mm) | 10 ± 2 | 10 ± 2 | 10 ± 2 | 0.87 |
| LVEDd (mm) | 65 ± 9 | 62 ± 8 | 67 ± 9 | 0.004 * |
| LVESd (mm) | 52 ± 12 | 49 ± 9 | 55 ± 12 | 0.01 * |
| LVEDVi (mL/m2) | 124 ± 37 | 115 ± 28 | 130 ± 42 | 0.04 * |
| LVESVi (mL/m2) | 80 ± 37 | 70 ± 27 | 88 ± 42 | 0.01 * |
| LVEF (%) | 38 ± 12 | 41 ± 11 | 35 ± 12 | 0.004 * |
| LV-CO (mL/min) | 6 ± 6 | 7 ± 5 | 6 ± 6 | 0.54 |
| LVMassEDi (g/m2) | 72 ± 19 | 69 ± 17 | 73 ± 20 | 0.2 |
| LAVI (mL/m2) | 50 ± 19 | 50 ± 2 | 50 ± 19 | 0.88 |
| Right ventricle and atrium | ||||
| RVEDVi (mL/m2) | 89 ± 27 | 89 ± 22 | 89 ± 30 | 0.96 |
| RVESVi (mL/m2) | 47 ± 26 | 48 ± 25 | 46 ± 27 | 0.74 |
| RVEF (%) | 50 ± 11 | 49 ± 10 | 50 ± 12 | 0.88 |
| RAVI (mL/m2) | 44 ± 19 | 47 ± 21 | 42 ± 18 | 0.18 |
| Parameters | Studied Population n = 131 | Patients Without CAD n = 50 | Patients with CAD n = 81 | p |
|---|---|---|---|---|
| LGE sequence, n (%) | 122 (93) | 47 (94) | 75 (93) | 0.76 |
| Presence of LGE, n (%) | 75 (61) | 26 (55) | 49 (65) | 0.14 |
| Subendocardial and transmural pattern of LGE, n (%) | 34 (28) | 10 (21) | 24 (32) | 0.20 |
| Cardiac segments with subendocardial and transmural LGE, n | 1.0 ± 2.1 | 0.6 ± 1.3 | 1.2 ± 2.5 | <0.001 * |
| Subepicardial pattern of LGE, n (%) | 9 (7) | 5 (11) | 4 (5) | 0.28 |
| Cardiac segments with subepicardial LGE, n | 0.3 ± 1.3 | 0.4 ± 1.3 | 0.3 ± 1.3 | 0.92 |
| Midmyocardial pattern of LGE, n (%) | 52 (43) | 19 (40) | 36 (44) | 0.70 |
| Cardiac segments with midmyocardial LGE, n | 1.4 ± 2.3 | 1.5 ± 2.9 | 1.3 ± 1.8 | <0.001 * |
| Subepicardial and midmyocardial pattern of LGE, n (%) | 55 (45) | 21 (45) | 34 (45) | 0.94 |
| Interpretation of myocardial damage | ||||
| Isolated ischemic myocardial damage, n (%) | 20 (16) | 5 (11) | 15 (20) | 0.17 |
| Isolated non-ischemic myocardial damage, n (%) | 41 (34) | 16 (34) | 25 (33) | 0.94 |
| Mixed myocardial damage, n (%) | 14 (11) | 5 (11) | 9 (12) | 0.82 |
| Normal CMR findings (LVEF ≧ 51% in men and >52% in women) | 4 (3) | 3 (6) | 1 (1) | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolska, J.M.; Logoń, K.; Pszczołowska, M.; Kosmala, W. Myocardial Damage Patterns in Patients with Left Ventricular Systolic Dysfunction with and Without Coronary Artery Disease Referred for Cardiac Magnetic Resonance. Biomedicines 2025, 13, 1612. https://doi.org/10.3390/biomedicines13071612
Sokolska JM, Logoń K, Pszczołowska M, Kosmala W. Myocardial Damage Patterns in Patients with Left Ventricular Systolic Dysfunction with and Without Coronary Artery Disease Referred for Cardiac Magnetic Resonance. Biomedicines. 2025; 13(7):1612. https://doi.org/10.3390/biomedicines13071612
Chicago/Turabian StyleSokolska, Justyna M., Katarzyna Logoń, Magdalena Pszczołowska, and Wojciech Kosmala. 2025. "Myocardial Damage Patterns in Patients with Left Ventricular Systolic Dysfunction with and Without Coronary Artery Disease Referred for Cardiac Magnetic Resonance" Biomedicines 13, no. 7: 1612. https://doi.org/10.3390/biomedicines13071612
APA StyleSokolska, J. M., Logoń, K., Pszczołowska, M., & Kosmala, W. (2025). Myocardial Damage Patterns in Patients with Left Ventricular Systolic Dysfunction with and Without Coronary Artery Disease Referred for Cardiac Magnetic Resonance. Biomedicines, 13(7), 1612. https://doi.org/10.3390/biomedicines13071612







