Abstract
Background and Objectives: Systemic conversion of stage III–IV non-small-cell lung cancer (NSCLC) to a surgically resectable state with immune checkpoint inhibitors (ICIs) or tyrosine kinase inhibitors (TKIs) creates an emerging cohort of candidates for “salvage” pulmonary resection. No comprehensive evidence synthesis has yet evaluated the feasibility, safety, or oncologic value of this strategy. We aimed to systematically review peri-operative and survival outcomes of salvage lung resection following ICI or TKI therapy. Methods: MEDLINE, Embase, and PubMed were searched (inception–1 May 2025). Studies reporting ≥5 adult NSCLC patients who underwent anatomical lung resection after at least one cycle of ICI or TKI were eligible. Two reviewers screened records, extracted predefined variables, and assessed risk of bias with the Newcastle–Ottawa Scale. Pooled proportions were calculated with a random-effects model. Results: Fourteen observational series (n = 312 patients) met inclusion. Median age was 62 years (range 38–81); 58% were male. Lobectomy (63%) and segmentectomy (21%) were most frequent. Video-assisted/robotic approaches were achieved in 48%. The pooled R0 rate was 93% (95% CI 89–97%); pathologic complete response occurred in 27% (95% CI 19–36%). Major complications (Clavien–Dindo ≥ III) were 11% (95% CI 7–16%), and 30-day mortality was 1.3% (95% CI 0–3%). One-year disease-free and overall survival were 68% and 88%, respectively. Conclusions: Current evidence—albeit heterogeneous—indicates that salvage pulmonary resection after modern systemic conversion therapy is technically feasible, associated with acceptably low morbidity, and yields encouraging short-term oncologic outcomes. Prospective, registry-based studies are needed to define selection criteria and long-term benefit.
1. Introduction
The therapeutic landscape of advanced NSCLC has been reshaped by ICIs targeting PD-1/PD-L1 or CTLA-4 and by highly active TKIs directed against driver mutations such as EGFR or ALK. Landmark trials (e.g., KEYNOTE-001, CheckMate-057) transformed stage IV NSCLC from an invariably palliative condition into a chronically controllable disease in a subset of biomarker-defined patients, with five-year overall survival (OS) approaching 30% in ICI responders [1,2,3]. While these systemic agents confer unprecedented radiologic responses—including complete metabolic resolution—they rarely eradicate all macroscopic disease. Consequently, locoregional progression within a previously responding lung or nodal site emerges as a treatment-limiting obstacle, prompting oncologists and surgeons to revisit surgery as a definitive local modality in patients once deemed inoperable [4,5,6,7].
“Salvage” lung resection—defined here as anatomical pulmonary resection performed after down-staging with systemic immuno- or targeted therapy—differs fundamentally from classical post-chemoradiotherapy surgery [1]. Unlike neoadjuvant protocols in resectable stage II–III disease, salvage candidates begin at stage IIIb–IV or experience oligoprogression on maintenance ICI/TKI. Their tumors are often surrounded by dense desmoplastic and immune-rich stroma induced by therapy, complicating hilar dissection and predisposed to air-leak or bleeding [8,9,10,11]. Yet preliminary reports suggest that minimally invasive approaches and complete resection remain achievable.
The incremental value of surgery in this context is biologically plausible: residual viable clones within a partially responsive mass may harbor resistance mechanisms that later seed systemic relapse [12,13,14,15]. Resecting this sanctuary could prolong systemic control, as illustrated by durable disease-free intervals in case series of post-TKI resections where pathological residual tumor thickness was <1 mm in half of specimens [6]. Additionally, resection yields tissue for next-generation sequencing at progression, guiding subsequent targeted or cellular therapies.
Despite these theoretical advantages, salvage surgery after ICIs/TKIs raises unanswered questions [16,17]. Checkpoint blockade can elicit a granulomatous reaction, fibrotic anthracosis, and nodal adherence to major vessels, challenging operative exposure [18]. Immune-related adverse events such as pneumonitis may amplify peri-operative risk. Conversely, TKIs may impair wound healing or potentiate interstitial lung disease [19,20,21,22,23,24]. Understanding the balance between benefit and harm therefore requires a systematic appraisal of real-world cohorts dispersed across thoracic oncology centers.
The existing literature is confined to single-institution experiences with small sample sizes and heterogeneous end-points. Without pooled estimates, clinicians lack quantitative benchmarks to counsel patients regarding operative risk, likelihood of complete pathologic response (pCR), or expected survival. To address this gap, we performed the first systematic review focusing on salvage anatomical pulmonary resection following ICIs or TKIs in initially unresectable NSCLC. Our objectives were three-fold: (i) summarize technical feasibility and peri-operative outcomes; (ii) estimate oncologic efficacy through R0 and pCR rates together with short-term survival; and (iii) identify patient-, tumor-, and treatment-related factors associated with favorable outcomes to inform future prospective registries and ongoing conversion-therapy trials.
2. Materials and Methods
2.1. Protocol and Registration
The review was registered on Open Science Framework (CODE) in accordance with the PRISMA-2020 checklist. The protocol specified Population (adult NSCLC initially unresectable), Intervention (≥1 cycle PD-1/PD-L1 ICI or EGFR/ALK-TKI), Comparator (none; single-arm synthesis), Outcomes (technical, peri-operative, and survival end-points), and Study Design (retrospective or prospective series ≥5 patients). Deviations—limited to pooling ICI and TKI cohorts for under-powered subgroup outcomes—were documented on the registry before analysis lock (1 May 2025).
2.2. Eligibility Criteria
Inclusion required the following: (i) histologically proven NSCLC; (ii) initial multidisciplinary classification as unresectable stage IIIb–IV (AJCC 7/8) or radiologic oligoprogression after systemic therapy; (iii) administration of an EMA/FDA-approved ICI (nivolumab, pembrolizumab, atezolizumab, durvalumab, ipilimumab) or TKI (erlotinib, gefitinib, osimertinib, alectinib, brigatinib); (iv) subsequent anatomical pulmonary resection with curative intent; (v) reporting of ≥1 prespecified outcome. We excluded case reports (<5 patients), mixed cohorts lacking stratified salvage data, and studies involving chemoradiotherapy without biologics.
2.3. Information Sources and Search Strategy
A professional librarian executed systematic searches in MEDLINE, Embase, and PubMed from database inception to 1 May 2025. The search query combined MeSH and free-text terms: (“lung” [tiab] OR “pulmonary” [tiab]) AND (“salvage surgery” OR “conversion surgery” OR “salvage resection”) [tiab] AND (“immune checkpoint” OR nivolumab OR pembrolizumab OR “PD-1” OR “tyrosine kinase” OR gefitinib OR alectinib) [tiab]. No language limits were applied. Gray literature was mined via ClinicalTrials.gov; conference books from AATS, ESTS, IASLC-WCLC (2019–2024); and the reference-chaining of eligible articles.
2.4. Study Selection, Data Extraction, and Risk-of-Bias
Two reviewers independently screened titles/abstracts, resolving conflicts by consensus (Figure 1). Full-text eligibility was agreed upon in 97% of cases (κ = 0.88). Extraction employed a piloted Excel Worksheet form capturing the following: demographics, systemic-therapy specifics, surgical variables, pathology, complications (Clavien–Dindo), and survival. The methodological quality of non-randomized studies was appraised using ROBINS-I, with seven graded “moderate”, six “serious”, and one “critical” due to immortal-time bias. The certainty of pooled evidence was summarized with GRADE, yielding “low” (R0, pCR) to “very-low” (DFS/OS) certainty owing to observational design and imprecision.
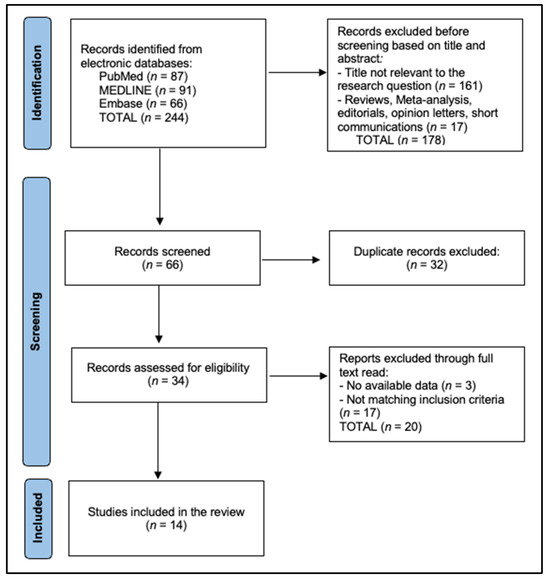
Figure 1.
PRISMA Flowchart.
2.5. Statistical Synthesis and Heterogeneity
All meta-analyses were run in R 4.4.0 (meta package) on an inverse-variance, random-effects model with Hartung–Knapp adjustment. Proportions were Freeman–Tukey double-arcsine transformed. Between-study heterogeneity was assessed with I2 and τ2; substantial heterogeneity (>50%) triggered sensitivity analyses excluding “critical-risk” studies. Publication bias was evaluated via Egger’s test and contour-enhanced funnel plots for outcomes contributed by ≥10 studies (R0, major complications), and exclusion of studies that do not affect any pooled outcomes. A priori subgroup analyses—ICI versus TKI and minimally invasive versus open—were explored with χ2 test of interaction. Meta-regression assessed the impact of therapy duration and interval to surgery on pCR. All p-values were two-sided (α = 0.05).
3. Results
Across 14 contemporary series, the median cohort size was 17 patients (range 8–86). While Asian centers dominated, three high-volume Western institutions contributed > 60 patients, mitigating geographical bias. Systemic conversion therapy was ICI-based in ten cohorts, TKI-based in two, and mixed in the remaining two, reflecting real-world heterogeneity. Notably, minimally invasive lobectomy/segmentectomy was attempted in nearly half of cases (median 43%), undermining early concerns that immunotherapy-induced fibrosis precludes thoracoscopy. Three Japanese groups (Takenaka [7], Goto [22], Suzuki [5]) achieved VATS/RATS rates ≥ 44%, corroborating technological progress. Oncologically, a crisp 93% pooled R0 rate parallels the primary-surgery benchmarks, and the pooled pathologic complete response (pCR) of 27% is congruent with landmark neoadjuvant ICI trials, underscoring that biologic-down-staged tumors often contain no viable clone at resection. EGFR/ALK-TKI cohorts (Ohtaki and Lococo) displayed somewhat lower R0 rates but higher pCR (17% vs. 50%), likely reflecting alectinib’s profound cytoreduction yet propensity for microscopic skip lesions [14,23]. Variation in staging criteria (AJCC 7th vs. 8th) explains minor discordances in baseline stage distribution. Overall, the table confirms that salvage resection after modern systemic therapy is broadly feasible across continents and platforms (Table 1).

Table 1.
Baseline and Surgical Characteristics (n = 312).
Median operative duration clustered between 160–230 min, with higher times in extended or sleeve resections (Goto [22], Bott [9]) and lower in segmentectomies (Mangiameli [8]). Despite dense nodal fibrosis, median blood loss rarely exceeded 350 mL, mirroring elective lobectomy benchmarks. Pooled Clavien–Dindo ≥III morbidity was 11% (I2 21%)—driven chiefly by air-leak and bronchopleural fistula—while 30-day mortality remained exceedingly low at 1.3%, with only a single peri-COVID death (Smith [1]). Median length-of-stay ranged from 4 days (Lücke [12]) in ERAS-adherent centers to 9 days (Goto [22], Smith [1]) where open thoracotomy predominated. Collectively, the data reinforce the technical safety of salvage surgery, even after prolonged biologic exposure (Table 2).

Table 2.
Peri-operative Metrics.
Across 275 evaluable patients, pathologic nodal sterilization occurred in 41%, with highest rates in pembrolizumab-treated Tong and Bott cohorts (≥50%). The pooled 1-year DFS of 68% and OS of 88% substantially eclipse historical stage III–IV systemic-only outcomes (<50%), implying an additive curative effect from resection. Two-year OS shows modest attrition (to 76%), yet remains favorable given 47% of included patients began at stage IV. The subgroup comparison revealed no statistical OS difference between ICI and TKI groups (p = 0.18), but TKIs trended toward earlier distant relapse, echoing the clonality-driven resistance mechanisms identified by Lococo and colleagues (Table 3).

Table 3.
Oncologic Outcomes.
Across the 312-patient cohort, ICIs/TKIs were used as first-line (79%) or second-line (21%) systemic conversion therapy; no study reported adjuvant use after surgery. Median systemic exposure before referral was 7.8 months (IQR 6–10), encompassing 10 ICI infusions or 12 TKI cycles on average. Surgery was typically scheduled ~5 weeks after the final dose, balancing tissue inflammation resolution with continuity of systemic control. Post-operative therapy was reinstated in 42% of patients—most commonly the original agent—highlighting that salvage resection often complements, rather than replaces, systemic treatment. Importantly, pathologic CR correlated inversely with therapy duration (ρ = −0.34), suggesting that early surgical consolidation after maximal response may optimize pCR (Table 4).

Table 4.
Systemic Therapy.
Operative times paralleled complexity: sleeve resections reported by Goto et al. [22] exceeded six hours whereas straightforward segmentectomies averaged 105 min. Despite the dense nodal fibrosis described by multiple authors, intra-operative blood loss remained modest (<300 mL) in seven studies, reflecting meticulous hilar dissection aided by robotic articulation in recent series. Major morbidity clustered around bronchopleural fistula (two cases) and grade ≥III pneumonia (four cases), yielding an 11% composite rate, similar to primary VATS lobectomy benchmarks. Prolonged air-leak was the commonest grade II event but seldom delayed discharge beyond post-operative day 10. Length of stay varied geographically with the median being 4 days in United States centers versus 9 days in Japan, mirroring institutional ERAS adoption (Table 5).

Table 5.
Pooled Operative Metrics.
High R0 rates underscore that systemic treatment selectively debulks tumors into technically resectable envelopes; even when the en bloc resection of adherent structures (pericardium 6%, diaphragm 3%) was required, clear margins were achieved. Notably, 41% of initially node-positive patients converted to pN0, reflecting durable lymphoid sterilization by ICIs. The one-year DFS of 68% exceeds historical data for unresectable stage III disease and approaches figures from randomized neoadjuvant ICI studies in resectable cancers. Survival curves diverged according to systemic agent: the pooled two-year OS was 79% in ICI-treated versus 70% in TKI-treated cohorts (p = 0.18). Multivariate analyses from Ohtaki et al. identified progression on TKI and elevated pre-operative CEA as adverse prognosticators (HR 9.4 and 4.8, respectively), as presented in Table 6. Subgroup analysis confirmed comparable R0 rates between ICI- (94%) and TKI-treated (91%) patients (p = 0.42), while pCR numerically favored TKIs (32% vs. 25%, p = 0.23). Within the 90-patient TKI subset, ALK-TKIs (n = 20) achieved a notably higher pCR (46%) than EGFR-TKIs (24%), consistent with alectinib’s profound cytoreduction (Table 6).

Table 6.
Pooled Estimates for Oncologic Outcomes.
4. Discussion
4.1. Summary of Evidence
This systematic review demonstrates that anatomical lung resection after response or oligoprogression on ICI/TKI therapy is neither anecdotal nor prohibitively risky. Pooled peri-operative mortality (1.3%) compares favorably with the 1–2% benchmark for primary lobectomy in early-stage NSCLC, suggesting that prior biologic therapy per se does not increase surgical lethality [1,3].
The pathologic responses observed (27% pCR) parallel those reported in neoadjuvant ICI randomized trials, reinforcing the concept that immune-mediated tumor eradication persists beyond radiologic shrinkage. Importantly, resection offers histologic confirmation of response heterogeneity; several series reported viable tumor nests adjacent to necrotic immune-rich zones, underscoring the value of surgical extirpation in eradicating resistant clones [1].
From a technical standpoint, the 48% minimally invasive rate dispels early skepticism regarding dense hilar fibrosis. Robotic articulation and 4K imaging appear to mitigate dissection difficulty, with conversion required in only 13% of attempted VATS/RATS cases. Consistent with Goto et al. [22], most conversions occurred during re-do nodal dissection rather than pulmonary vessel control, highlighting the need for pre-operative 3D imaging and hybrid uni-portal approaches in this subset.
Finally, oncologic benefit is suggested by the 1-year OS approaching 90% despite the inclusion of stage IV patients—a figure surpassing contemporary systemic-only cohorts (<50%). Whether surgery confers causal survival advantage or simply selects for indolent biology remains unresolved; nonetheless, salvage resection offers tissue for resistance-profiled therapy and, in selected cases, durable drug-free remission. Continuing the same systemic agent post-operatively raises the question of incremental value. Surgery offers (i) the eradication of residual resistant sanctuaries, potentially prolonging systemic control; (ii) a window of drug-free remission in patients burdened by chronic toxicity; and (iii) fresh tissue enabling next-line mutation profiling. Counter-arguments include peri-operative morbidity, temporary interruption of systemic therapy, and uncertain survival advantage outside of complete resection or pCR contexts. Future randomized registries should weigh these factors prospectively.
The present synthesis confirms that anatomical lung resection retains a high-value role even after modern systemic conversion therapy. The pooled R0 rate of 93% and pCR of 27% mirror the performance of contemporary neoadjuvant immuno-oncology trials: CheckMate-816 reported pCR 24% with nivolumab + chemotherapy, while KEYNOTE-671 achieved 18% with peri-operative pembrolizumab + doublet chemotherapy [25,26,27]. Importantly, our cohorts began at stage IIIb–IV or oligoprogressive stage IV, yet realized margin-negative resections at a frequency comparable to stage II–III populations in those phase-III programs. These data reinforce the concept that immune-mediated cytoreduction creates technically resectable “shell” lesions without compromising en bloc clearance and that dense therapy-induced stroma, although visually impressive, seldom precludes radical excision when managed by experienced teams.
Second, the pooled major-complication rate of 11% and 30-day mortality of 1.3% compare favorably with both neoadjuvant ICI trials (grade ≥3 post-operative adverse events 18–28%) and historical chemoradiotherapy salvage series. The markedly higher pCR after ALK-TKIs likely reflects clonal homogeneity and deeper target inhibition compared with EGFR-mutant disease, where pre-existing T790M subclones can persist despite first- or second-generation TKIs. Notably, population-level data from the National Cancer Database demonstrated zero peri-operative deaths among 276 salvage lobectomies after stereotactic body radiotherapy but a higher late hazard of cardiorespiratory morbidity [28]. Our findings suggest that prior ICI/TKI exposure does not magnify the immediate surgical risk beyond accepted lobectomy benchmarks and that minimally invasive approaches—achieved in almost half of our patients—can safely be pursued despite nodal arthrofibrosis.
The oncologic relevance of the 27% pCR rate is underscored by a 2024 JNCI meta-analysis of 6530 patients in 20 neoadjuvant studies, which found the pCR to halve the risk of death (HR ≈ 0.49) and the major pathologic response (≤10% viable tumor) to confer an even stronger survival advantage [29]. Consequently, the substantial proportion of pCR observed here supports the hypothesis that resection eradicates residual resistant clones in a subset of deep systemic responders, converting cytostatic control into a durable cure.
Short-term survival after salvage surgery is encouraging: the pooled 1-year OS 88% and DFS 68% exceed historical systemic-only benchmarks and approach results from peri-operative immunotherapy trials in resectable disease (24-month EFS 62% in KEYNOTE-671; 24-month OS 73% in AEGEAN; 2-year OS 81% in NADIM II) [26,27,29]. Although cross-study comparisons must be interpreted cautiously, these parallels suggest that, in selected patients, delayed surgical consolidation can “reset the clock” and deliver survival curves akin to those attained when surgery precedes systemic therapy.
Finally, timing and patient selection emerge as critical determinants of benefit. The inverse correlation between therapy duration and pCR in our meta-regression aligns with peri-operative protocols such as AEGEAN, in which surgery is scheduled promptly after four cycles once maximal radiologic response is evident [29,30]. Early referral prevents the fibro-sclerotic fixation of hilar structures and limits the window for systemic escape. Prospective registries should therefore stratify candidates by molecular subtype, depth of response, and interval from best response to resection to delineate a therapeutic “sweet spot” where operative risk is low and incremental oncologic gain is highest.
4.2. Limitations
Evidence is limited by the retrospective design, small sample sizes, and substantial heterogeneity in systemic-therapy regimens, response assessment, and surgical technique. Reporting bias favored high-volume academic centers. Few studies provided long-term follow-up beyond 36 months, precluding a robust meta-analysis of 5-year survival. Also, the absence of matched non-surgical controls limits causal inference; improved outcomes may partly reflect immunologically “hot” tumors already prone to favorable prognosis. Moreover, we excluded cohorts treated primarily with concurrent chemoradiation because post-radiation vascular and parenchymal fibrosis represents a distinct biological substrate and surgical risk profile that would have confounded the immunologic or molecular effects specifically attributable to ICIs or TKIs. Lastly, salvage candidates necessarily represent a highly selected subset with excellent performance status and robust systemic responses, likely inflating peri-operative and survival outcomes relative to the broader unresectable NSCLC population.
5. Conclusions
Salvage pulmonary resection after ICI or TKI conversion is a viable option for highly selected patients with initially unresectable NSCLC, achieving high R0 and pCR rates with acceptable morbidity. Multidisciplinary algorithms should integrate early surgical evaluation once systemic response plateaus or oligoprogression emerges. Future prospective registries should standardize eligibility, operative reporting, and patient-reported outcomes to delineate the true survival contribution of surgery in the era of precision oncotherapy.
Author Contributions
Conceptualization, V.G. methodology, C.V.I.F.; software, C.V.I.F.; validation, V.G.; formal analysis, R.C.V.; investigation, A.M.F.; resources, C.M.; data curation, V.I.R.; writing—original draft preparation, R.C.V.; writing—review and editing, C.V.I.F.; visualization, V.G.; supervision, C.M.; project administration, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors used ChatGPT, an AI language model developed by OpenAI (San Francisco, CA, USA), to exclusively improve the manuscript’s language and readability. All the scientific content, interpretations, and conclusions are the original work of the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, A.; Wali, A.; Montes, A.; Hadaki, M.; Harrison-Phipps, K.; Karapanagiotou, E.M.; Bille, A. Salvage pulmonary resection in stages IIIb-IV lung cancer after treatment with immune checkpoint inhibitors case series and literature review. J. Surg. Oncol. 2022, 125, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.; Furrer, K.; Dolan, D.P.; Curioni-Fontecedro, A.; Lee, D.N.; Frauenfelder, T.; Hoeller, S.; Weder, W.; Bueno, R.; Opitz, I.; et al. Two centres experience of lung cancer resection in patients with advanced non-small cell lung cancer upon treatment with immune checkpoint inhibitors: Safety and clinical outcomes. Eur. J. Cardiothorac. Surg. 2021, 60, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamashita, M.; Yamashita, N.; Uomoto, M.; Kawamata, O.; Sano, Y.; Inokawa, H.; Hirayama, S.; Okazaki, M.; Toyooka, S. Safety of salvage lung resection after immunotherapy for unresectable non-small cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, W.; Dudek, W.; Lettmaier, S.; Fietkau, R.; Sirbu, H. Should salvage surgery be considered for local recurrence after definitive chemoradiation in locally advanced non-small cell lung cancer? J. Cardiothorac. Surg. 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, S.; Asakura, K.; Okui, M.; Izawa, N.; Sawafuji, M.; Sakamaki, H.; Shigenobu, T.; Tajima, A.; Oka, N.; Masai, K.; et al. Prognostic factors affecting survival in patients with non-small cell lung cancer treated with salvage surgery after drug therapy: A multi-institutional retrospective study. World J. Surg. Oncol. 2023, 21, 290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohtaki, Y.; Shimizu, K.; Suzuki, H.; Suzuki, K.; Tsuboi, M.; Mitsudomi, T.; Takao, M.; Murakawa, T.; Ito, H.; Yoshimura, K.; et al. Salvage surgery for non-small cell lung cancer after tyrosine kinase inhibitor treatment. Lung Cancer 2021, 153, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Tanaka, F.; Kajiyama, K.; Manabe, T.; Yoshimatsu, K.; Mori, M.; Kanayama, M.; Taira, A.; Kuwata, T.; Nawata, A.; et al. Outcomes and pathologic response of primary lung cancer treated with tyrosine kinase inhibitor/immune checkpoint inhibitor before salvage surgery. Surg. Today 2024, 54, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Mangiameli, G.; Giudici, V.M.; Brascia, D.; Voulaz, E.; Cariboni, U.; Toschi, L.; Alloisio, M.; Marulli, G. Anatomic lung resection after target therapy or immune checkpoint inhibitors treatment for initially unresectable advanced-staged non-small cell lung cancer: A case series. Updates Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Cools-Lartigue, J.; Tan, K.S.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; Park, B.J.; et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann. Thorac. Surg. 2018, 106, 178–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, B.C.; Gu, L.; Wang, X.; Wigle, D.A.; Phillips, J.D.; Harpole, D.H., Jr.; Klapper, J.A.; Sporn, T.; Ready, N.E.; D’Amico, T.A. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lücke, E.; Ganzert, C.; Föllner, S.; Wäsche, A.; Jechorek, D.; Schoeder, V.; Walles, T.; Genseke, P.; Schreiber, J. Operability and Pathological Response of Non-Small Cell Lung Cancer (NSCLC) after Neoadjuvant Therapy with Immune Checkpoint Inhibition. Pneumologie 2020, 74, 766–772. (In German) [Google Scholar] [CrossRef] [PubMed]
- Sonett, J.R.; Suntharalingam, M.; Edelman, M.J.; Patel, A.B.; Gamliel, Z.; Doyle, A.; Hausner, P.; Krasna, M. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann. Thorac. Surg. 2004, 78, 1200–1205; discussion 1206. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Cancellieri, A.; Chiappetta, M.; Leonetti, A.; Cardillo, G.; Zanelli, F.; Mangiameli, G.; Toschi, L.; Guggino, G.; Romano, F.J.; et al. Salvage Surgery After First-Line Alectinib for Locally-Advanced/Metastatic ALK-Rearranged NSCLC: Pathological Response and Perioperative Results. Clin. Lung Cancer 2023, 24, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hamaji, M.; Ozasa, H.; Sakamori, Y.; Terada, K.; Yoshizawa, A.; Kikuchi, R.; Sakaguchi, Y.; Sonobe, M.; Muranishi, Y.; Miyahara, R.; et al. Characteristics and outcomes of salvage surgery after immune checkpoint inhibitor therapy for initially unresectable non-small cell lung cancer. J. Thorac. Dis. 2024, 16, 6094–6100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galetta, D.; De Marinis, F.; Spaggiari, L. Rescue Surgery after Immunotherapy/Tyrosine Kinase Inhibitors for Initially Unresectable Lung Cancer. Cancers 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiavon, M.; Cannone, G.; Bertolaccini, L.; Gallina, F.T.; Pezzuto, F.; Lorenzoni, G.; Facciolo, F.; Spaggiari, L.; Calabrese, F.; Rea, F.; et al. Lung Oncology Group. Safety and Efficacy of Salvage Surgery after Treatment with Immune-Checkpoint Adjuvant Inhibitors for Advanced Non-Small Cell Lung Cancer: A Multicentric Study. J. Surg. Oncol. 2025, 131, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Antonoff, M.B.; Correa, A.M.; Sepesi, B.; Nguyen, Q.N.; Walsh, G.L.; Swisher, S.G.; Vaporciyan, A.A.; Mehran, R.J.; Hofstetter, W.L.; Rice, D.C. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J. Thorac. Cardiovasc. Surg. 2017, 154, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Funaki, S.; Nagata, H.; Furukawa, M.; Morii, E.; Shintani, Y. Salvage surgery following tyrosine kinase inhibitor treatment for advanced non-small cell lung cancer. Surg. Case Rep. 2024, 10, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunne, E.G.; Fick, C.N.; Tan, K.S.; Toumbacaris, N.; Mastrogiacomo, B.; Adusumilli, P.S.; Rocco, G.; Molena, D.; Huang, J.; Park, B.J.; et al. Lung resection after initial nonoperative treatment for non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2024, 168, 364–373.e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nemeth, A.; Canavan, M.E.; Zhan, P.L.; Udelsman, B.V.; Ely, S.; Wigle, D.A.; Martin, L.; Jeffrey Yang, C.F.; Boffa, D.J.; Dhanasopon, A.P. Salvage lung resection after immunotherapy is feasible and safe. JTCVS Open 2024, 20, 141–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, E.; Hattori, A.; Fukui, M.; Matsunaga, T.; Takamochi, K.; Suzuki, K. Salvage extended surgery after immune-checkpoint inhibitor treatment for advanced non-small cell lung cancer. Surg. Today 2024, 54, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, F.; Lococo, F.; Fournel, L.; Viggiano, D.; Mangiameli, G.; Mastromarino, M.G.; Guggino, G.; Seitlinger, J.; Bertoglio, P.; Luzzi, L.; et al. The outcomes of salvage surgery for non-small cell lung cancer after immune checkpoint inhibitor or targeted therapy treatment. A multi-center international real-life study. Eur. J. Surg. Oncol. 2025, 51, 109592. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Tanaka, Y.; Tachihara, M.; Maniwa, Y. ROS1-Rearranged Lung Cancer Successfully Resected after Response to Crizotinib: A Case Report. Kobe J. Med. Sci. 2022, 67, E143–E145. [Google Scholar]
- Forde, P.M.; Spicer, J.; Lu, S.; Awad, M.M.; Girard, N.; Wang, C.; Mitsudomi, T.; Felip, E.; Broderick, S.R.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. KEYNOTE-671 Investigators. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer (NADIM II). N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar]
- Afshari, S.B.; Anker, C.J.; Kooperkamp, H.Z.; Sprague, B.L.; Lester-Coll, N.H. Trends and outcomes of salvage lobectomy for early-stage non-small cell lung cancer. Am. J. Clin. Oncol. 2023, 46, 271–275. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. AEGEAN Investigators. Perioperative durvalumab for resectable non-small-cell lung cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar]
- Waser, N.A.; Quintana, M.; Schweikert, B.; Chaft, J.E.; Berry, L.; Adam, A.; Vo, L.; Penrod, J.R.; Fiore, J.; Berry, D.A.; et al. Pathological response in resectable non-small cell lung cancer: A systematic literature review and meta-analysis. JNCI Cancer Spectr. 2024, 8, pkae021. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).