Advances in Mechanical Circulatory Support (MCS): Literature Review
Abstract
1. Introduction
1.1. Overview of Heart Failure
1.2. The Role of Mechanical Circulatory Support (MCS)
1.3. Objectives of the Paper
2. Current Approaches in Mechanical Circulatory Support
2.1. Types of MCS Devices
2.1.1. LVADs
2.1.2. BiVADs
2.1.3. Impella
2.1.4. TAH
2.1.5. IABP
2.1.6. ECMO
2.2. Technological Advancements
2.2.1. Enhanced Durability and Reliability
2.2.2. Improved Biocompatibility
2.2.3. Electrophysiological Impact of MCS
3. Challenges and Limitations of Current MCS Technologies
3.1. Complications
3.1.1. LVADs
3.1.2. BiVADs
3.1.3. Impella
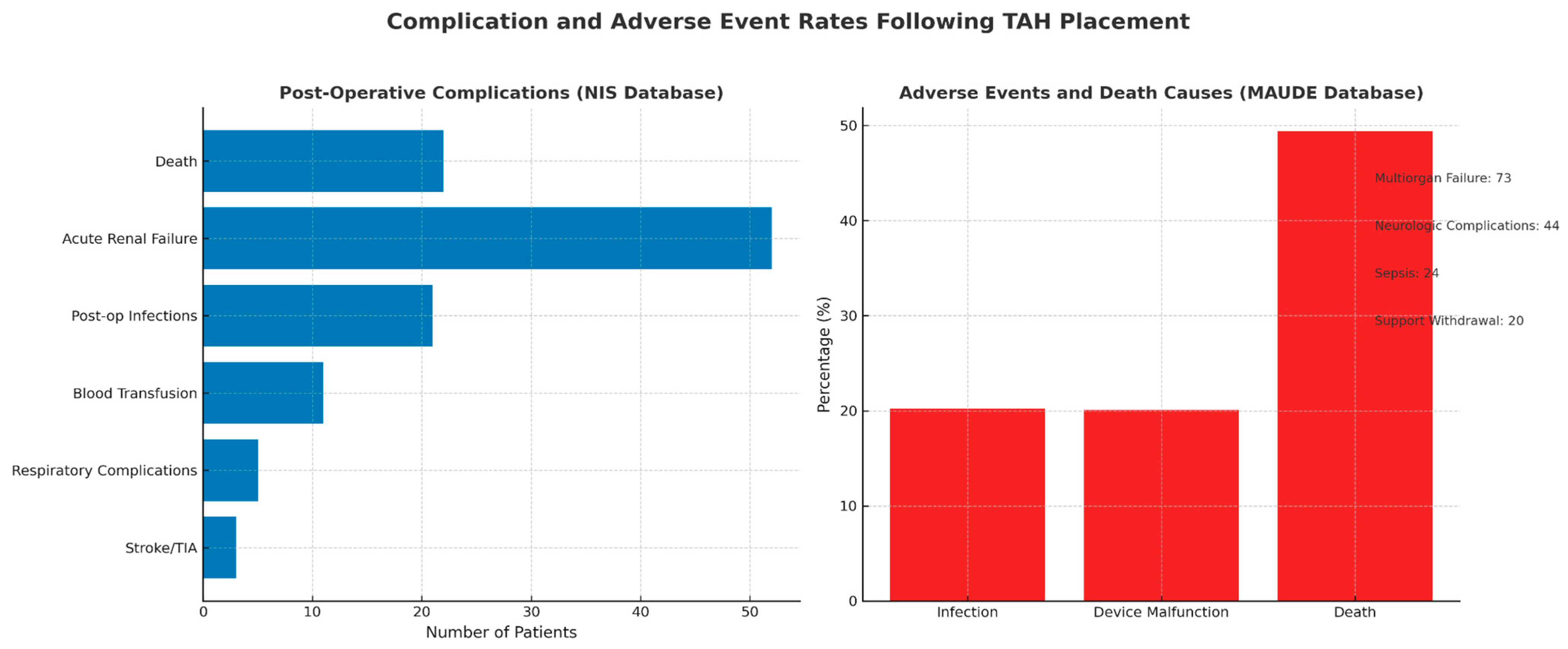
3.1.4. TAH
3.1.5. IABP
3.1.6. ECMO
3.2. Limited Long-Term Data
3.2.1. LVAD Long-Term Survival Outcomes and Device Performance
3.2.2. BIVAD Long-Term Survival Outcomes and Device Performance
3.2.3. Impella Long-Term Survival Outcomes and Device Performance
3.2.4. TAH Long-Term Survival Outcomes and Device Performance
3.2.5. IABP Survival Outcomes and Device Performance
3.2.6. ECMO Survival Outcomes and Device Performance
3.3. Cost and Accessibility
4. Future Directions in Mechanical Circulatory Support
4.1. Next-Generation Devices
4.1.1. Fully Implantable and Wearable Devices
4.1.2. Bioengineered and Biological Solutions
4.1.3. AI and Machine Learning Integration
4.2. Stem Cell Therapy and Regenerative Approaches
4.3. The Role of Gene Therapy
4.4. Combining MCS with Other Therapies
4.5. Xenotransplantation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Dual, S.A.; Cowger, J.; Roche, E.; Nayak, A. The Future of Durable Mechanical Circulatory Support: Emerging Technological Innovations and Considerations to Enable Evolution of the Field. J. Card. Fail. 2024, 30, 596–609. [Google Scholar] [CrossRef]
- Albulushi, A.; Al-Riyami, M.B.; Al-Rawahi, N.; Al-Mukhaini, M. Effectiveness of mechanical circulatory support devices in reversing pulmonary hypertension among heart transplant candidates: A systematic review. Curr. Probl. Cardiol. 2024, 49, 102579. [Google Scholar] [CrossRef]
- Castagna, F.; Stöhr, E.J.; Pinsino, A.; Cockcroft, J.R.; Willey, J.; Garan, A.R.; Topkara, V.K.; Colombo, P.C.; Yuzefpolskaya, M.; McDonnell, B.J. The Unique Blood Pressures and Pulsatility of LVAD Patients: Current Challenges and Future Opportunities. Curr. Hypertens. Rep. 2017, 19, 85. [Google Scholar] [CrossRef]
- Frigerio, M. Left Ventricular Assist Device: Indication, Timing, and Management. Heart Fail. Clin. 2021, 17, 619–634. [Google Scholar] [CrossRef]
- Llerena-Velastegui, J.; Santafe-Abril, G.; Villacis-Lopez, C.; Hurtado-Alzate, C.; Placencia-Silva, M.; Santander-Aldean, M.; Trujillo-Delgado, M.; Freire-Oña, X.; Santander-Fuentes, C.; Velasquez-Campos, J. Efficacy and Complication Profiles of Left Ventricular Assist Devices in Adult Heart Failure Management: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2024, 49 Pt C, 102118. [Google Scholar] [CrossRef]
- Teuteberg, J.J.; Cleveland, J.C.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef]
- Berardi, C.; Bravo, C.A.; Li, S.; Khorsandi, M.; Keenan, J.E.; Auld, J.; Rockom, S.; Beckman, J.A.; Mahr, C. The History of Durable Left Ventricular Assist Devices and Comparison of Outcomes: HeartWare, HeartMate II, HeartMate 3, and the Future of Mechanical Circulatory Support. J. Clin. Med. 2022, 11, 2022. [Google Scholar] [CrossRef]
- Arabía, F.A.; Murray, C.F.; Cantor, R.; Deng, L.; Gopalan, R.; Amabile, O.; Kalya, A.; Tasset, M.R.; Colón, M.J.; Smith, R.; et al. Heart Transplant Outcomes After Total Artificial Heart. Transplant. Proc. 2023, 55, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Hariri, I.M.; Dardas, T.; Kanwar, M.; Cogswell, R.; Gosev, I.; Molina, E.; Myers, S.L.; Kirklin, J.K.; Shah, P.; Pagani, F.D.; et al. Long-term survival on LVAD support: Device complications and end-organ dysfunction limit long-term success. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2022, 41, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.C.; Naftel, D.C.; Reece, T.B.; Murray, M.; Antaki, J.; Pagani, F.D.; Kirklin, J.K. Survival after biventricular assist device implantation: An analysis of the Interagency Registry for Mechanically Assisted Circulatory Support database. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2011, 30, 862–869. [Google Scholar] [CrossRef]
- Bravo, C.A.; Navarro, A.G.; Dhaliwal, K.K.; Khorsandi, M.; Keenan, J.E.; Mudigonda, P.; O’BRien, K.D.; Mahr, C. Right heart failure after left ventricular assist device: From mechanisms to treatments. Front. Cardiovasc. Med. 2022, 9, 1023549. [Google Scholar] [CrossRef] [PubMed]
- Farag, J.; Woldendorp, K.; McNamara, N.; Bannon, P.G.; Marasco, S.F.; Loforte, A.; Potapov, E.V. Contemporary outcomes of continuous-flow biventricular assist devices. Ann. Cardiothorac. Surg. 2021, 10, 311–328. [Google Scholar] [CrossRef]
- Maynes, E.J.; O’mAlley, T.J.; Luc, J.G.Y.; Weber, M.P.; Horan, D.P.; Choi, J.H.; Patel, S.; Rizvi, S.-S.A.; Morris, R.J.; Entwistle, J.W.; et al. Comparison of SynCardia total artificial heart and HeartWare HVAD biventricular support for management of biventricular heart failure: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2020, 9, 69–80. [Google Scholar] [CrossRef]
- McGiffin, D.; Kure, C.; McLean, J.; Marasco, S.; Bergin, P.; Hare, J.L.; Leet, A.; Patel, H.; Zimmet, A.; Rix, J.; et al. The results of a single-center experience with HeartMate 3 in a biventricular configuration. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2021, 40, 193–200. [Google Scholar] [CrossRef]
- Lavee, J.; Mulzer, J.; Krabatsch, T.; Marasco, S.; McGiffin, D.; Garbade, J.; Schmitto, J.D.; Zimpfer, D.; Potapov, E.V. An international multicenter experience of biventricular support with HeartMate 3 ventricular assist systems. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 1399–1402. [Google Scholar] [CrossRef]
- Masiero, G.; Arturi, F.; Panza, A.; Tarantini, G. Mechanical Circulatory Support with Impella: Principles, Evidence, and Daily Practice. J. Clin. Med. 2024, 13, 4586. [Google Scholar] [CrossRef]
- Glazier, J.J.; Kaki, A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int. J. Angiol. 2018, 28, 118–123. [Google Scholar] [CrossRef]
- Pahuja, M.; Johnson, A.; Kabir, R.; Bhogal, S.; Wermers, J.P.; Bernardo, N.L.; Ben-Dor, I.; Hashim, H.; Satler, L.F.; Sheikh, F.H.; et al. Randomized Trials of Percutaneous Microaxial Flow Pump Devices: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 2028–2049. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Dudek, D.; Hassager, C.; Combes, A.; Gramegna, M.; Halvorsen, S.; Huber, K.; Kunadian, V.; Maly, J.; Møller, J.E.; et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. EuroInterv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2021, 17, e274–e286. [Google Scholar] [CrossRef]
- Barnard, C.N. The operation. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S. Afr. Med. J. Suid-Afr. Tydskr. Geneeskd. 1967, 41, 1271–1274. [Google Scholar]
- Atti, V.; Narayanan, M.A.; Patel, B.; Balla, S.; Siddique, A.; Lundgren, S.; Velagapudi, P. A Comprehensive Review of Mechanical Circulatory Support Devices. Heart Int. 2022, 16, 37–48. [Google Scholar] [CrossRef]
- Gajanan, G.; Brilakis, E.S.; Siller-Matula, J.M.; Zolty, R.L.; Velagapudi, P. The Intra-Aortic Balloon Pump. J. Vis. Exp. JoVE 2021, 168, e62132. [Google Scholar] [CrossRef]
- Poirier, Y.; Voisine, P.; Plourde, G.; Rimac, G.; Perez, A.B.; Costerousse, O.; Bertrand, O.F. Efficacy and safety of preoperative intra-aortic balloon pump use in patients undergoing cardiac surgery: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 207, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Zeymer, U.; Neumann, F.-J.; Ferenc, M.; Olbrich, H.-G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Thelemann, N.; Neumann, F.-J.; Hausleiter, J.; Abdel-Wahab, M.; Meyer-Saraei, R.; Fuernau, G.; Eitel, I.; Hambrecht, R.; et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction. Circulation 2018, 139, 395–403. [Google Scholar] [CrossRef]
- Kuno, T.; Takagi, H.; Ando, T.; Kodaira, M.; Numasawa, Y.; Fox, J.; Bangalore, S. Safety and efficacy of mechanical circulatory support with Impella or intra-aortic balloon pump for high-risk percutaneous coronary intervention and/or cardiogenic shock: Insights from a network meta-analysis of randomized trials. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, E636–E645. [Google Scholar] [CrossRef]
- Zeng, P.; Yang, C.; Chen, J.; Fan, Z.; Cai, W.; Huang, Y.; Xiang, Z.; Yang, J.; Zhang, J.; Yang, J. Comparison of the Efficacy of ECMO With or Without IABP in Patients With Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 917610. [Google Scholar] [CrossRef]
- Rabah, H.; Rabah, A. Extracorporeal Membrane Oxygenation (ECMO): What We Need to Know. Cureus 2022, 14, e26735. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Peek, G.J.; Hajage, D.; Hardy, P.; Abrams, D.; Schmidt, M.; Dechartres, A.; Elbourne, D. ECMO for severe ARDS: Systematic review and individual patient data meta-analysis. Intensive Care Med. 2020, 46, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Guirand, D.M.; Okoye, O.T.; Schmidt, B.S.; Mansfield, N.J.; Aden, J.K.; Martin, R.S.; Cestero, R.F.; Hines, M.H.; Pranikoff, T.; Inaba, K.; et al. Venovenous extracorporeal life support improves survival in adult trauma patients with acute hypoxemic respiratory failure: A multicenter retrospective cohort study. J. Trauma Acute Care Surg. 2014, 76, 1275–1281. [Google Scholar] [CrossRef]
- Healy, A.H.; McKellar, S.H.; Drakos, S.G.; Koliopoulou, A.; Stehlik, J.; Selzman, C.H. Physiologic effects of continuous-flow left ventricular assist devices. J. Surg. Res. 2016, 202, 363–371. [Google Scholar] [CrossRef]
- Davis, M.E.; Haglund, N.A.; Tricarico, N.M.; Keebler, M.E.; Maltais, S. Development of acquired von Willebrand syndrome during short-term micro axial pump support: Implications for bleeding in a patient bridged to a long-term continuous-flow left ventricular assist device. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2014, 60, 355–357. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Cleveland, J.C.; Cowger, J.A.; Hall, S.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Chuang, J.; Wang, A.; et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef]
- Racodon, M.; Hermand, É.; Lemahieu, J.-M.; Blairon, P.M.; Vanhove, P.; Secq, A. Prehabilitation Using a Cardiac Rehabilitation Program for a Patient With a Total Artificial Heart Prior to Heart Transplantation. J. Cardiopulm. Rehabil. Prev. 2024, 44, 137–140. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; Ghionzoli, N.; Mandoli, G.E.; D’ascenzi, F.; Focardi, M.; Valente, S.; Cameli, M. Biomarkers in Patients with Left Ventricular Assist Device: An Insight on Current Evidence. Biomolecules 2022, 12, 334. [Google Scholar] [CrossRef]
- Poitier, B.; Chocron, R.; Peronino, C.; Philippe, A.; Pya, Y.; Rivet, N.; Richez, U.; Bekbossynova, M.; Gendron, N.; Grimmé, M.; et al. Bioprosthetic Total Artificial Heart in Autoregulated Mode Is Biologically Hemocompatible: Insights for Multimers of von Willebrand Factor. Arter. Thromb. Vasc. Biol. 2022, 42, 470–480. [Google Scholar] [CrossRef]
- Han, J.J. Aeson—The Carmat total artificial heart is approved for enrollment in the United States. Artif. Organs 2021, 45, 445–446. [Google Scholar] [CrossRef]
- Cohrs, N.H.; Petrou, A.; Loepfe, M.; Yliruka, M.; Schumacher, C.M.; Kohll, A.X.; Starck, C.T.; Daners, M.S.; Meboldt, M.; Falk, V.; et al. A Soft Total Artificial Heart—First Concept Evaluation on a Hybrid Mock Circulation. Artif. Organs 2017, 41, 948–958. [Google Scholar] [CrossRef]
- Guex, L.G.; Jones, L.S.; Kohll, A.X.; Walker, R.; Meboldt, M.; Falk, V.; Daners, M.S.; Stark, W.J. Increased Longevity and Pumping Performance of an Injection Molded Soft Total Artificial Heart. Soft Robot. 2021, 8, 588–593. [Google Scholar] [CrossRef]
- Vis, A.; Arfaee, M.; Khambati, H.; Slaughter, M.S.; Gummert, J.F.; Overvelde, J.T.B.; Kluin, J. The ongoing quest for the first total artificial heart as destination therapy. Nat. Rev. Cardiol. 2022, 19, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Pierucci, N.; Cipollone, P.; Vignaroli, W.; Piro, A.; Compagnucci, P.; Matteucci, A.; Chimenti, C.; Pandozi, C.; Russo, A.D.; et al. Mechanical Circulatory Support Systems in the Management of Ventricular Arrhythmias: A Contemporary Overview. J. Clin. Med. 2024, 13, 1746. [Google Scholar] [CrossRef]
- Genovese, E.A.; Dew, M.A.; Teuteberg, J.J.; Simon, M.A.; Kay, J.; Siegenthaler, M.P.; Bhama, J.K.; Bermudez, C.A.; Lockard, K.L.; Winowich, S.; et al. Incidence and patterns of adverse event onset during the first 60 days after ventricular assist device implantation. Ann. Thorac. Surg. 2009, 88, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Ziv, O.; Dizon, J.; Thosani, A.; Naka, Y.; Magnano, A.R.; Garan, H. Effects of Left Ventricular Assist Device Therapy on Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2005, 45, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Jezovnik, M.K.; Gregoric, I.D.; Poredos, P. Medical complications in patients with LVAD devices. Eur. Soc. Cardiol. 2017, 14, 37. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-14/Medical-complications-in-patients-with-LVAD-devices (accessed on 29 April 2025).
- Kushnir, V.M.; Sharma, S.; Ewald, G.A.; Seccombe, J.; Novak, E.; Wang, I.-W.; Joseph, S.M.; Gyawali, C.P. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest. Endosc. 2012, 75, 973–979. [Google Scholar] [CrossRef]
- Boyle, A.J.; Russell, S.D.; Teuteberg, J.J.; Slaughter, M.S.; Moazami, N.; Pagani, F.D.; Frazier, O.H.; Heatley, G.; Farrar, D.J.; John, R. Low Thromboembolism and Pump Thrombosis With the HeartMate II Left Ventricular Assist Device: Analysis of Outpatient Anti-coagulation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2009, 28, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; LaRue, S.J.; Raymer, D.S.; Novak, E.; Vader, J.M.; Ewald, G.A.; Gage, B.F. Relationship Between Anticoagulation Intensity and Thrombotic or Bleeding Outcomes Among Outpatients With Continuous-Flow Left Ventricular Assist Devices. Circ. Heart Fail. 2016, 9, e002680. [Google Scholar] [CrossRef]
- Haglund, N.A.; Davis, M.E.; Tricarico, N.M.; Keebler, M.E.; Maltais, S. Readmissions After Continuous Flow Left Ventricular Assist Device Implantation: Differences Observed Between Two Contemporary Device Types. ASAIO J. 2015, 61, 410–416. [Google Scholar] [CrossRef]
- Levy, D.; Guo, Y.; Simkins, J.; Puius, Y.; Muggia, V.; Goldstein, D.; D’ALessandro, D.; Minamoto, G. Left ventricular assist device exchange for persistent infection: A case series and review of the literature. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2014, 16, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.V.; Sharma, V.; Cho, Y.H.; Shah, I.K.; Park, S.J. De novo aortic insufficiency during long-term support on a left ventricular assist device: A systematic review and meta-analysis. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2014, 60, 183–188. [Google Scholar] [CrossRef]
- Saeed, D.; Westenfeld, R.; Maxhera, B.; Keymel, S.; Sherif, A.; Sadat, N.; Petrov, G.; Albert, A.; Lichtenberg, A. Prevalence of De Novo Aortic Valve Insufficiency in Patients After HeartWare VAD Implantation with an Intermittent Low-Speed Algorithm. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2016, 62, 565–570. [Google Scholar] [CrossRef]
- Furukawa, K.; Motomura, T.; Nosé, Y. Right Ventricular Failure After Left Ventricular Assist Device Implantation: The Need for an Implantable Right Ventricular Assist Device. Artif. Organs 2005, 29, 369–377. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Jacobs, J.P.; Meece, L.E.; Jeng, E.I.; Bleiweis, M.S.; Cantor, R.S.; Singletary, B.; Kirklin, J.K.; Slaughter, M.S. Timing and Outcomes of Concurrent and Sequential Biventricular Assist Device Implantation: A Society of Thoracic Surgeons Intermacs Analysis. Ann. Thorac. Surg. 2023, 116, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ancona, M.B.; Montorfano, M.; Masiero, G.; Burzotta, F.; Briguori, C.; Pagnesi, M.; Pazzanese, V.; Trani, C.; Piva, T.; De Marco, F.; et al. Device-related complications after Impella mechanical circulatory support implantation: An IMP-IT observational multicentre registry substudy. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 999–1006. [Google Scholar] [CrossRef]
- Pasha, A.K.; Lee, J.Z.; Desai, H.; Hashemzadeh, M.; Movahed, M.R. In-hospital complications associated with total artificial heart implantation in the United States between 2004 to 2011. Am. J. Cardiovasc. Dis. 2022, 12, 278–282. [Google Scholar]
- Tan, M.C.; Yeo, Y.H.; Tham, J.W.; Tan, J.L.; Fong, H.K.; Tan, B.E.-X.; Lee, K.S.; Lee, J.Z. Adverse Events in Total Artificial Heart for End-Stage Heart Failure: Insight From the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE). Int. J. Heart Fail. 2023, 6, 76–81. [Google Scholar] [CrossRef]
- Khan, T.M.; Siddiqui, A.H. Intra-Aortic Balloon Pump. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK542233/ (accessed on 28 April 2025).
- Stone, G.W.; Ohman, E.; Miller, M.F.; Joseph, D.L.; Christenson, J.T.; Cohen, M.; Urban, P.M.; Reddy, R.C.; Freedman, R.J.; Staman, K.L.; et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: The benchmark registry. J. Am. Coll. Cardiol. 2003, 41, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Sokolovic, M.; Pratt, A.K.; Vukicevic, V.; Sarumi, M.; Johnson, L.S.; Shah, N.S. Platelet Count Trends and Prevalence of Heparin-Induced Thrombocytopenia in a Cohort of Extracorporeal Membrane Oxygenator Patients. Crit. Care Med. 2016, 44, e1031–e1037. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.V.; Barsness, G.W.; Vallabhajosyula, S.; Vallabhajosyula, S. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiol. Ther. 2019, 8, 211–228. [Google Scholar] [CrossRef]
- Bisdas, T.; Beutel, G.; Warnecke, G.; Hoeper, M.M.; Kuehn, C.; Haverich, A.; Teebken, O.E. Vascular Complications in Patients Undergoing Femoral Cannulation for Extracorporeal Membrane Oxygenation Support. Ann. Thorac. Surg. 2011, 92, 626–631. [Google Scholar] [CrossRef]
- Russo, J.J.; Aleksova, N.; Pitcher, I.; Couture, E.; Parlow, S.; Faraz, M.; Visintini, S.; Simard, T.; Di Santo, P.; Mathew, R.; et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Feiger, B.; Kochar, A.; Gounley, J.; Bonadonna, D.; Daneshmand, M.; Randles, A. Determining the impacts of venoarterial extracorporeal membrane oxygenation on cerebral oxygenation using a one-dimensional blood flow simulator. J. Biomech. 2020, 104, 109707. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Schroeder, S.E.; Houston, B.A.; Robinson, M.R.; Gosev, I.; Reyentovich, A.; Koehl, D.; Cantor, R.; Jorde, U.P.; Kirklin, J.K.; et al. The Society of Thoracic Surgeons Intermacs 2022 Annual Report: Focus on the 2018 Heart Transplant Allocation System. Ann. Thorac. Surg. 2023, 115, 311–327. [Google Scholar] [CrossRef]
- Tedford, R.J.; Leacche, M.; Lorts, A.; Drakos, S.G.; Pagani, F.D.; Cowger, J. Durable Mechanical Circulatory Support. JACC 2023, 82, 1464–1481. [Google Scholar] [CrossRef]
- Abraham, J.; Anderson, M.; Silvestry, S.; Soltesz, E.G.; Ono, M.; Mody, K.; Esmailian, F.; Kilic, A.; Bharmi, R.; Chhim, R.; et al. Outcomes of Surgically Implanted Impella Microaxial Flow Pumps in Heart Failure-Related Cardiogenic Shock. J. Card. Fail. 2025. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, M.; Sibbing, D.; Bauer, I.; Fröhlich, G.; Bott-Flügel, L.; Byrne, R.; Dirschinger, J.; Kastrati, A.; Schömig, A. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 52, 1584–1588. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Eriksen, E.; Sjauw, K.D.; van Dongen, I.M.; Hirsch, A.; Packer, E.J.; Vis, M.M.; Wykrzykowska, J.J.; Koch, K.T.; Baan, J.; et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 278–287. [Google Scholar] [CrossRef]
- Dhruva, S.S.; Ross, J.S.; Mortazavi, B.J.; Hurley, N.C.; Krumholz, H.M.; Curtis, J.P.; Berkowitz, A.; Masoudi, F.A.; Messenger, J.C.; Parzynski, C.S.; et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump With In-Hospital Mortality and Major Bleeding Among Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA 2020, 323, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Moriguchi, J.; Shah, K.B.; Anyanwu, A.C.; Mahr, C.; Skipper, E.; Cossette, M.; Noly, P.-E. Outcomes after heart transplantation and total artificial heart implantation: A multicenter study. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2021, 40, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Dziewierz, A.; Siudak, Z.; Rakowski, T.; Kleczyński, P.; Zasada, W.; Dudek, D. Original paper Impact of intra-aortic balloon pump on long-term mortality of unselected patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock. Adv. Interv. Cardiol. Kardiol. Interwencyjnej 2014, 10, 175–180. [Google Scholar] [CrossRef]
- Teaima, T.; Gajjar, R.; Jha, V.; Aziz, I.; Shoura, S.; Shilbayeh, A.-R.; Battikh, N.; Sqour, H.; Gomez-Valencia, J. Impact of right ventricular dysfunction on outcomes in patients requiring intra-aortic balloon pump placement: A retrospective nationwide analysis (2016–2020). Curr. Probl. Cardiol. 2024, 49, 102611. [Google Scholar] [CrossRef]
- Registry Dashboard. ECMO. Extracorporeal Membrane Oxygenation. Available online: https://www.elso.org/registry/elsoliveregistrydashboard.aspx (accessed on 8 June 2025).
- Rossong, H.; Debreuil, S.; Yan, W.; Hiebert, B.M.; Singal, R.K.; Arora, R.C.; Yamashita, M.H. Long-term survival and quality of life after extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2023, 166, 555–566.e2. [Google Scholar] [CrossRef]
- Malik, A.; Basu, T.; VanAken, G.; Aggarwal, V.; Lee, R.; Abdul-Aziz, A.; Birati, E.Y.; Basir, M.B.; Nallamothu, B.K.; Shore, S. National Trends for Temporary Mechanical Circulatory Support Utilization in Patients With Cardiogenic Shock From Decompensated Chronic Heart Failure: Incidence, Predictors, Outcomes, and Cost. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101177. [Google Scholar] [CrossRef]
- Malone, G.; Abdelsayed, G.; Bligh, F.; Al Qattan, F.; Syed, S.; Varatharajullu, P.; Msellati, A.; Mwipatayi, D.; Azhar, M.; Malone, A.; et al. Advancements in left ventricular assist devices to prevent pump thrombosis and blood coagulopathy. Am. J. Anat. 2022, 242, 29–49. [Google Scholar] [CrossRef]
- Aigner, P.; Schlöglhofer, T.; Plunger, L.C.; Beitzke, D.; Wielandner, A.; Schima, H.; Zimpfer, D.; Moscato, F. Pump position and thrombosis in ventricular assist devices: Correlation of radiographs and CT data. Int. J. Artif. Organs 2021, 44, 956–964. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Liu, L.; Li, L.; Hua, Y.; Zhang, J.; Ishida, M.; Yoshida, N.; Tabata, A.; Sougawa, N.; et al. Developing Thick Cardiac Tissue with a Multilayer Fiber Sheet for Treating Myocardial Infarction. Adv. Fiber Mater. 2023, 5, 1905–1918. [Google Scholar] [CrossRef]
- Khan, M.S.; Smego, D.; Li, J.; Ishidoya, Y.; Offei, E.; Castillo, M.S.R.; Hirahara, A.M.; Balmaceda, P.; Hunter, J.; Athavale, A.; et al. AAV9-cBIN1 gene therapy rescues chronic heart failure due to ischemic cardiomyopathy in a canine model. Commun. Med. 2025, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Bridges, C.; Katz, M.G.; Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart J. 2017, 38, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Chen, Q. Mesenchymal stem cell therapy for heart failure: A meta-analysis. Herz 2020, 45, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’aMato, A.; Prosperi, S.; Costi, B.; Angotti, D.; Birtolo, L.I.; Chimenti, C.; Lavalle, C.; Maestrini, V.; Mancone, M.; et al. Sodium-glucose cotransporter 2 inhibitors and heart failure: The best timing for the right patient. Heart Fail. Rev. 2023, 28, 709–721. [Google Scholar] [CrossRef]
- Ryczek, N.; Hryhorowicz, M.; Zeyland, J.; Lipiński, D.; Słomski, R. CRISPR/Cas Technology in Pig-to-Human Xenotransplantation Research. Int. J. Mol. Sci. 2021, 22, 3196. [Google Scholar] [CrossRef]




| Device | Primary Indications | Common Complications | Survival Outcomes | Typical Patient Population |
|---|---|---|---|---|
| LVAD | - Refractory left-sided HF - Bridge to transplantation - Destination therapy | - Bleeding (GI, perioperative) - Thrombosis - Stroke - Infection (driveline, pump) - RV failure - Aortic insufficiency |
- 1 year: ~84–87%
- 5 years: ~52% - Improved QoL (NYHA class, walk test) | - Patients with end-stage HF not eligible for transplant or are awaiting transplant |
| BiVAD |
- Biventricular HF
- Bridge to transplant |
- Higher rate of infection
- Bleeding - Neurologic events - Device failure - Renal dysfunction |
- 6 months: ~56%
- 1 year: ~50% - Concomitant implant is better compared to sequential implant | - Critically ill patients with RHF + LHF, often not candidates for isolated LVAD |
| TAH |
- Replacement for both ventricles
- Bridge to transplant (not a destination therapy in U.S.) |
- Infection
- Device malfunction - Renal failure - Stroke |
- 1 year: 75%
- 2 years: 64% - 5 years: 58% - 63.5% successfully transitioned to heart transplant | - Patients with end-stage biventricular HF unsuitable for LVAD/BiVAD and awaiting transplant |
| Impella |
- Cardiogenic shock
- High-risk PCI |
- Bleeding
- Hemolysis - Limb ischemia |
- 1 month: ~48%
- No survival benefit over IABP | - Acute cardiogenic shock patients during/after intervention |
| IABP |
- Cardiogenic shock, especially post-MI
- Supportive therapy post-CABG |
- Limb ischemia
- Stroke - Balloon leak - Bleeding | - Overall, IABP mortality ~0.05% | - Used for transient support in patients with ischemic cardiogenic shock or low output post-surgery |
| ECMO (VA/VV) |
- VA: cardiogenic shock, cardiac arrest
- VV: refractory ARDS |
- Bleeding
- Thrombosis - Stroke - Hemolysis - Limb ischemia - LV distension |
- 5 years (overall): ~33% (VA) and ~36% (VV)
- 5 years (after first 30-day survival): ~73% (VA) and ~71% (VV) | - Patients in severe respiratory/circulatory failure or multiorgan dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugal, J.K.; Malhi, A.S.; Singh, Y.; Razmi, R.; Vance, J.; Sharma, D. Advances in Mechanical Circulatory Support (MCS): Literature Review. Biomedicines 2025, 13, 1580. https://doi.org/10.3390/biomedicines13071580
Dugal JK, Malhi AS, Singh Y, Razmi R, Vance J, Sharma D. Advances in Mechanical Circulatory Support (MCS): Literature Review. Biomedicines. 2025; 13(7):1580. https://doi.org/10.3390/biomedicines13071580
Chicago/Turabian StyleDugal, Jasmine K., Arpinder S. Malhi, Yuvraj Singh, Rooz Razmi, Joshua Vance, and Divyansh Sharma. 2025. "Advances in Mechanical Circulatory Support (MCS): Literature Review" Biomedicines 13, no. 7: 1580. https://doi.org/10.3390/biomedicines13071580
APA StyleDugal, J. K., Malhi, A. S., Singh, Y., Razmi, R., Vance, J., & Sharma, D. (2025). Advances in Mechanical Circulatory Support (MCS): Literature Review. Biomedicines, 13(7), 1580. https://doi.org/10.3390/biomedicines13071580





