Interactions of Nedaplatin with Nucleobases and Purine Alkaloids: Their Role in Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Study
- B3LYP/6-31G(d,p) [86,87]: This hybrid functional combines Becke’s 1988 exchange functional [88] with the Lee–Yang–Parr correlation functional [89], incorporating some Hartree–Fock exchange. It is widely used for its versatility across various molecular systems. For heavy atoms such as platinum, the LanL2DZ basis set was used [90,91], which includes relativistic effective core potentials to account for heavy-element effects.
2.2. Experimental Study (UV-Vis Spectroscopy)
3. Results and Discussion
3.1. In Silico Study
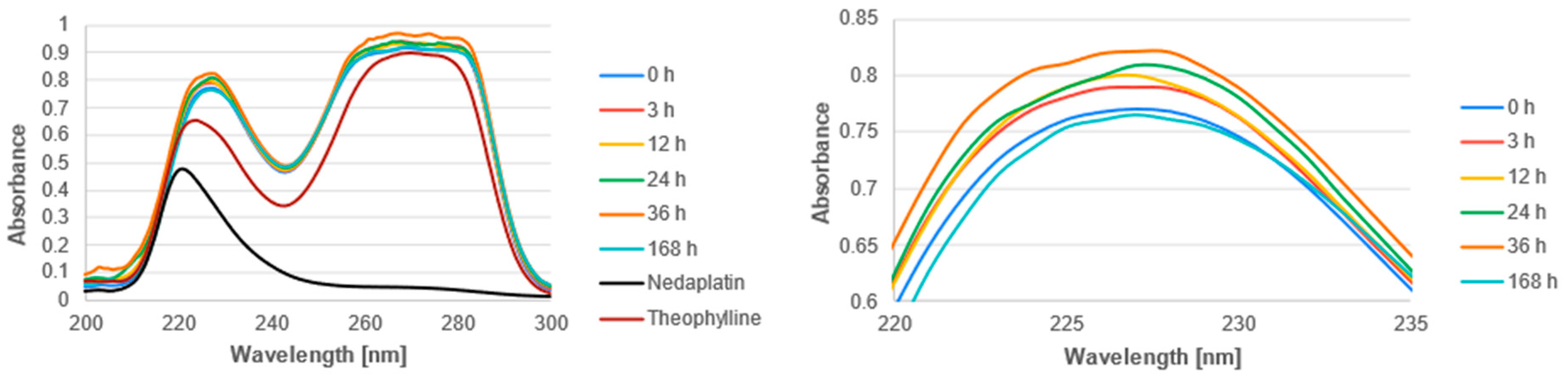
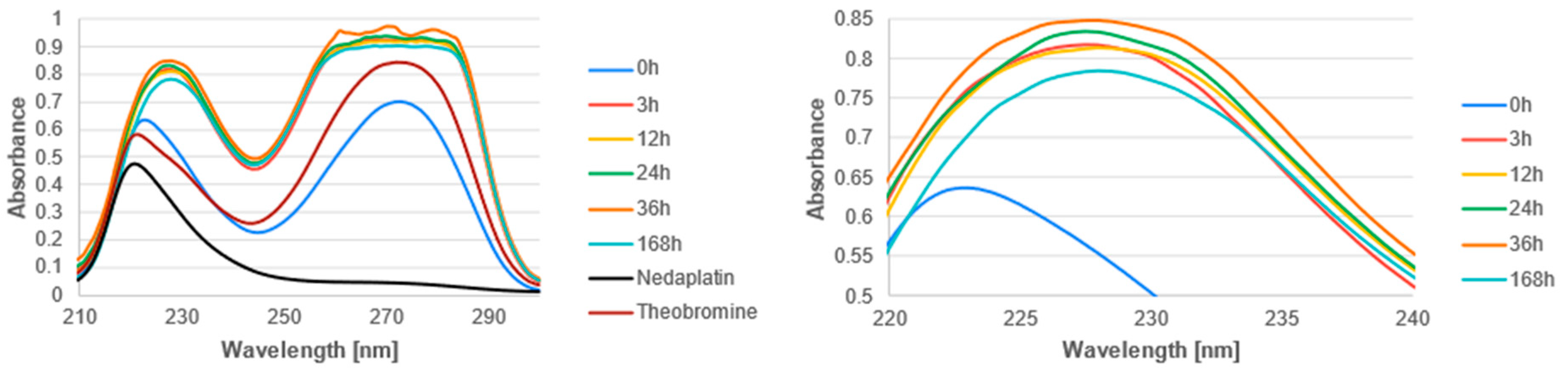
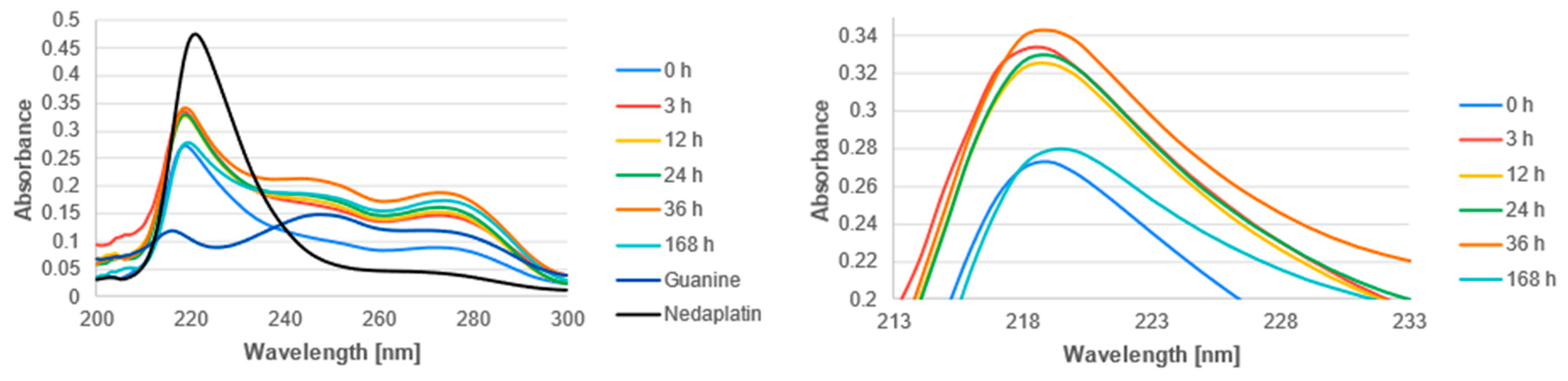
3.2. Experimental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimada, M.; Itamochi, H.; Kigawa, J. Nedaplatin: A Cisplatin Derivative in Cancer Chemotherapy. Cancer Manag. Res. 2013, 5, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ota, K. [Nedaplatin]. Gan Kagaku Ryoho 1996, 23, 379–387. [Google Scholar]
- Zhong, L.-Z.; Xu, H.-Y.; Zhao, Z.-M.; Zhang, G.-M.; Lin, F.-W. Comparison of Efficacy and Toxicity between Nedaplatin and Cisplatin in Treating Malignant Pleural Effusion. OncoTargets Ther. 2018, 11, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; Loos, W.J.; Eechoute, K.; Verweij, J.; Mathijssen, R.H.J.; Wiemer, E.A.C. Drug Transporters of Platinum-Based Anticancer Agents and Their Clinical Significance. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2011, 14, 22–34. [Google Scholar] [CrossRef]
- Kozelka, J.; Legendre, F.; Reeder, F.; Chottard, J.-C. Kinetic Aspects of Interactions between DNA and Platinum Complexes. Coord. Chem. Rev. 1999, 190–192, 61–82. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Zhang, W.; Shen, Z.; Hu, X.; Zhu, X. Molecular Mechanisms of Cisplatin Resistance in Cervical Cancer. Drug Des. Devel. Ther. 2016, 10, 1885–1895. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Zoń, A.; Bednarek, I. Cisplatin in Ovarian Cancer Treatment—Known Limitations in Therapy Force New Solutions. Int. J. Mol. Sci. 2023, 24, 7585. [Google Scholar] [CrossRef]
- Bischin, C.; Lupan, A.; Taciuc, V.; Silaghi-Dumitrescu, R. Interactions between Proteins and Platinum-Containing Anti-Cancer Drugs. Mini Rev. Med. Chem. 2011, 11, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Yoo, H.; Lin, W.; Brooks, J.G.; Lippard, S.J. Pt(IV) Prodrugs Designed to Bind Non-Covalently to Human Serum Albumin for Drug Delivery. J. Am. Chem. Soc. 2014, 136, 8790–8798. [Google Scholar] [CrossRef]
- Anufrieva, N.V.; Morozova, E.A.; Kulikova, V.V.; Bazhulina, N.P.; Manukhov, I.V.; Degtev, D.I.; Gnuchikh, E.Y.; Rodionov, A.N.; Zavilgelsky, G.B.; Demidkina, T.V. Sulfoxides, Analogues of L-Methionine and L-Cysteine as Pro-Drugs against Gram-Positive and Gram-Negative Bacteria. Acta Naturae 2015, 7, 128–135. [Google Scholar] [CrossRef]
- Potęga, A. Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules 2022, 27, 5252. [Google Scholar] [CrossRef]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent Advances in Targeting the “Undruggable” Proteins: From Drug Discovery to Clinical Trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, G.; Kojić, V.; Srdić, T.; Jakimov, D.; Djuran, M.I.; Bugarcić, Z.D.; Baltić, M.; Baltić, V.V. Growth Effects of Some Platinum(II) Complexes with Sulfur-Containing Carrier Ligands on MCF7 Human Breast Cancer Cell Line upon Simultaneous Administration with Taxol. Met.-Based Drugs 2002, 9, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lilley, D.M.J. Cisplatin Adducts in DNA: Distortion and Recognition. JBIC J. Biol. Inorg. Chem. 1996, 1, 189–191. [Google Scholar] [CrossRef]
- Malinge, J.M.; Giraud-Panis, M.J.; Leng, M. Interstrand Cross-Links of Cisplatin Induce Striking Distortions in DNA. J. Inorg. Biochem. 1999, 77, 23–29. [Google Scholar] [CrossRef]
- Zhu, G.; Myint, M.; Ang, W.H.; Song, L.; Lippard, S.J. Monofunctional Platinum-DNA Adducts Are Strong Inhibitors of Transcription and Substrates for Nucleotide Excision Repair in Live Mammalian Cells. Cancer Res. 2012, 72, 790–800. [Google Scholar] [CrossRef]
- Kanzawa, F.; Koizumi, F.; Koh, Y.; Nakamura, T.; Tatsumi, Y.; Fukumoto, H.; Saijo, N.; Yoshioka, T.; Nishio, K. In Vitro Synergistic Interactions between the Cisplatin Analogue Nedaplatin and the DNA Topoisomerase I Inhibitor Irinotecan and the Mechanism of This Interaction. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 202–209. [Google Scholar]
- Kawanishi, K.; Miyagi, Y.; Yamamoto, J.; Miyagi, Y.; Nakamura, K.; Kodama, J.; Hongo, A.; Yoshinouchi, M.; Kudo, T. Cytocidal Effect and DNA Damage of Nedaplatin in Vitro by Simulating Pharmacokinetic Parameters. Cancer Chemother. Pharmacol. 2001, 47, 303–308. [Google Scholar] [CrossRef]
- Alberto, M.E.; Butera, V.; Russo, N. Which One among the Pt-Containing Anticancer Drugs More Easily Forms Monoadducts with G and A DNA Bases? A Comparative Study among Oxaliplatin, Nedaplatin, and Carboplatin. Inorg. Chem. 2011, 50, 6965–6971. [Google Scholar] [CrossRef]
- Deepa, P.; Kolandaivel, P.; Senthilkumar, K. Structural Properties and the Effect of Platinum Drugs with DNA Base Pairs. Struct. Chem. 2013, 24, 583–595. [Google Scholar] [CrossRef]
- Pizarro, A.M.; Barry, N.P.E.; Sadler, P.J. Metal–DNA Coordination Complexes. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; pp. 751–784. ISBN 978-0-08-096529-1. [Google Scholar]
- Brabec, V.; Hrabina, O.; Kasparkova, J. Cytotoxic Platinum Coordination Compounds. DNA Binding Agents. Coord. Chem. Rev. 2017, 351, 2–31. [Google Scholar] [CrossRef]
- Szupryczyński, K.; Czeleń, P.; Jeliński, T.; Szefler, B. What Is the Reason That the Pharmacological Future of Chemotherapeutics in the Treatment of Lung Cancer Could Be Most Closely Related to Nanostructures? Platinum Drugs in Therapy of Non-Small and Small Cell Lung Cancer and Their Unexpected, Possible Interactions. The Review. Int. J. Nanomed. 2024, 19, 9503–9547. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Bridgewater, H.E.; Song, J.-I.; Smith, N.A.; Barry, N.P.E.; Bagley, I.; Sadler, P.J.; Romero-Canelón, I. In Vivo Selectivity and Localization of Reactive Oxygen Species (ROS) Induction by Osmium Anticancer Complexes That Circumvent Platinum Resistance. J. Med. Chem. 2018, 61, 9246–9255. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Wang, D.; Li, S.; Sun, K.; Wan, X.; Taylor, E.W.; Zhang, J. Inhibition of Glutathione Synthesis Eliminates the Adaptive Response of Ascitic Hepatoma 22 Cells to Nedaplatin That Targets Thioredoxin Reductase. Toxicol. Appl. Pharmacol. 2012, 265, 342–350. [Google Scholar] [CrossRef]
- Iida, M.; Doi, H.; Asamoto, S.; Sugiyama, H.; Sakagami, H.; Kuribayashi, N.; Takeda, M.; Okamura, Y.; Matsumoto, K. Effect of Glutathione-Modulating Compounds on Platinum Compounds-Induced Cytotoxicity in Human Glioma Cell Lines. Anticancer Res. 1999, 19, 5383–5384. [Google Scholar]
- Mirzaei, S.; Hushmandi, K.; Zabolian, A.; Saleki, H.; Torabi, S.M.R.; Ranjbar, A.; SeyedSaleh, S.; Sharifzadeh, S.O.; Khan, H.; Ashrafizadeh, M.; et al. Elucidating Role of Reactive Oxygen Species (ROS) in Cisplatin Chemotherapy: A Focus on Molecular Pathways and Possible Therapeutic Strategies. Molecules 2021, 26, 2382. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Nizami, Z.N.; Aburawi, H.E.; Semlali, A.; Muhammad, K.; Iratni, R. Oxidative Stress Inducers in Cancer Therapy: Preclinical and Clinical Evidence. Antioxidants 2023, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.K.; Taylor, D.D.; Wan, T.; O’Connor, D.M.; Doering, D.L.; Gerçel-Taylor, C. Apoptosis as a Measure of Chemosensitivity to Cisplatin and Taxol Therapy in Ovarian Cancer Cell Lines. Gynecol. Oncol. 1997, 65, 13–22. [Google Scholar] [CrossRef]
- Gonzalez, V.M.; Fuertes, M.A.; Alonso, C.; Perez, J.M. Is Cisplatin-Induced Cell Death Always Produced by Apoptosis? Mol. Pharmacol. 2001, 59, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-T.; Ke, L.-X.; Wu, X.-Y.; Tian, H.-T.; Deng, H.-Z.; Xu, L.-Y.; Li, E.-M.; Long, L. TRIP13 Induces Nedaplatin Resistance in Esophageal Squamous Cell Carcinoma by Enhancing Repair of DNA Damage and Inhibiting Apoptosis. BioMed Res. Int. 2022, 2022, 7295458. [Google Scholar] [CrossRef]
- Duffull, S.B.; Robinson, B.A. Clinical Pharmacokinetics and Dose Optimisation of Carboplatin. Clin. Pharmacokinet. 1997, 33, 161–183. [Google Scholar] [CrossRef]
- Scheff, J.D.; Almon, R.R.; DuBois, D.C.; Jusko, W.J.; Androulakis, I.P. Assessment of Pharmacologic Area Under the Curve When Baselines Are Variable. Pharm. Res. 2011, 28, 1081–1089. [Google Scholar] [CrossRef]
- Duan, F.; Simeone, S.; Wu, R.; Grady, J.; Mandoiu, I.; Srivastava, P.K. Area under the Curve as a Tool to Measure Kinetics of Tumor Growth in Experimental Animals. J. Immunol. Methods 2012, 382, 224–228. [Google Scholar] [CrossRef]
- Ota, K.; Oguma, T.; Shimamura, K. Pharmacokinetics of Platinum in Cancer Patients Following Intravenous Infusion of Cis-Diammine(Glycolato)Platinum, 254-S. Anticancer Res. 1994, 14, 1383–1387. [Google Scholar]
- Zhang, K.; Qin, H.; Pan, F.; Liu, E.; Liang, H.; Ruan, Z. Nedaplatin or Oxaliplatin Combined with Paclitaxel and Docetaxel as First-Line Treatment for Patients with Advanced Non-Small Cell Lung Cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 2830–2836. [Google Scholar] [CrossRef]
- Oshita, F.; Yamada, K.; Saito, H.; Noda, K. Phase II Study of Nedaplatin and Irinotecan Followed by Gefitinib for Elderly Patients with Unresectable Non-Small Cell Lung Cancer. Cancer Chemother. Pharmacol. 2008, 62, 465–470. [Google Scholar] [CrossRef]
- Fu, J.; Fang, S.; Wen, Y.; Wang, Y.; Yin, X.; Wang, D. The Efficacy and Safety of Irinotecan Combined with Nedaplatin in the Treatment of Small Cell Lung Cancer. J. BUON Off. J. Balk. Union Oncol. 2020, 25, 1707–1713. [Google Scholar]
- Teramoto, K.; Asada, Y.; Ozaki, Y.; Suzumura, Y.; Nakano, Y.; Sawai, S.; Tezuka, N.; Inoue, S.; Fujino, S. A Phase II Study of Docetaxel plus Nedaplatin in Patients with Metastatic Non-Small-Cell Lung Cancer. Cancer Chemother. Pharmacol. 2012, 70, 531–537. [Google Scholar] [CrossRef]
- Yang, S. A Pooled Study on Combination of Gemcitabine and Nedaplatin for Treating Patients with Non-Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 5963–5966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Igawa, S.; Otani, S.; Nakahara, Y.; Ryuge, S.; Hiyoshi, Y.; Fukui, T.; Mitsufuji, H.; Kubota, M.; Katagiri, M.; Sato, Y.; et al. Phase I Study of Nedaplatin, a Platinum Based Antineoplastic Drug, Combined with Nab-Paclitaxel in Patients with Advanced Squamous Non-Small Cell Lung Cancer. Investig. New Drugs 2018, 36, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mai, W.; Hao, B.; Jiang, W.; Geng, Q. Promising Response to a PD-1 Inhibitor (Sintilimab) in Non-Small Cell Lung Cancer: A Case Report. Medicine 2020, 99, e19790. [Google Scholar] [CrossRef]
- Kenmotsu, Y.; Oshita, F.; Sugiura, M.; Murakami, S.; Kondo, T.; Saito, H.; Yamada, K. Nedaplatin and Irinotecan in Patients with Large-Cell Neuroendocrine Carcinoma of the Lung. Anticancer Res. 2012, 32, 1453–1456. [Google Scholar]
- Kasai, T.; Mori, K.; Sugiyama, T.; Koyama, N.; Nakamura, Y.; Ohyanagi, F.; Fukuda, H.; Hoshi, E.; Kobayashi, K.; Nakayama, M. Phase I/II Study of Nedaplatin and Nab-Paclitaxel for Patients with Previously Untreated Advanced Squamous Cell Lung Cancer: Kanto Respiratory Disease Study Group (KRSG) 1302. Int. J. Clin. Oncol. 2022, 27, 1841–1848. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Z.; Hu, C.; Zhang, J.; Chen, Y.; Song, Y.; Zhao, Q.; Fan, Y.; Wu, G.; Ma, Z.; et al. Nedaplatin Plus Docetaxel Versus Cisplatin Plus Docetaxel as First-Line Chemotherapy for Advanced Squamous Cell Carcinoma of the Lung—A Multicenter, Open-Label, Randomized, Phase III Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1743–1749. [Google Scholar] [CrossRef]
- Masago, K.; Fujita, S.; Kim, Y.H.; Hatachi, Y.; Fukuhara, A.; Irisa, K.; Nagai, H.; Sakamori, Y.; Togashi, Y.; Mio, T.; et al. Phase I Study of the Combination of Nedaplatin and Gemcitabine in Previously Untreated Advanced Squamous Cell Lung Cancer. Cancer Chemother. Pharmacol. 2011, 67, 325–330. [Google Scholar] [CrossRef]
- Yamada, K.; Saito, H.; Kondo, T.; Murakami, S.; Masuda, N.; Yamamoto, M.; Igawa, S.; Katono, K.; Takiguchi, Y.; Iwasawa, S.; et al. Multicenter Phase II Study of Nedaplatin and Irinotecan for Patients with Squamous Cell Carcinoma of the Lung: Thoracic Oncology Research Group 0910. Anticancer Res. 2015, 35, 6705–6711. [Google Scholar] [PubMed]
- Tang, Z.; Fang, R.; Tong, G.; Liu, P.; Ou, Z.; Tang, Y. Overcoming Resistance to Anti-PD-1 Immunotherapy in Lymphoepithelioma-like Carcinoma: A Case Report and Review of the Literature. Lung Cancer 2020, 146, 335–340. [Google Scholar] [CrossRef]
- Kagabu, M.; Shoji, T.; Murakami, K.; Omi, H.; Honda, T.; Miura, F.; Yokoyama, Y.; Tokunaga, H.; Takano, T.; Ohta, T.; et al. Clinical Efficacy of Nedaplatin-Based Concurrent Chemoradiotherapy for Uterine Cervical Cancer: A Tohoku Gynecologic Cancer Unit Study. Int. J. Clin. Oncol. 2016, 21, 735–740. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mochizuki, K.; Hirayama, T.; Ikeda, M.; Nishi, M.; Tabata, K.; Okazaki, M.; Fujita, T.; Taoka, Y.; Iwamura, M. Gemcitabine plus Nedaplatin as Salvage Therapy Is a Favorable Option for Patients with Progressive Metastatic Urothelial Carcinoma after Two Lines of Chemotherapy. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Taguchi, K.; Kunishima, Y.; Yanase, M.; Takahashi, A.; Shigyo, M.; Tanaka, T.; Mutoh, M.; Fukuta, F.; Masumori, N.; et al. Paclitaxel, Ifosfamide, and Nedaplatin as Second-Line Treatment for Patients with Metastatic Urothelial Carcinoma: A Phase II Study of the SUOC Group. Cancer Sci. 2011, 102, 1171–1175. [Google Scholar] [CrossRef]
- Ueda, H.; Kawakami, H.; Nonagase, Y.; Takegawa, N.; Okuno, T.; Takahama, T.; Takeda, M.; Chiba, Y.; Tamura, T.; Nakagawa, K. Phase II Trial of 5-Fluorouracil, Docetaxel, and Nedaplatin (UDON) Combination Therapy for Recurrent or Metastatic Esophageal Cancer. Oncologist 2019, 24, 163-e76. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Takahashi, K.; Muguruma, K.; Ohira, M.; Nagayama, K. Establishment of Nedaplatin Safety Dose Formula Based on Renal Function for Combination Chemotherapy with Nedaplatin and 5-Fluorouracil in Patients with Esophageal Cancer. Int. J. Clin. Oncol. 2022, 27, 348–353. [Google Scholar] [CrossRef]
- Heianna, J.; Makino, W.; Hirakawa, H.; Agena, S.; Tomita, H.; Ariga, T.; Ishikawa, K.; Takehara, S.; Maemoto, H.; Murayama, S. Therapeutic Efficacy of Selective Intra-Arterial Chemoradiotherapy with Docetaxel and Nedaplatin for Fixed Bulky Nodal Disease in Head and Neck Cancer of Unknown Primary. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol.-Head Neck Surg. 2022, 279, 3105–3113. [Google Scholar] [CrossRef]
- Qiu, X.; Li, J.; Zhou, H.; Zhang, M.; Jiang, C.; Shen, Z.; Zhu, X.; Li, A.; Che, Y.; Wu, T.; et al. Concurrent Chemoradiotherapy with Raltitrexed and Nedaplatin Regimen for Esophageal Squamous Cell Carcinoma. Medicine 2020, 99, e18732. [Google Scholar] [CrossRef]
- Fujita, Y.; Hiramatsu, M.; Kawai, M.; Sumiyoshi, K.; Nishimura, H.; Tanigawa, N. Evaluation of Combined Docetaxel and Nedaplatin Chemotherapy for Recurrent Esophageal Cancer Compared with Conventional Chemotherapy Using Cisplatin and 5-Fluorouracil: A Retrospective Study. Dis. Esophagus 2008, 21, 496–501. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ojima, H.; Fukuchi, M.; Sakai, M.; Sohda, M.; Tanaka, N.; Suzuki, S.; Ieta, K.; Saito, K.; Sano, A.; et al. Phase II Study of Docetaxel, Nedaplatin, and 5-Fluorouracil Combined Chemotherapy for Advanced Esophageal Cancer. Ann. Surg. Oncol. 2015, 22, 3653–3658. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Abe, O.; Nakagawa, K. Involved-Field Irradiation Concurrently Combined with Nedaplatin/5-Fluorouracil for Inoperable Esophageal Cancer on Basis of 18FDG-PET Scans: A Long Follow-up Results of Phase II Study. Radiother. Oncol. 2017, 123, 488. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Wang, D.-S.; Wang, F.-H.; Ren, C.; Tan, Q.; Li, Y.-H. Recombinant Human Endostatin plus Paclitaxel/Nedaplatin for Recurrent or Metastatic Advanced Esophageal Squamous Cell Carcinoma: A Prospective, Single-Arm, Open-Label, Phase II Study. Investig. New Drugs 2021, 39, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhuang, Q.; Cao, Z.; Yin, R.; Zhu, Y.; Zhu, L.; Xie, X.; Zhang, Y.; Li, L.; Wu, Q.; et al. Paclitaxel plus Nedaplatin vs. Paclitaxel plus Carboplatin in Women with Epithelial Ovarian Cancer: A Multi-Center, Randomized, Open-Label, Phase III Trial. Oncol. Lett. 2018, 15, 3646–3652. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.; Czeleń, P.; Szefler, B.; Cysewski, P. Theoretical Studies on the Interaction between Chalcone Dyes and Concanavalin A—The Reactive Group Effects on the Photophysical and Biological Properties of the Fluorescence Probe. J. Photochem. Photobiol. Chem. 2017, 346, 327–337. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P.; Krawczyk, P. The Affinity of Carboplatin to B-Vitamins and Nucleobases. Int. J. Mol. Sci. 2021, 22, 3634. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P.; Kruszewski, S.; Siomek-Górecka, A.; Krawczyk, P. The Assessment of Physicochemical Properties of Cisplatin Complexes with Purines and Vitamins B Group. J. Mol. Graph. Model. 2022, 113, 108144. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P.; Wojtkowiak, K.; Jezierska, A. Affinities to Oxaliplatin: Vitamins from B Group vs. Nucleobases. Int. J. Mol. Sci. 2022, 23, 10567. [Google Scholar] [CrossRef]
- Szefler, B.; Czeleń, P. Will the Interactions of Some Platinum (II)-Based Drugs with B-Vitamins Reduce Their Therapeutic Effect in Cancer Patients? Comparison of Chemotherapeutic Agents Such as Cisplatin, Carboplatin and Oxaliplatin-A Review. Int. J. Mol. Sci. 2023, 24, 1548. [Google Scholar] [CrossRef]
- Tilley, S.L. Methylxanthines in Asthma. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 439–456. [Google Scholar] [CrossRef]
- Monteiro, J.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Pharmacological Potential of Methylxanthines: Retrospective Analysis and Future Expectations. Crit. Rev. Food Sci. Nutr. 2019, 59, 2597–2625. [Google Scholar] [CrossRef]
- Ritter, J.M.; Flower, R.J.; Henderson, G.; Loke, Y.K.; MacEwan, D. Rang y Dale. Farmacología; Elsevier: Amsterdam, The Netherlands, 2024; ISBN 978-84-13-82690-5. [Google Scholar]
- Feoktistov, I.; Biaggioni, I. Adenosine A2b Receptors Evoke Interleukin-8 Secretion in Human Mast Cells. An Enprofylline-Sensitive Mechanism with Implications for Asthma. J. Clin. Investig. 1995, 96, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Elmowafi, M.; Mohsen, N.; Nour, I.; Nasef, N. Prophylactic versus Therapeutic Caffeine for Apnea of Prematurity: A Randomized Controlled Trial. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2022, 35, 6053–6061. [Google Scholar] [CrossRef] [PubMed]
- Renner, B.; Clarke, G.; Grattan, T.; Beisel, A.; Mueller, C.; Werner, U.; Kobal, G.; Brune, K. Caffeine Accelerates Absorption and Enhances the Analgesic Effect of Acetaminophen. J. Clin. Pharmacol. 2007, 47, 715–726. [Google Scholar] [CrossRef]
- Laska, E.M.; Sunshine, A.; Zighelboim, I.; Roure, C.; Marrero, I.; Wanderling, J.; Olson, N. Effect of Caffeine on Acetaminophen Analgesia. Clin. Pharmacol. Ther. 1983, 33, 498–509. [Google Scholar] [CrossRef]
- Diener, H.C.; Gaul, C.; Lehmacher, W.; Weiser, T. Aspirin, Paracetamol (Acetaminophen) and Caffeine for the Treatment of Acute Migraine Attacks: A Systemic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Eur. J. Neurol. 2022, 29, 350–357. [Google Scholar] [CrossRef]
- MeSH Browser. Available online: https://meshb.nlm.nih.gov/record/ui?ui=D013805 (accessed on 4 July 2024).
- Smit, H.J. Theobromine and the Pharmacology of Cocoa. In Methylxanthines; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 201–234. ISBN 978-3-642-13443-2. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Citation|Gaussian.Com. Available online: https://gaussian.com/citation/ (accessed on 9 July 2024).
- Ortiz Quantum Chemistry Group: Collaboration with Gaussian Inc. Available online: https://www.auburn.edu/academic/cosam/faculty/chemistry/ortiz/research/info_gaussian.html (accessed on 9 July 2024).
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Baik, M.-H.; Friesner, R.A.; Lippard, S.J. Theoretical Study of Cisplatin Binding to Purine Bases: Why Does Cisplatin Prefer Guanine over Adenine? J. Am. Chem. Soc. 2003, 125, 14082–14092. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Yu, H.S.; He, X.; Li, S.L.; Truhlar, D.G. MN15: A Kohn–Sham Global-Hybrid Exchange–Correlation Density Functional with Broad Accuracy for Multi-Reference and Single-Reference Systems and Noncovalent Interactions. Chem. Sci. 2016, 7, 5032–5051. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A New Definition of Cavities for the Computation of Solvation Free Energies by the Polarizable Continuum Model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Cysewski, P.; Szefler, B.; Kozłowska, K. Solvent Impact on the Aromaticity of Benzene Analogues: Implicit versus Explicit Solvent Approach. J. Mol. Model. 2009, 15, 731–738. [Google Scholar] [CrossRef]
- Cysewski, P.; Szefler, B.; Szatyłowicz, H.; Krygowski, T.M. An Explicit Solvent Quantum Chemistry Study on the Water Environment Influence on the Interactions of Fluoride with Phenol. New J. Chem. 2009, 33, 831–837. [Google Scholar] [CrossRef]
- Cysewski, P.; Szefler, B. Environment Influences on the Aromatic Character of Nucleobases and Amino Acids. J. Mol. Model. 2010, 16, 1709–1720. [Google Scholar] [CrossRef]
- Szefler, B.; Diudea, M.V. On Molecular Dynamics of the Diamond D5 Seeds. Struct. Chem. 2012, 23, 717–722. [Google Scholar] [CrossRef]
- Senn, H.M.; Thiel, W. QM/MM Methods for Biomolecular Systems. Angew. Chem. Int. Ed. 2009, 48, 1198–1229. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Fischer, J. Specific Detection of Nucleotides, Creatine Phosphate, and Their Derivatives from Tissue Samples in a Simple, Isocratic, Recycling, Low-Volume System. LC-GC Int. 1995, 8, 254–264. [Google Scholar]
- Belay, A.; Ture, K.; Redi, M.; Asfaw, A. Measurement of Caffeine in Coffee Beans with UV/Vis Spectrometer. Food Chem. 2008, 108, 310–315. [Google Scholar] [CrossRef]
- Guerra, J.M.; Hernández, A.R.; Garrido, L.D.G.; Silva, M.T.R. New Insights about Equilibrium Constants, pKa, of Purine Nitrogenous Bases: The Case of Adenine and Guanine. A UV-Vis Spectrophotometric Study at I = 0.4 M. J. Mex. Chem. Soc. 2025, 69, 70–77. [Google Scholar] [CrossRef]
- Barbatti, M.; Aquino, A.J.A.; Lischka, H. The UV Absorption of Nucleobases: Semi-Classical Ab Initio Spectra Simulations. Phys. Chem. Chem. Phys. 2010, 12, 4959–4967. [Google Scholar] [CrossRef]
- Kaspar, F.; Giessmann, R.T.; Krausch, N.; Neubauer, P.; Wagner, A.; Gimpel, M. A UV/Vis Spectroscopy-Based Assay for Monitoring of Transformations Between Nucleosides and Nucleobases. Methods Protoc. 2019, 2, 60. [Google Scholar] [CrossRef]
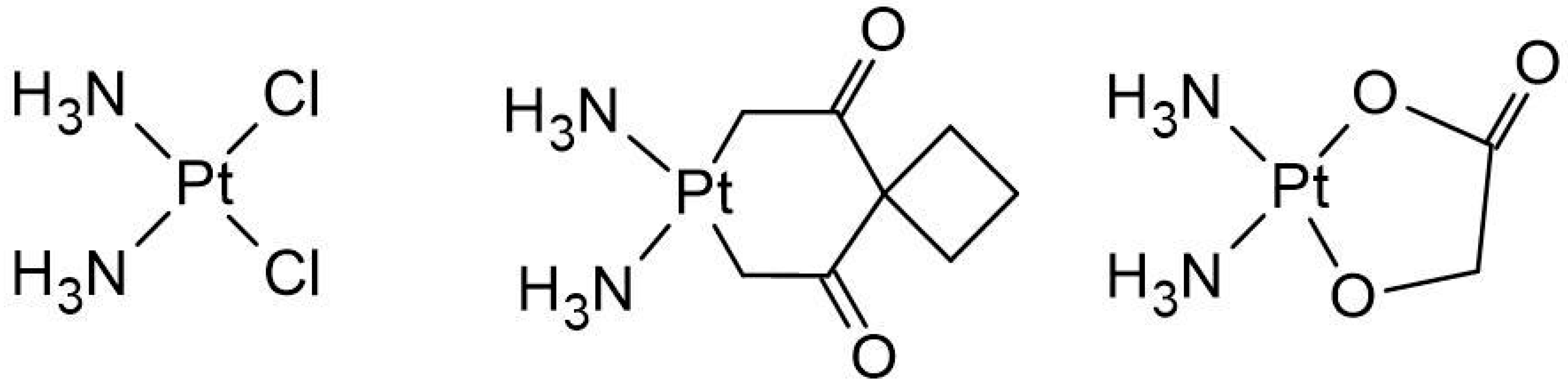
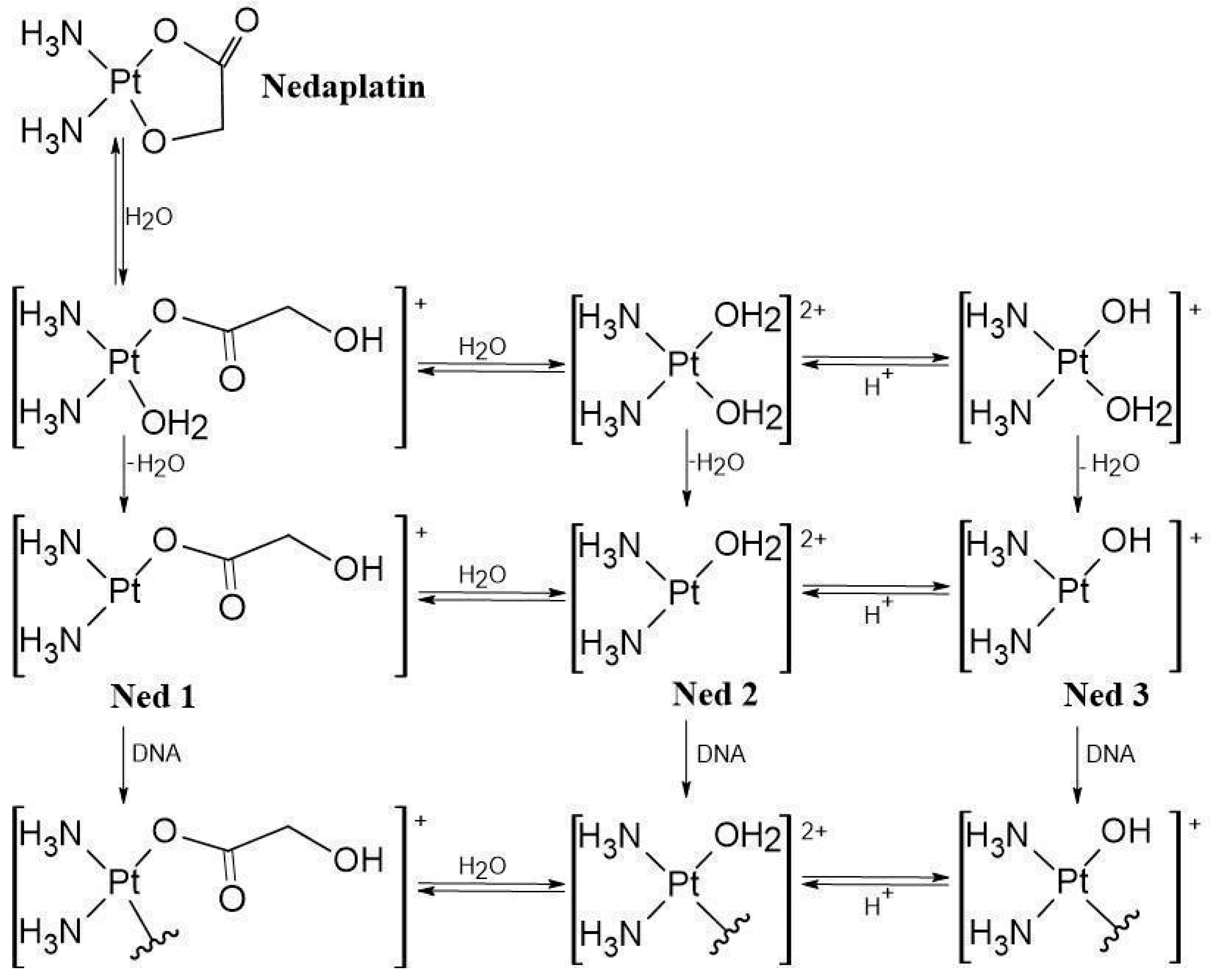

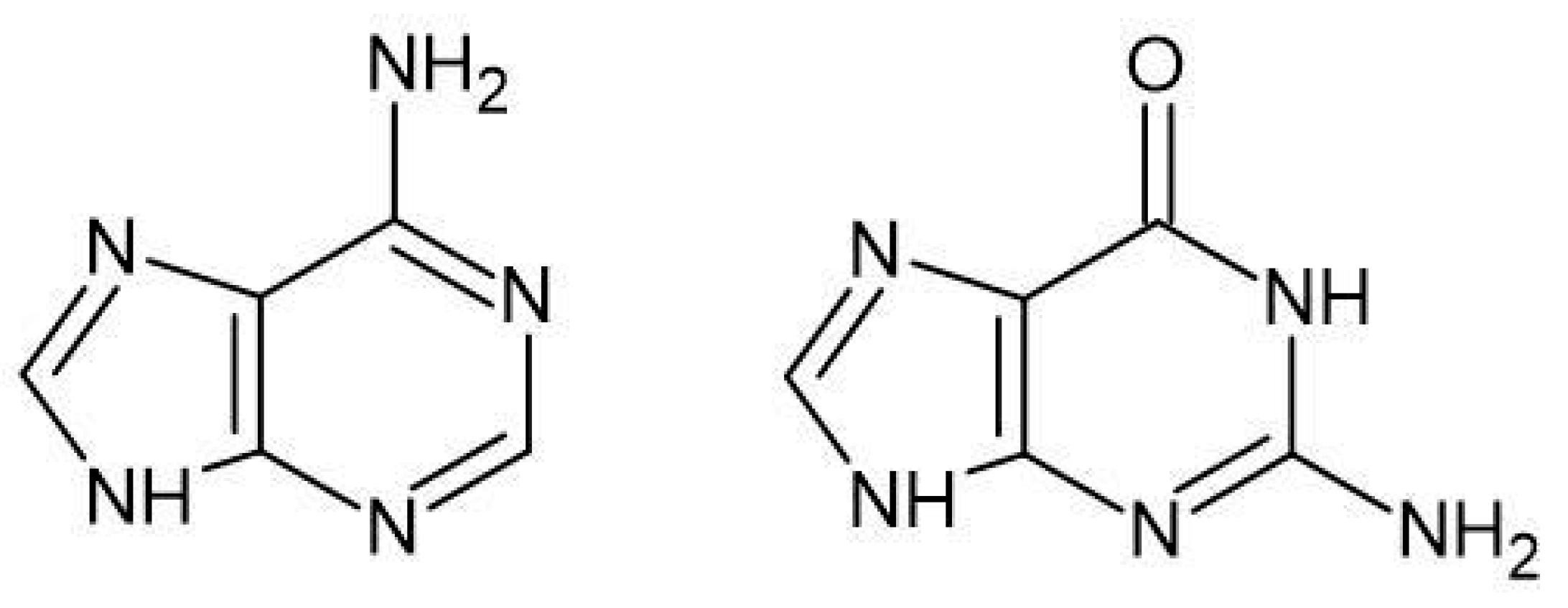
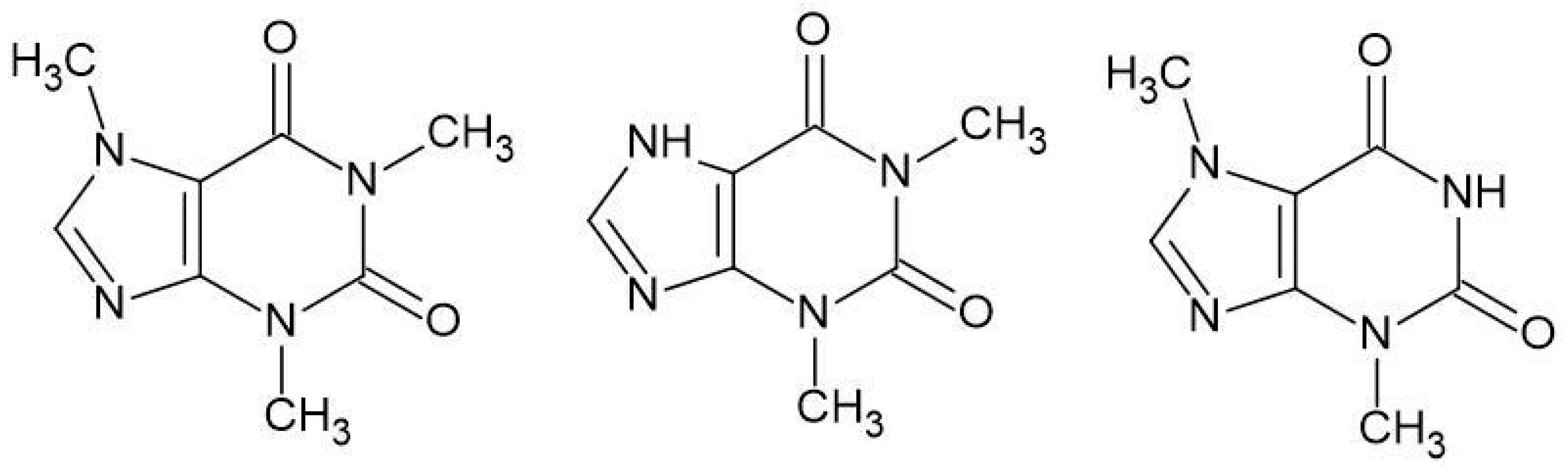

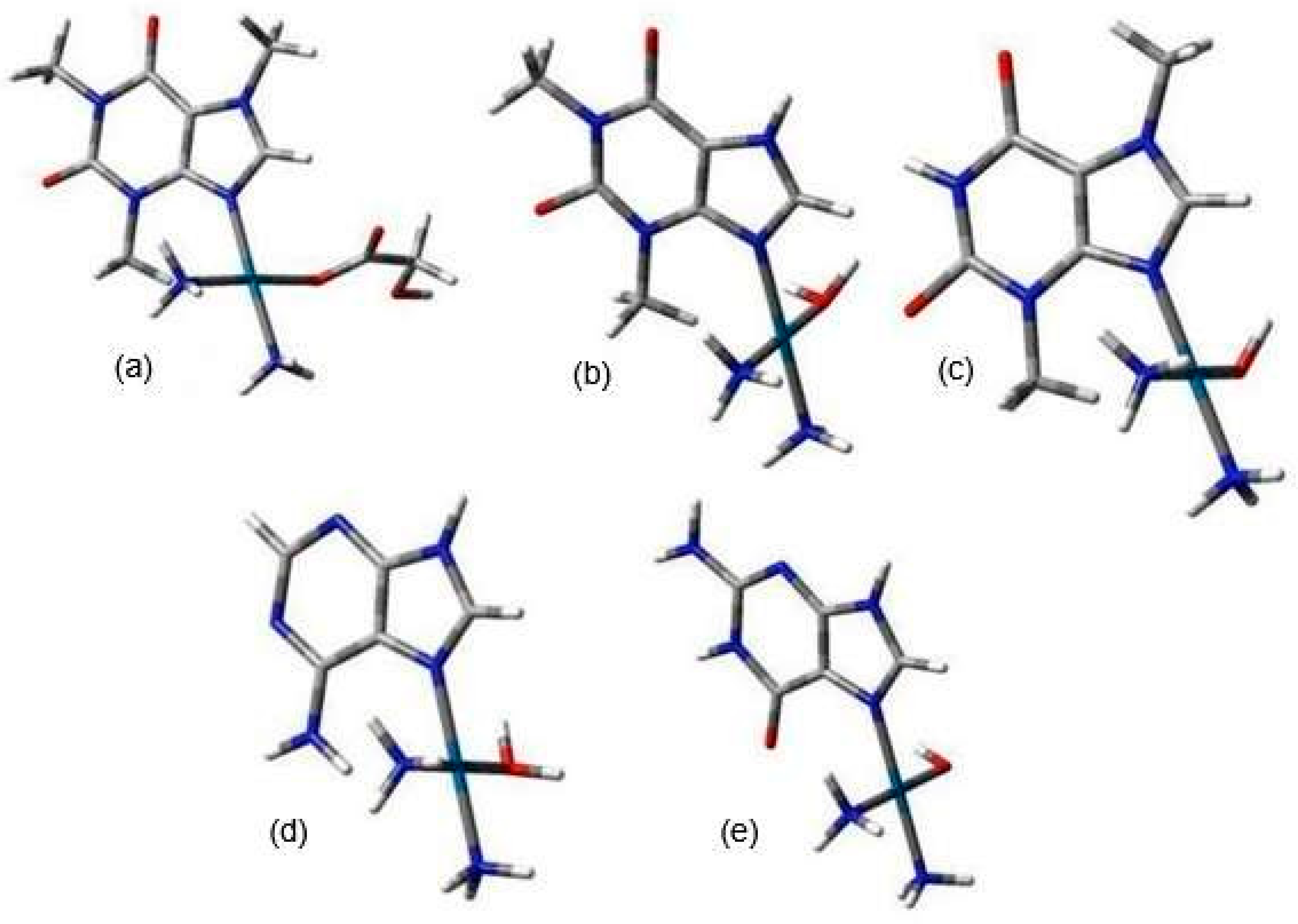
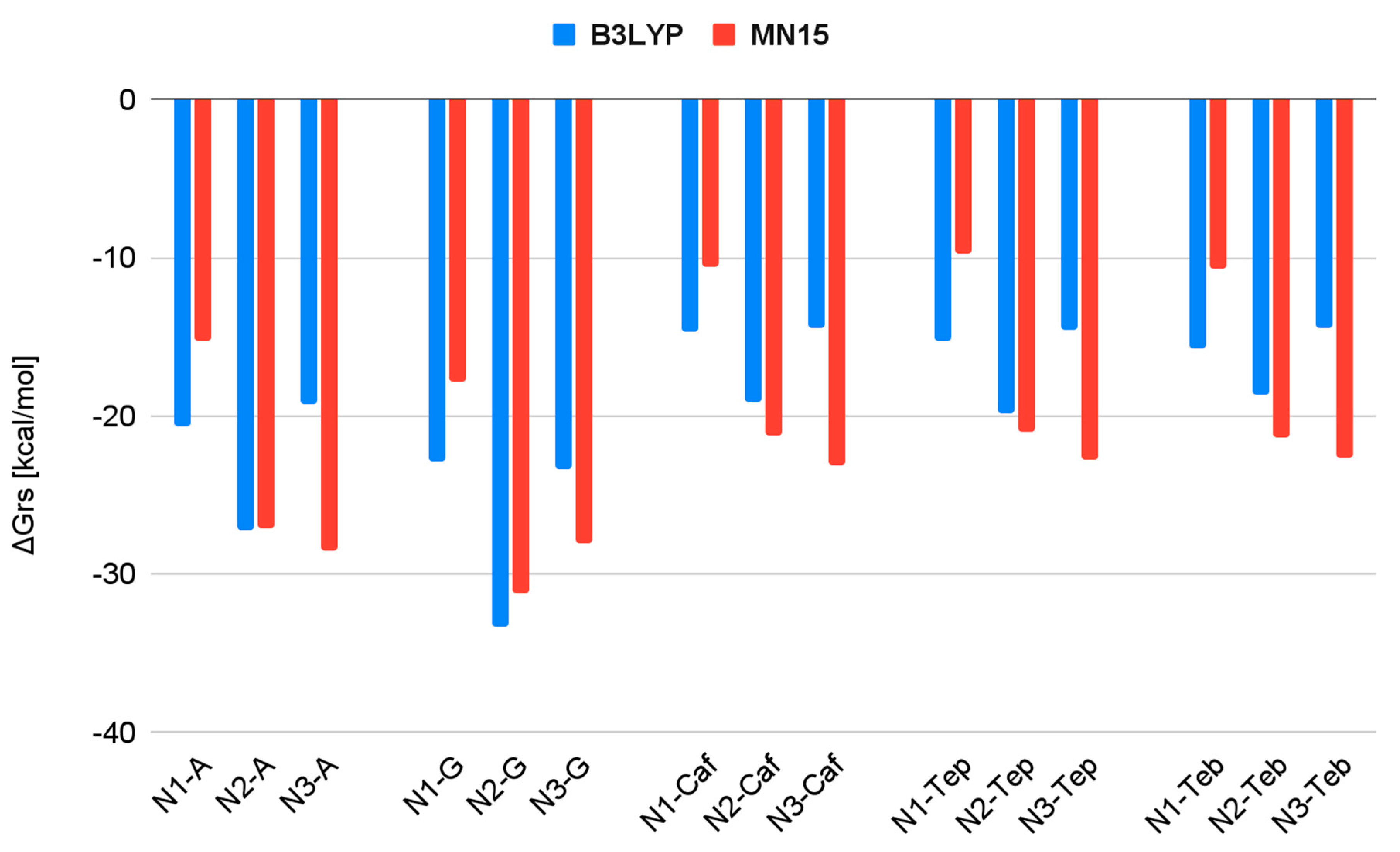
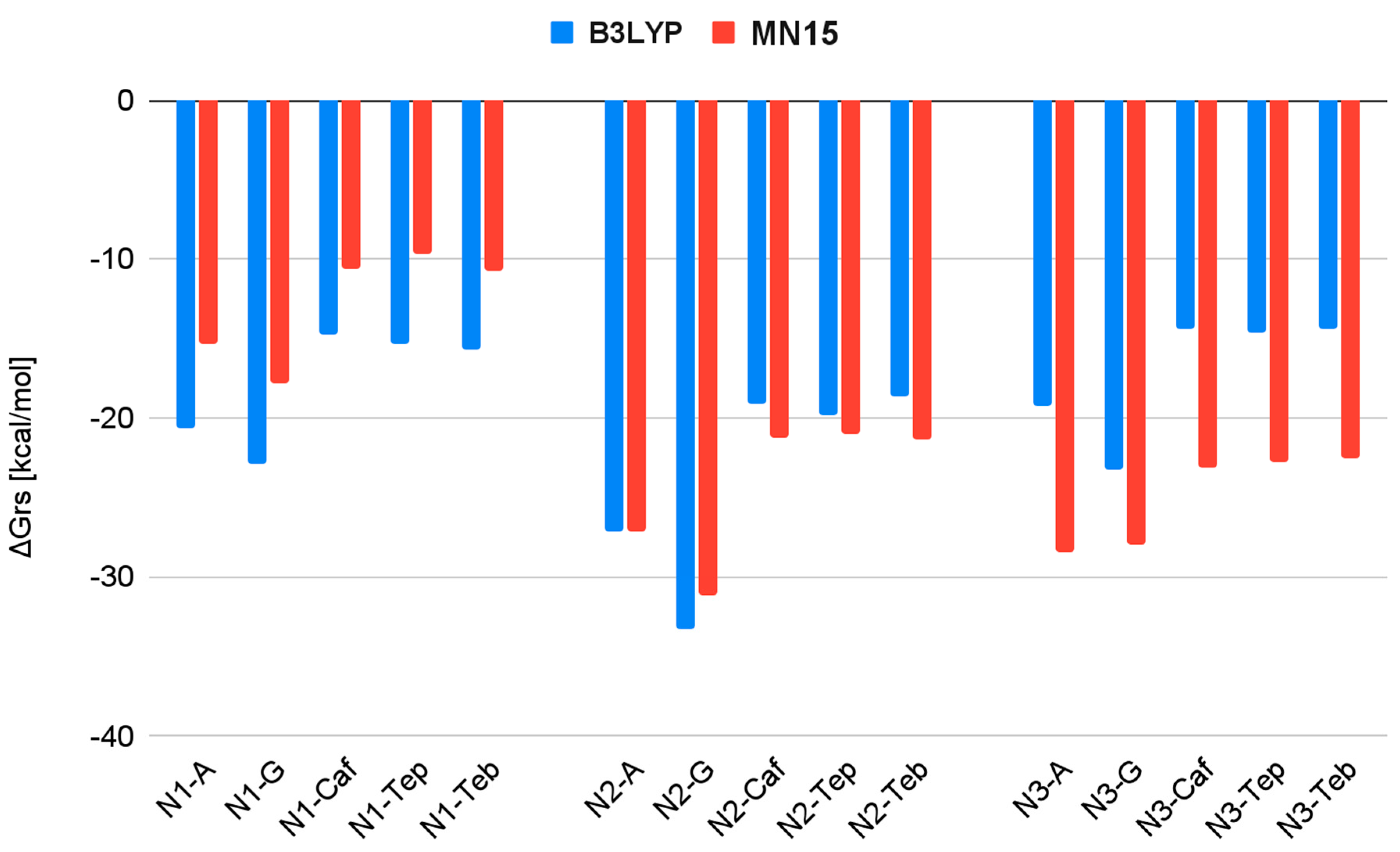
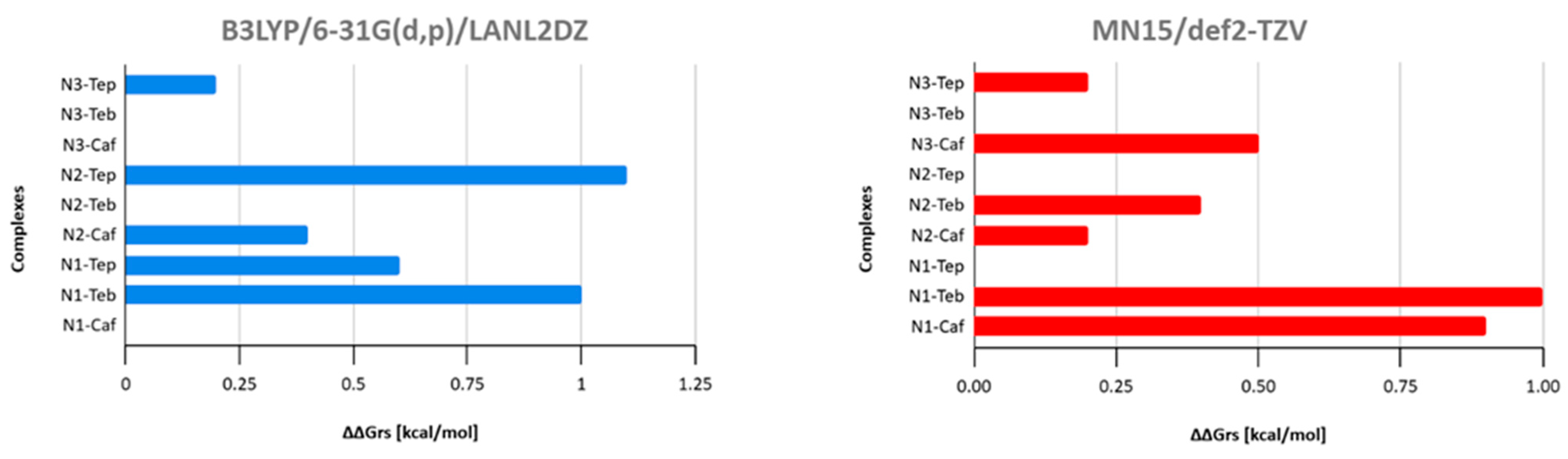



| Reaction | ΔGrs [kcal/mol] | |
|---|---|---|
| B3LYP/6-31G(d,p)/LANL2DZ | MN15/def2-TZV | |
| N1 + A → N1-A | −20.6 | −15.3 |
| N1 + G → N1-G | −22.9 | −17.8 |
| N1 + Caf → N1-Caf | −14.7 | −10.6 |
| N1 + Teb → N1-Teb | −15.7 | −10.7 |
| N1 + Tep → N1-Tep | −15.3 | −9.7 |
| N2 + A → N2-A | −27.2 | −27.1 |
| N2 + G → N2-G | −33.3 | −31.2 |
| N2 + Caf → N2-Caf | −19.1 | −21.2 |
| N2 + Teb → N2-Teb | −18.7 | −21.4 |
| N2 + Tep → N2-Tep | −19.8 | −21.0 |
| N3 + A → N3-A | −19.3 | −28.5 |
| N3 + G → N3-G | −23.3 | −28.0 |
| N3 + Caf → N3-Caf | −14.4 | −23.1 |
| N3 + Teb → N3-Teb | −14.4 | −22.6 |
| N3 + Tep → N3-Tep | −14.6 | −22.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szupryczyński, K.; Szefler, B. Interactions of Nedaplatin with Nucleobases and Purine Alkaloids: Their Role in Cancer Therapy. Biomedicines 2025, 13, 1551. https://doi.org/10.3390/biomedicines13071551
Szupryczyński K, Szefler B. Interactions of Nedaplatin with Nucleobases and Purine Alkaloids: Their Role in Cancer Therapy. Biomedicines. 2025; 13(7):1551. https://doi.org/10.3390/biomedicines13071551
Chicago/Turabian StyleSzupryczyński, Kamil, and Beata Szefler. 2025. "Interactions of Nedaplatin with Nucleobases and Purine Alkaloids: Their Role in Cancer Therapy" Biomedicines 13, no. 7: 1551. https://doi.org/10.3390/biomedicines13071551
APA StyleSzupryczyński, K., & Szefler, B. (2025). Interactions of Nedaplatin with Nucleobases and Purine Alkaloids: Their Role in Cancer Therapy. Biomedicines, 13(7), 1551. https://doi.org/10.3390/biomedicines13071551







