Initial Low-Density Lipoprotein Cholesterol and Inflammation Status Predicts Long-Term Mortality in Patients with Acute Coronary Syndrome in the Chinese Population
Abstract
1. Introduction
2. Materials and Methods
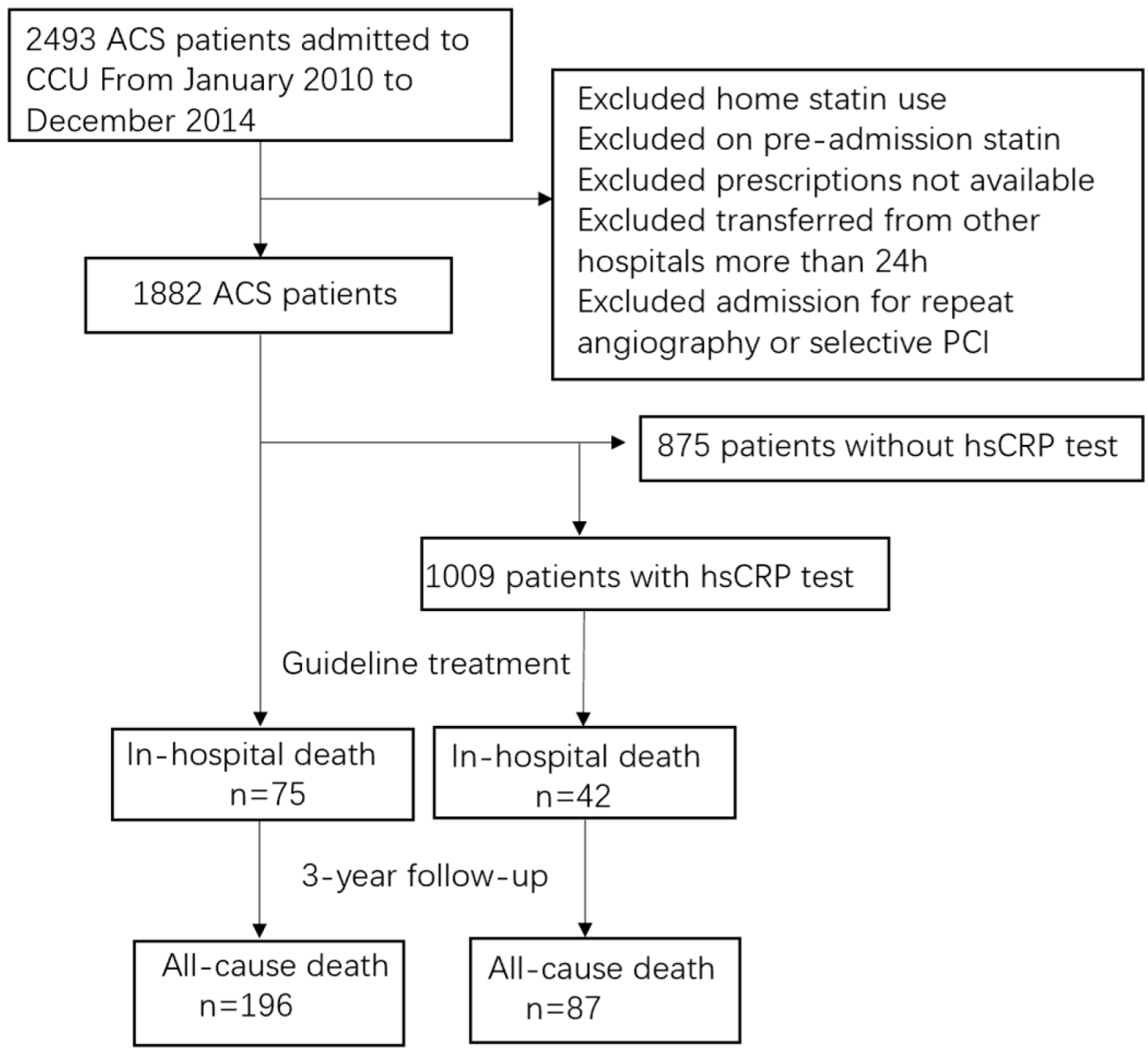
2.1. Study Population
2.2. Patient and Public Involvement
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics in Statin-Null ACS Patients with Different LDL-C Level
3.2. LDL-C Paradox in the Prognosis of Statin-Null ACS Patients
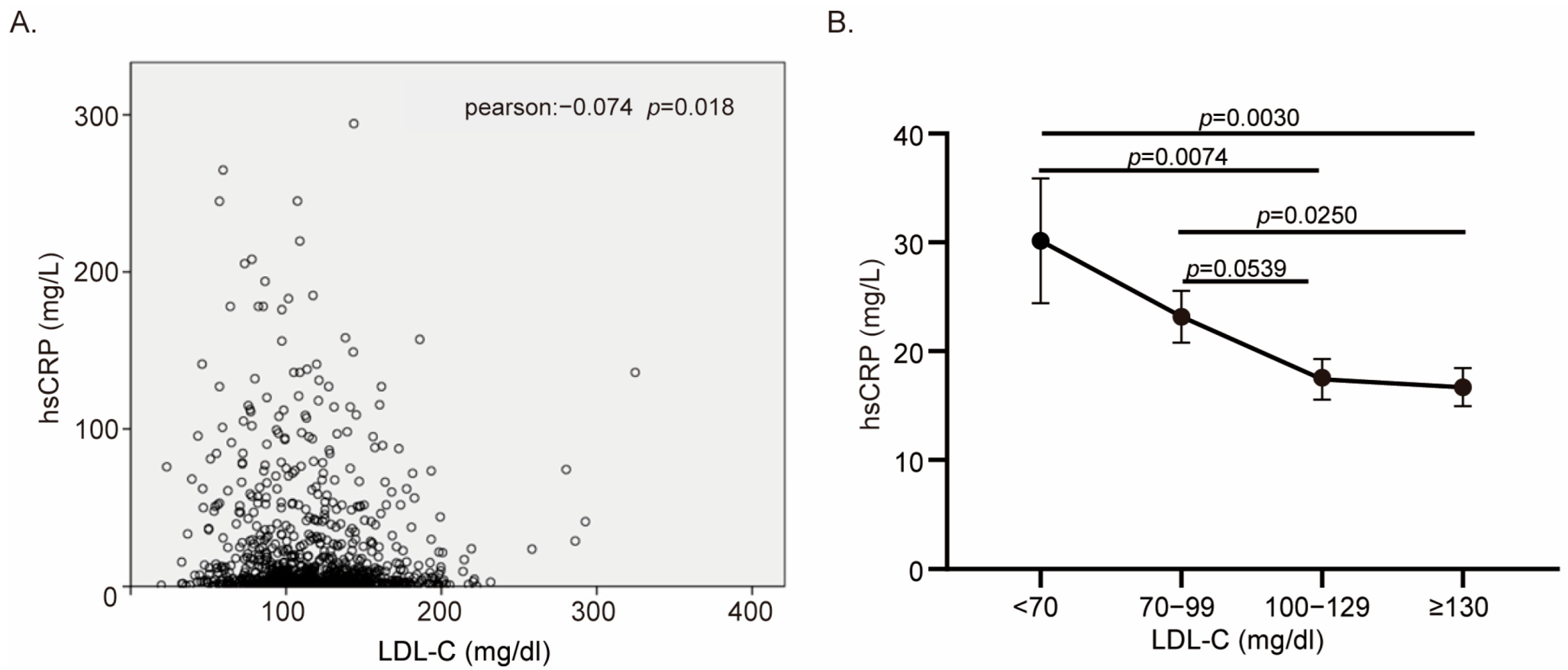
3.3. Heterogeneity of CRP Level in Low LDL-C Group Explained for Lipid Paradox
3.4. The Predictive Ability of the CRP–LDL-C Ratio in Prognosis
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDL-C | Low-density lipoprotein cholesterol |
| ACS | Acute coronary syndrome |
| hs-CRP | High-sensitivity C-reactive protein |
| STEMI | ST-elevation myocardial infarction |
| NSTE-ACS | Non-ST-segment elevation acute coronary syndrome |
| PCI | Percutaneous coronary intervention |
| HDL-C | High-density lipoprotein cholesterol |
| ACEI | Angiotensin-converting enzyme inhibitors |
| ARB | Angiotensin II receptor blockers |
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality From Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health 2018, 18, 975. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, D.; Pei, C.; Zeng, Y.; Wang, Z.; Li, Z.; Hao, Y.; Song, X.; Li, Y.; Liu, G.; et al. LDL cholesterol levels and in-hospital bleeding in patients on high-intensity antithrombotic therapy: Findings from the CCC-ACS project. Eur. Heart J. 2021, 42, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Apoprotein E and Reverse Cholesterol Transport. Int. J. Mol. Sci. 2018, 19, 3479. [Google Scholar] [CrossRef] [PubMed]
- Averna, M.; Banach, M.; Bruckert, E.; Drexel, H.; Farnier, M.; Gaita, D.; Magni, P.; März, W.; Masana, L.; Mello, E.S.A.; et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: A statement from a European Atherosclerosis Society Task Force. Atherosclerosis 2021, 325, 99–109. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Claessen, B.E.; Guedeney, P.; Gibson, C.M.; Angiolillo, D.J.; Cao, D.; Lepor, N.; Mehran, R. Lipid Management in Patients Presenting with Acute Coronary Syndromes: A Review. J. Am. Heart Assoc. 2020, 9, e018897. [Google Scholar] [CrossRef]
- Cheng, K.H.; Chu, C.S.; Lin, T.H.; Lee, K.T.; Sheu, S.H.; Lai, W.T. Lipid paradox in acute myocardial infarction-the association with 30-day in-hospital mortality. Crit. Care Med. 2015, 43, 1255–1264. [Google Scholar] [CrossRef]
- Al-Mallah, M.H.; Hatahet, H.; Cavalcante, J.L.; Khanal, S. Low admission LDL-cholesterol is associated with increased 3-year all-cause mortality in patients with non ST segment elevation myocardial infarction. Cardiol. J. 2009, 16, 227–233. [Google Scholar]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, B.; Zheng, T.; Yang, L.; Zhang, X.; Cheng, L.; Tuerhongjiang, G.; Yuan, Z.; Wu, Y. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 2023, 14, 929. [Google Scholar] [CrossRef] [PubMed]
- Denegri, A.; Boriani, G. High Sensitivity C-reactive Protein (hsCRP) and its Implications in Cardiovascular Outcomes. Curr. Pharm. Des. 2021, 27, 263–275. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, V.J.; Umans, V.; Brankovic, M.; Oemrawsingh, R.M.; Asselbergs, F.W.; van der Harst, P.; Hoefer, I.E.; Kietselaer, B.; Crijns, H.; Lenderink, T.; et al. Stabilization patterns and variability of hs-CRP, NT-proBNP and ST2 during 1 year after acute coronary syndrome admission: Results of the BIOMArCS study. Clin. Chem. Lab. Med. 2020, 58, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Carrero, J.J.; Andersson Franko, M.; Obergfell, A.; Gabrielsen, A.; Jernberg, T. hsCRP Level and the Risk of Death or Recurrent Cardiovascular Events in Patients With Myocardial Infarction: A Healthcare-Based Study. J. Am. Heart Assoc. 2019, 8, e012638. [Google Scholar] [CrossRef]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: A collaborative analysis of three randomised trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef]
- O’Brien, E.C.; Simon, D.N.; Roe, M.T.; Wang, T.Y.; Peterson, E.D.; Alexander, K.P. Statin Treatment by Low-Density Lipoprotein Cholesterol Levels in Patients with Non-ST-Segment Elevation Myocardial Infarction/Unstable Angina Pectoris (from the CRUSADE Registry). Am. J. Cardiol. 2015, 115, 1655–1660. [Google Scholar] [CrossRef]
- Cullen, P.; Schulte, H.; Assmann, G. The Münster Heart Study (PROCAM): Total mortality in middle-aged men is increased at low total and LDL cholesterol concentrations in smokers but not in nonsmokers. Circulation 1997, 96, 2128–2136. [Google Scholar] [CrossRef]
- Iseki, K.; Yamazato, M.; Tozawa, M.; Takishita, S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002, 61, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Zuliani, G.; Guralnik, J.M.; Palmieri, E.; Fellin, R. The inverse association between age and cholesterol level among older patients: The role of poor health status. Gerontology 2001, 47, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Leveille, S.G.; Corti, M.C.; Harris, T.B.; Guralnik, J.M. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J. Am. Geriatr. Soc. 2001, 49, 1142–1147. [Google Scholar] [CrossRef]
- Schatz, I.J.; Masaki, K.; Yano, K.; Chen, R.; Rodriguez, B.L.; Curb, J.D. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: A cohort study. Lancet 2001, 358, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Güder, G.; Frantz, S.; Bauersachs, J.; Allolio, B.; Wanner, C.; Koller, M.T.; Ertl, G.; Angermann, C.E.; Störk, S. Reverse epidemiology in systolic and nonsystolic heart failure: Cumulative prognostic benefit of classical cardiovascular risk factors. Circ. Heart Fail. 2009, 2, 563–571. [Google Scholar] [CrossRef]
- Miura, M.; Yamasaki, M.; Uemura, Y.; Yoshikawa, M.; Miyauchi, K.; Tanaka, H.; Miyachi, H.; Yamashita, J.; Suzuki, M.; Yamamoto, T.; et al. Effect of Statin Treatment and Low-Density Lipoprotein-Cholesterol on Short-Term Mortality in Acute Myocardial Infarction Patients Undergoing Primary Percutaneous Coronary Intervention—Multicenter Registry from Tokyo CCU Network Database. Circ. J. 2016, 80, 461–468. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, M.H.; Kim, H.M.; Ahn, Y.; Kim, J.H.; Chae, S.C.; Kim, Y.J.; Hur, S.H.; Seong, I.W.; Hong, T.J.; et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J. Am. Coll. Cardiol. 2011, 58, 1664–1671. [Google Scholar] [CrossRef]
- Cho, K.H.; Jeong, M.H.; Ahn, Y.; Kim, Y.J.; Chae, S.C.; Hong, T.J.; Seong, I.W.; Chae, J.K.; Kim, C.J.; Cho, M.C.; et al. Low-density lipoprotein cholesterol level in patients with acute myocardial infarction having percutaneous coronary intervention (the cholesterol paradox). Am. J. Cardiol. 2010, 106, 1061–1068. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.; Song, X.; Liu, H.; Li, Y.; Hao, Y.; Teng, T.; Liu, J.; Liu, J.; Zhao, D.; et al. Revisiting the lipid paradox in ST-elevation myocardial infarction in the Chinese population: Findings from the CCC-ACS project. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 978–987. [Google Scholar] [CrossRef]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blömstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- Chen, Z.M.; Pan, H.C.; Chen, Y.P.; Peto, R.; Collins, R.; Jiang, L.X.; Xie, J.X.; Liu, L.S. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet 2005, 366, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Nissen, S.E.; Libby, P.; Shao, M.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.; Raichlen, J.S.; Uno, K.; et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation 2013, 128, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Jeong, M.H.; Ahmed, K.; Hachinohe, D.; Choi, H.S.; Chang, S.Y.; Kim, M.C.; Hwang, S.H.; Park, K.H.; Lee, M.G.; et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am. J. Cardiol. 2011, 107, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, J.; Inoue, T.; Saino, Y.; Kobayashi, H.; Murotani, K.; Mori, N.; Maeda, K. Diagnosis and prevalence of cachexia in Asians: A scoping review. Nutrition 2024, 119, 112301. [Google Scholar] [CrossRef]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]


| All Patients (N = 1882) | LDL-C Level at Presentation (mg/dL) | p Value | ||||
|---|---|---|---|---|---|---|
| <70 | 70–99 | 100–129 | ≥130 | |||
| (N = 144) | (N = 551) | (N = 639) | (N = 548) | |||
| Demographics | ||||||
| Age, median (IQR), y | 62 (55–72) | 66 (57–75) | 63 (56–73) | 62 (56–72) | 62 (54–71) | 0.004 |
| Female sex, n (%) | 383 (20.4) | 22 (15.3) | 89 (16.2) | 127 (19.9) | 145 (26.5) | <0.001 |
| Cardiac risk factors, n (%) | ||||||
| Current smoker | 845 (44.9) | 69 (47.9) | 256 (46.5) | 270 (42.3) | 250 (45.6) | 0.392 |
| Hypertension | 1161 (61.7) | 86 (59.7) | 336 (61.0) | 399 (62.4) | 340 (62.0) | 0.911 |
| Diabetes mellitus | 462 (24.5) | 43 (29.9) | 151 (27.4) | 135 (21.1) | 133 (24.3) | 0.034 |
| Family history of CAD | 91 (4.8) | 6 (4.2) | 24 (4.4) | 35 (5.5) | 26 (4.7) | 0.802 |
| Prior stroke | 217 (11.5) | 18 (12.5) | 70 (12.7) | 74 (11.6) | 55 (10.0) | 0.556 |
| Prior MI | 69 (3.7) | 11 (7.6) | 21 (3.8) | 20 (3.1) | 17 (3.1) | 0.058 |
| Prior PCI | 73 (3.9) | 10 (6.9) | 26 (4.7) | 19 (3.0) | 18 (3.3) | 0.086 |
| Prior heart failure | 34 (1.8) | 6 (4.2) | 12 (2.2) | 9 (1.4) | 7 (1.3) | 0.094 |
| BMI (kg/m2) | 24.4 (3.2) | 23.9 (3.4) | 24.1 (3.2) | 24.5 (3.1) | 24.7 (3.2) | 0.037 |
| Diagnosis | 0.230 | |||||
| STEMI | 1284 (68.2) | 91 (63.2) | 363 (65.9) | 447 (70.0) | 383 (69.9) | |
| NSTE-ACS | 598 (31.8) | 53 (8.9) | 188 (31.4) | 192 (32.1) | 165 (27.6) | |
| In-hospital treatment | ||||||
| Cardiac catheterization | 1783 (94.7) | 131 (91.0) | 518 (94.0) | 614 (96.1) | 520 (94.9) | 0.071 |
| PCI | 1640 (87.1) | 115 (79.9) | 457 (82.9) | 574 (89.8) | 494 (90.1) | <0.001 |
| Thrombolytic therapy | 170 (9.0) | 9 (6.3) | 41 (7.4) | 66 (10.3) | 54 (9.9) | 0.186 |
| Heparin administration | ||||||
| IV unfractionated | 1417 (75.3) | 106 (73.6) | 391 (71.0) | 491 (76.8) | 429 (78.3) | 0.026 |
| Low molecular weight | 1289 (68.5) | 82 (56.9) | 398 (72.2) | 445 (69.6) | 364 (66.4) | 0.003 |
| Medications w/in 24 hours | ||||||
| Aspirin | 1863 (99.0) | 140 (97.2) | 546 (99.1) | 634 (99.2) | 543 (99.1) | 0.318 |
| Beta-blocker | 937 (49.8) | 55 (38.2) | 253 (45.9) | 330 (51.6) | 299 (54.6) | 0.001 |
| ACEI/ARB | 1142 (60.7) | 66 (45.8) | 329 (59.7) | 413 (64.6) | 334 (60.9) | 0.001 |
| Statin | 1817 (96.5) | 133 (92.4) | 525 (95.3) | 621 (97.2) | 538 (98.2) | 0.003 |
| Intensive statin | 104 (19.0) | 16 (11.1) | 82 (14.9) | 105 (16.4) | 104 (19.0) | 0.088 |
| LDL-C Level at Presentation (mg/dL) | p Value | ||||
|---|---|---|---|---|---|
| <70 (N = 144) | 70–99 (N = 551) | 100–129 (N = 639) | ≥130 (N = 548) | ||
| In-hospital mortality, n (%) | 14 (9.7) | 25 (4.5) | 17 (2.7) | 19 (3.5) | 0.001 |
| In-hospital MACE, n (%) | 56 (38.9) | 233 (42.3) | 219 (34.3) | 201 (36.7) | 0.037 |
| Long-term mortality, n (%) | 30 (20.8) | 72 (13.1) | 51 (8.0) | 43 (7.8) | <0.001 |
| Parameter | p Value | HR | 95% CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| On-admission LDL-C level | ||||
| <70 mg/dL | 0.002 | 2.09 | 1.30 | 3.36 |
| 70–89 mg/dL | 0.169 | 1.31 | 0.89 | 1.91 |
| 90–129 mg/dL | 0.382 | 0.83 | 0.55 | 1.26 |
| ≥130 mg/dL | Reference | |||
| Demographics | ||||
| Age | <0.001 | 1.07 | 1.05 | 1.09 |
| Cardiac risk factors | ||||
| Diabetes mellitus | 0.008 | 1.50 | 1.11 | 2.02 |
| Clinical presentation | ||||
| Heart failure | <0.001 | 2.29 | 1.69 | 3.11 |
| Diagnosis | ||||
| STEMI | 0.007 | 1.59 | 1.14 | 2.23 |
| In-hospital treatment | ||||
| PCI | <0.001 | 0.28 | 0.21 | 0.38 |
| ACEI/ARB in first 24 h | 0.003 | 0.65 | 0.48 | 0.87 |
| Statin in first 24 h | 0.001 | 0.42 | 0.25 | 0.70 |
| Low LDL-C < 100 | p Value | High LDL-C ≥ 100 | p Value | |||
|---|---|---|---|---|---|---|
| Low CRP (<2) | High CRP (≥2) | Low CRP (<2) | High CRP (≥2) | |||
| In-hospital mortality | 1 (1.2) | 20 (7.7) | 0.030 | 1 (0.5) | 20 (4.3) | 0.009 |
| In-hospital MACE | 19 (22.6) | 120 (46.2) | <0.001 | 47 (23.0) | 165 (35.8) | <0.001 |
| Long-term mortality | 1 (1.2) | 39 (15.5) | 0.001 | 5 (2.6) | 42 (9.4) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Sun, Y.; Miao, Y.; Liu, Z.; Shen, L.; He, B. Initial Low-Density Lipoprotein Cholesterol and Inflammation Status Predicts Long-Term Mortality in Patients with Acute Coronary Syndrome in the Chinese Population. Biomedicines 2025, 13, 1534. https://doi.org/10.3390/biomedicines13071534
Lu Y, Sun Y, Miao Y, Liu Z, Shen L, He B. Initial Low-Density Lipoprotein Cholesterol and Inflammation Status Predicts Long-Term Mortality in Patients with Acute Coronary Syndrome in the Chinese Population. Biomedicines. 2025; 13(7):1534. https://doi.org/10.3390/biomedicines13071534
Chicago/Turabian StyleLu, Yanqiao, Yujun Sun, Yutong Miao, Zhitong Liu, Lan Shen, and Ben He. 2025. "Initial Low-Density Lipoprotein Cholesterol and Inflammation Status Predicts Long-Term Mortality in Patients with Acute Coronary Syndrome in the Chinese Population" Biomedicines 13, no. 7: 1534. https://doi.org/10.3390/biomedicines13071534
APA StyleLu, Y., Sun, Y., Miao, Y., Liu, Z., Shen, L., & He, B. (2025). Initial Low-Density Lipoprotein Cholesterol and Inflammation Status Predicts Long-Term Mortality in Patients with Acute Coronary Syndrome in the Chinese Population. Biomedicines, 13(7), 1534. https://doi.org/10.3390/biomedicines13071534








