Network Pharmacology Approaches to Myocardial Infarction Reperfusion Injury: Exploring Mechanisms, Pathophysiology, and Novel Therapies
Abstract
1. Introduction
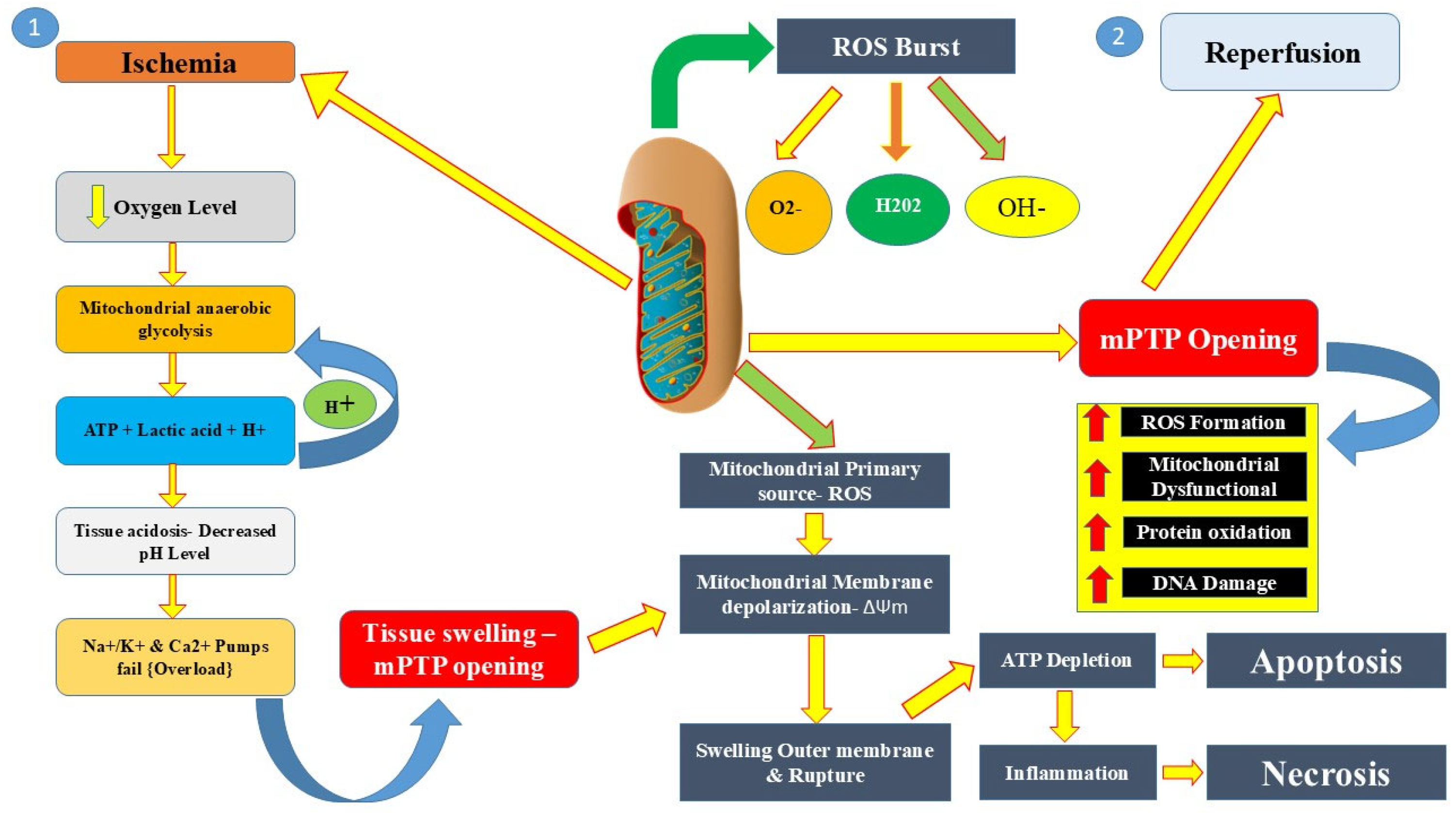
2. Pathophysiology of Reperfusion Injury
2.1. Cellular and Molecular Mechanisms
2.1.1. Oxidative Stress: Role of ROS in Tissue Damage
2.1.2. Calcium Overload: Dysregulated Calcium Homeostasis Leading to Myocyte Injury
2.1.3. Inflammatory Response: Activation of Neutrophils, Cytokines, and Endothelial Dysfunction
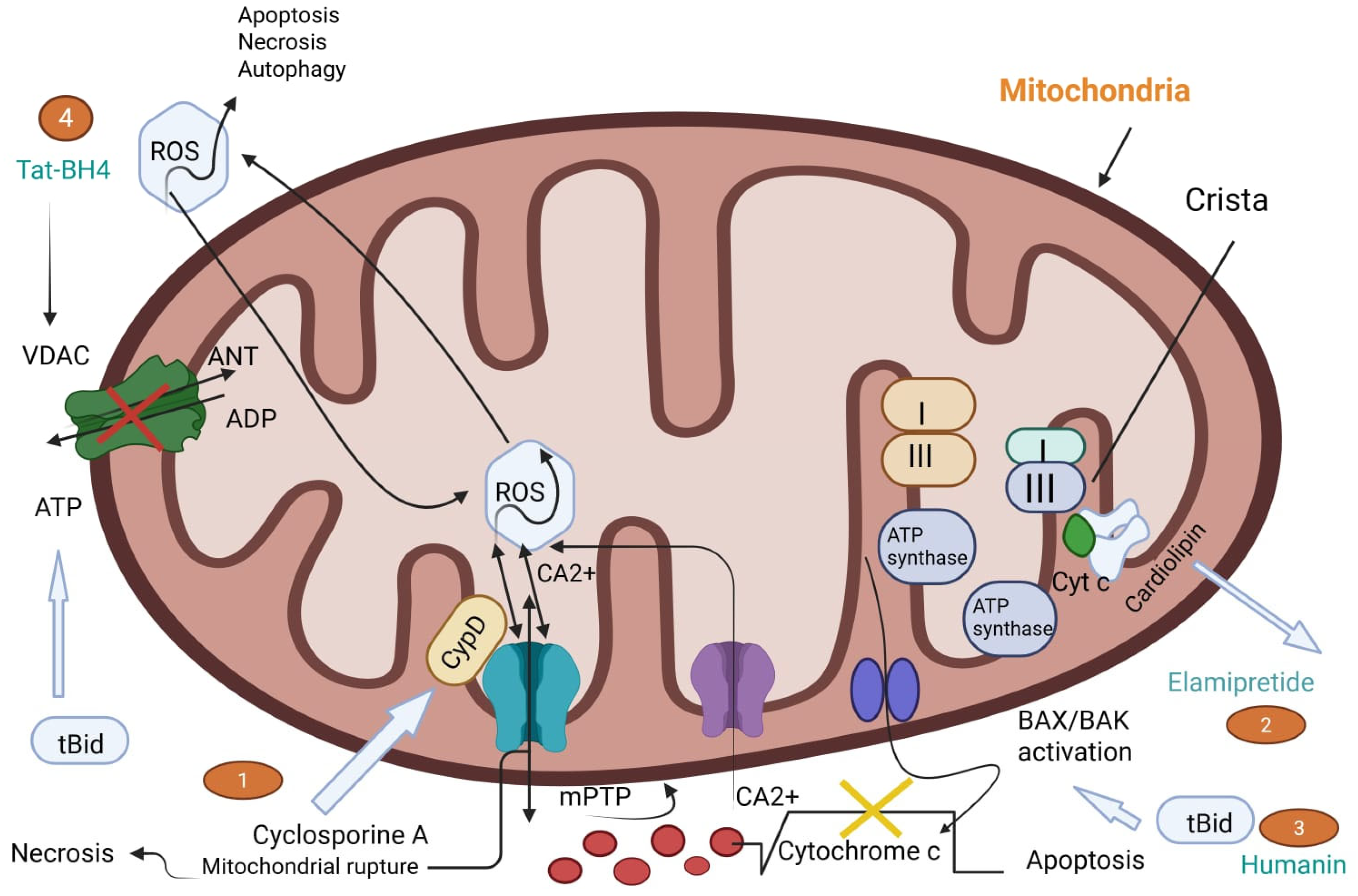
2.1.4. Mitochondrial Dysfunction: Opening of the Mitochondrial Permeability Transition Pore (mPTP)
2.1.5. Autophagy and Apoptosis: Cell Death Pathways in Reperfusion Injury
2.2. Hemodynamic and Structural Changes
2.2.1. Microvascular Obstruction (No-Reflow Phenomenon)
2.2.2. Endothelial Dysfunction and Capillary Leakage Materials and Methods
2.2.3. Myocardial Stunning and Contractile Dysfunction Materials and Methods
2.2.4. Preclinical Models and Investigated Therapeutic Strategies
3. Clinical Implications and Diagnosis
3.1. Cardiac Troponins
3.2. Lactate Dehydrogenase (LDH)
3.3. Imaging Techniques to Evaluate Myocardial Damage
3.3.1. Cardiac MRI
3.3.2. Echocardiography
| Trial Name/ID | Phase | Intervention/Therapy | Patient Population | Targeted Mechanism | Outcome/Findings | Reference |
|---|---|---|---|---|---|---|
| CIRCUS (NCT01502774) | Phase III | Cyclosporine A | STEMI patients undergoing PCI | Mitochondrial permeability transition pore inhibition | No significant reduction in infarct size or MACE | [59] |
| MITOCARE (NCT01374321) | Phase II | TRO40303 (Mitochondrial protector) | STEMI patients | Mitochondrial membrane stabilization | No significant cardioprotective effect observed | [60] |
| DANAMI-3-iPOST (NCT01435408) | Phase III | Ischemic postconditioning (iPOST) | STEMI patients undergoing PCI | Reduction of reperfusion injury via brief intermittent reperfusion | No significant benefit in clinical outcomes | [61] |
| ELIXIR (NCT00091637) | Phase III | Exenatide (GLP-1 agonist) | STEMI patients | Cardiomyocyte protection, metabolic modulation | Modest reduction in infarct size | [74] |
| NOMI trial | Phase II | Nitric oxide inhalation | STEMI patients during PCI | Microvascular protection, vasodilation | Improved myocardial perfusion but mixed results on infarct size | [75] |
4. Current and Emerging Therapeutic Strategies
4.1. Pharmacological Interventions
4.1.1. Antioxidants and ROS Scavengers
4.1.2. Calcium Channel Blockers and Mitochondrial Stabilizers
4.1.3. Anti-Inflammatory Agents
4.1.4. Remote Ischemic Conditioning and Preconditioning Strategies
4.2. Non-Pharmacological Strategies
4.2.1. Hypothermia and Metabolic Modulation
4.2.2. Gene Therapy and Regenerative Approaches
4.2.3. Stem Cell-Based Interventions for Myocardial Repair
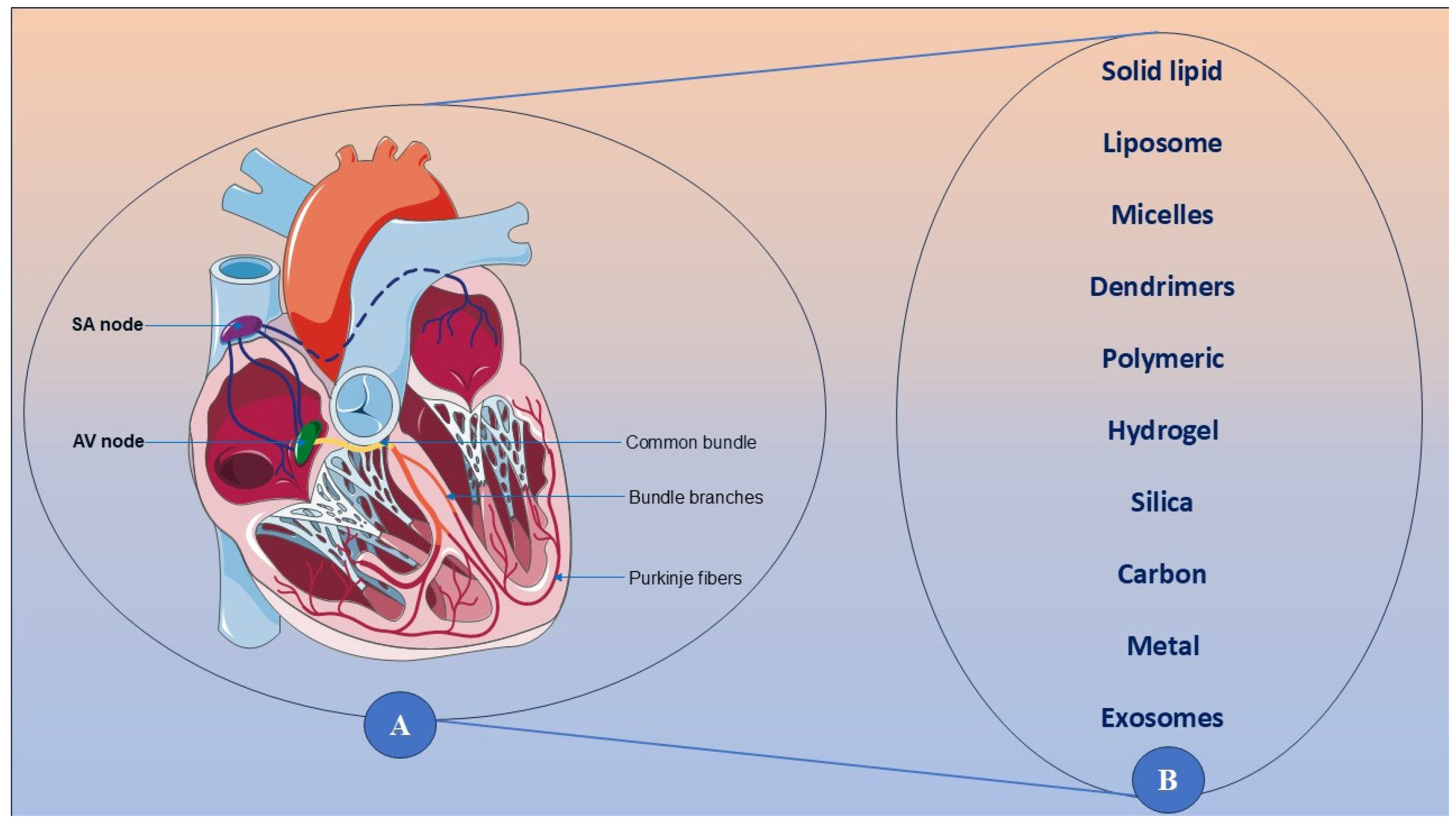
4.3. Advanced Nanomedicine Approaches
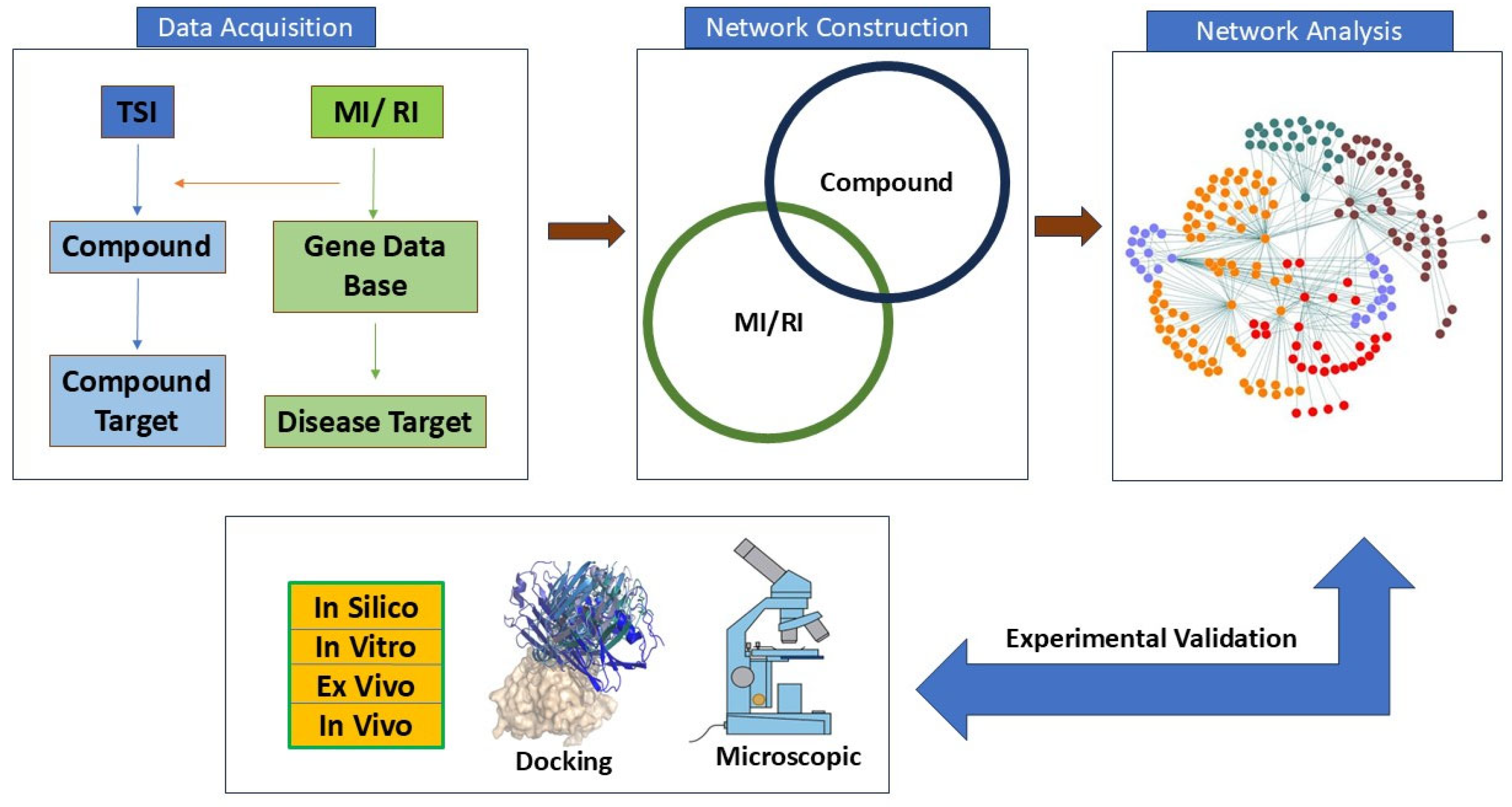
5. Network Pharmacology Approaches in MIRI
5.1. Methodology
5.1.1. Molecular Target
5.1.2. Gene Enrichment Analysis
5.1.3. KEGG Pathway Analysis
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labounty, T.; Eagle, K.A. The nature of the problem: An overview of acute coronary syndromes and myocardial infarction. Biol. Rhythm Res. 2007, 38, 143–153. [Google Scholar] [CrossRef]
- Dilip, D.; Lokendra, K.; Jia, L.-L. Diagnosis and management of acute myocardial infarction: An overview. Vasc. Investig. Ther. 2019, 2, 98. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial Ischemia Reperfusion Injury. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Mach, F.; Montecucco, F. Reactive Oxygen-Induced Cardiac Intracellular Pathways During Ischemia and Reperfusion. Curr. Signal Transduct. Ther. 2012, 7, 89–95. [Google Scholar] [CrossRef]
- Halestrap, A.P. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006, 34, 232. [Google Scholar] [CrossRef]
- Ibáñez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving Therapies for Myocardial Ischemia/Reperfusion Injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Sagris, M.; Apostolos, A.; Theofilis, P.; Ktenopoulos, N.; Katsaros, O.; Tsalamandris, S.; Tsioufis, K.; Toutouzas, K.; Tousoulis, D. Myocardial Ischemia–Reperfusion Injury: Unraveling Pathophysiology, Clinical Manifestations, and Emerging Prevention Strategies. Biomedicines 2024, 12, 802. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Girn, H.R.S.; Ahilathirunayagam, S.; Mavor, A.I.D.; Homer-Vanniasinkam, S. Reperfusion Syndrome: Cellular Mechanisms of Microvascular Dysfunction and Potential Therapeutic Strategies. Vasc. Endovasc. Surg. 2007, 41, 277–293. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Bagheri, F.; Khori, V.; Alizadeh, A.M.; Khalighfard, S.; Khodayari, S.; Khodayari, H. Reactive oxygen species-mediated cardiac-reperfusion injury: Mechanisms and therapies. Life Sci. 2016, 165, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.K.; Zhou, Y.; Xu, T.T.; Wu, Q. Ferroptosis: Opportunities and challenges in myocardial ischemia-reperfusion injury. Oxidative Med. Cell. Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Wang, T. Reactive oxygen species (ROS)-responsive biomaterials for treating myocardial ischemia-reperfusion injury. Front. Bioeng. Biotechnol. 2024, 12, 1469393. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, Y.; Han, R.; He, J.; Liu, D.; Xia, W.; Cai, Y.; Perek, B.; Xia, Z. Ferroptosis and its potential determinant role in myocardial susceptibility to ischemia/reperfusion injury in diabetes. Rev. Cardiovasc. Med. 2024, 25, 360. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Luo, J.; Cai, H.; Li, C.; Lei, Z.; Lu, Y.; Ni, L.; Cao, J.; Cheng, B.; Hu, X. Expression pattern and molecular mechanism of oxidative stress-related genes in myocardial ischemia–reperfusion injury. J. Cardiovasc. Dev. Dis. 2023, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; He, Q.; Xiong, Y.; Zeng, X. Systematic pharmacology reveals the antioxidative stress and anti-inflammatory mechanisms of resveratrol intervention in myocardial ischemia-reperfusion injury. Evid.-Based Complement. Altern. Med. 2021, 2021, 5515396. [Google Scholar] [CrossRef]
- Satoh, H.; Katoh, H.; Terada, H.; Hayashi, H. Involvement of Na+/Ca2+ Exchange in Normal Cardiac Excitation-Contraction Coupling and in Ca2+ Overload during Ischemia and Reperfusion. In Myocardial Ischemia and Preconditioning; Springer: Boston, MA, USA, 2003; pp. 481–503. [Google Scholar] [CrossRef]
- Xing, Y.; Xie, S.-Y.; Deng, W.; Tang, Q.-Z. Cardiolipin in myocardial ischaemia-reperfusion injury: From molecular mechanisms to clinical strategies. Biomed. Pharmacother. 2024, 176, 116936. [Google Scholar] [CrossRef]
- Pittas, K.; Vrachatis, D.A.; Angelidis, C.; Tsoucala, S.; Giannopoulos, G.; Deftereos, S. The role of calcium handling mechanisms in reperfusion injury. Curr. Pharm. Des. 2018, 24, 4077–4089. [Google Scholar] [CrossRef]
- Liu, G.Y.; Xie, W.L.; Wang, Y.T.; Chen, L.; Xu, Z.Z.; Lv, Y.; Wu, Q.P. Calpain: The regulatory point of myocardial ischemia-reperfusion injury. Front. Cardiovasc. Med. 2023, 10, 1194402. [Google Scholar] [CrossRef]
- Jiang, Z. Mechanism research of Salvia miltiorrhiza on treating myocardial ischemia reperfusion injury according to network pharmacology combined with molecular docking technique. Medicine 2021, 100, e28132. [Google Scholar] [CrossRef]
- Nah, D.-Y.; Rhee, M.-Y. The Inflammatory Response and Cardiac Repair After Myocardial Infarction. Korean Circ. J. 2009, 39, 393. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.B.; Puett, D.W.; Virmani, R. Endothelial and myocardial injury during ischemia and reperfusion: Pathogenesis and therapeutic implications. J. Am. Coll. Cardiol. 1989, 13, 450–459. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Li, T.; Wang, H. NETosis in myocardial ischemia-reperfusion injury: From mechanisms to therapies. Biomed. Rep. 2025, 23, 113. [Google Scholar] [CrossRef]
- Li, N.; Gu, X.; Liu, F.; Zhang, Y.; Sun, Y.; Gao, S.; Wang, B.; Zhang, C. Network pharmacology-based analysis of potential mechanisms of myocardial ischemia-reperfusion injury by total salvianolic acid injection. Front. Pharmacol. 2023, 14, 1202718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, X.; Zhao, Z.; Li, H.; Lu, J.; Xie, L.; Zheng, G.; Jiang, T. Identification of regulator gene and pathway in myocardial ischemia-reperfusion injury: A bioinformatics and biological validation study. Hereditas 2025, 162, 35. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Fan, J.; Zhang, J.; Ren, M.; Feng, L. Immune in myocardial ischemia/reperfusion injury: Potential mechanisms and therapeutic strategies. Front. Immunol. 2025, 16, 1558484. [Google Scholar] [CrossRef]
- Halestrap, A.P.; O’Toole, A.; Lim, K. The Mitochondrial Permeability Transition: A‘Pore’Way to Die. In Mechanisms of Organ Dysfunction in Critical Illness; Update in Intensive Care and Emergency Medicine; Springer: Berlin/Heidelberg, Germany, 2002; pp. 17–39. [Google Scholar] [CrossRef]
- Tsurusaki, S.; Kizana, E. Mechanisms and Therapeutic Potential of Multiple Forms of Cell Death in Myocardial Ischemia–Reperfusion Injury. Int. J. Mol. Sci. 2024, 25, 13492. [Google Scholar] [CrossRef]
- Wang, D.; Yu, W.; Liu, Y.; Zhong, G.; Zhao, Z.; Yan, X.; Liu, Q. Roles of Autophagy in Ischemic Heart Diseases and the Modulatory Effects of Chinese Herbal Medicine. Am. J. Chin. Med. 2017, 45, 1401–1419. [Google Scholar] [CrossRef]
- Huang, A.; Wang, Z.; Tang, H.; Jia, Z.; Ji, X.; Yang, X.; Jiang, W. Bardoxolone methyl ameliorates myocardial ischemia/reperfusion injury by activating the Nrf2/HO-1 signaling pathway. Cardiovasc. Ther. 2023, 2023, 5693732. [Google Scholar] [CrossRef]
- Ucar, B.I.; Ucar, G.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Pharmacological protection against ischemia-reperfusion injury by regulating the Nrf2-Keap1-ARE signaling pathway. Antioxidants 2021, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, G.; Tian, Y.; Li, J.; Meng, L.; Jiang, X.; Xin, Y. Sulforaphane inhibits angiotensin II-induced cardiomyocyte apoptosis by acetylation modification of Nrf2. Aging 2022, 14, 6740. [Google Scholar] [CrossRef] [PubMed]
- Van Hout, G.P.; Bosch, L.; Ellenbroek, G.H.; De Haan, J.J.; Van Solinge, W.W.; Cooper, M.A.; Arslan, F.; De Jager, S.C.; Robertson, A.A.; Pasterkamp, G.; et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 2017, 38, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, B.; Zhou, Y.; Yang, Z.; Jiang, L.; Kou, Z.; Li, J.; Ma, X.; Song, J. NLRP3 inflammasome inhibitor MCC950 reduces cerebral ischemia/reperfusion-induced neuronal ferroptosis. Neurosci. Lett. 2023, 795, 137032. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zeng, Z.; Niu, C.; Liu, D.; Lin, S.; Liu, X.; Szabó, G.; Lu, J.; Zheng, S.; Zhou, P. Normothermic ex vivo heart perfusion with NLRP3 inflammasome inhibitor MCC950 treatment improves cardiac function of circulatory death hearts after transplantation. Front. Cardiovasc. Med. 2023, 10, 1126391. [Google Scholar] [CrossRef]
- Papur, O.S.; Glatz, J.F.; Luiken, J.J. Protein kinase-D1 and downstream signaling mechanisms involved in GLUT4 translocation in cardiac muscle. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1861, 119748. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; Chakraborty, P.; Gangopadhyay, M.; Sahu, R.; Medala, V.; John, A.; Reddy, P.H.; De Feo, V.; et al. The emerging role of HDACs: Pathology and therapeutic targets in diabetes mellitus. Cells 2021, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Ge, J.; Li, H. SIRT6 in aging, metabolism, inflammation and cardiovascular diseases. Aging Dis. 2022, 13, 1787. [Google Scholar] [CrossRef]
- Bekkers, S.C.A.M.; Yazdani, S.K.; Virmani, R.; Waltenberger, J. Microvascular Obstruction. J. Am. Coll. Cardiol. 2010, 55, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Bouleti, C.; Mewton, N.; Germain, S. The no-reflow phenomenon: State of the art. Arch. Cardiovasc. Dis. 2015, 108, 661–674. [Google Scholar] [CrossRef]
- del Zoppo, G.J.; Schmid-Schönbein, G.W.; Mori, E.; Copeland, B.R.; Chang, C.M. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 1991, 22, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R.; Perry, M.A. Leukocyte—Endothelial cell adhesion induced by ischemia and reperfusion. Can. J. Physiol. Pharmacol. 1993, 71, 67–75. [Google Scholar] [CrossRef]
- Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Alvarenga, J.M.G.; Nobrega, J.; Cerqueira, R.J.; Mancio, J.; Leite-Moreira, A.F.; Roncon-Albuquerque, R. Myocardial oedema: Pathophysiological basis and implications for the failing heart. ESC Heart Fail. 2022, 9, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.L.; Kukreja, R.C. Free radicals, calcium homeostasis, heat shock proteins, and myocardial stunning. Ann. Thorac. Surg. 1995, 60, 760–766. [Google Scholar] [CrossRef]
- Bolli, R.; Marbán, E. Molecular and Cellular Mechanisms of Myocardial Stunning. Physiol. Rev. 1999, 79, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Kahan, J.; Kelesidis, I.; Makani, H.; Wever-Pinzon, O.; Medina, H.; Garcia, M.J. CMR imaging for the evaluation of myocardial stunning after acute myocardial infarction: A meta-analysis of prospective trials. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1080–1091. [Google Scholar] [CrossRef]
- Martin, T.P.; MacDonald, E.A.; Elbassioni, A.A.M.; O’Toole, D.; Zaeri, A.A.I.; Nicklin, S.A.; Gray, G.A.; Loughrey, C.M. Preclinical models of myocardial infarction: From mechanism to translation. Br. J. Pharmacol. 2022, 179, 770–791. [Google Scholar] [CrossRef]
- Buja, L.M. Pathobiology of Myocardial Ischemia and Reperfusion Injury: Models, Modes, Molecular Mechanisms, Modulation, and Clinical Applications. Cardiol. Rev. 2023, 31, 252–264. [Google Scholar] [CrossRef]
- Fordyce, C.B.; Gersh, B.J.; Stone, G.W.; Granger, C.B. Novel therapeutics in myocardial infarction: Targeting microvascular dysfunction and reperfusion injury. Trends Pharmacol. Sci. 2015, 36, 605–616. [Google Scholar] [CrossRef]
- Rezzani, R.; Rodella, L.F.; Bonomini, F.; Tengattini, S.; Bianchi, R.; Reiter, R.J. Beneficial effects of melatonin in protecting against cyclosporine A-induced cardiotoxicity are receptor mediated. J. Pineal Res. 2006, 41, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Bolling, S.F.; Schirmer, W.J.; Gott, V.L.; Flaherty, J.T.; Bulkley, B.H.; Gardner, T.J. Enhanced myocardial protection with verapamil prior to postischemic reflow. Surgery 1983, 94, 283–290. [Google Scholar]
- Odenstedt, J. Porcine Myocardial Ischemia-Reperfusion Studies on Cardioprotection, Ventricular Arrhythmia and Electrophysiology; Gothenburg University Publications Electronic Archive: Gothenburg, Sweden, 2009. [Google Scholar]
- Dreyer, W.J.; Michael, L.H.; Nguyen, T.; Smith, C.W.; Anderson, D.C.; Entman, M.L.; Rossen, R.D. Kinetics of C5a release in cardiac lymph of dogs experiencing coronary artery ischemia-reperfusion injury. Circ. Res. 1992, 71, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Bergey, J.L.; Wendt, R.L.; Nocella, K.; McCallum, J.D. Acute coronary artery occlusion-reperfusion arrhythmias in pigs: Antiarrhythmic and antifibrillatory evaluation of verapamil, nifedipine, prenylamine and propranolol. Eur. J. Pharmacol. 1984, 97, 95–103. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT01502774?term=NCT01502774&rank=1 (accessed on 10 January 2025).
- Available online: https://clinicaltrials.gov/study/NCT01374321?term=NCT01374321&rank=1 (accessed on 15 January 2025).
- Available online: https://clinicaltrials.gov/study/NCT01435408?term=NCT01435408&rank=1 (accessed on 11 January 2025).
- Saeed, M.; Hetts, S.; Wilson, M. Reperfusion injury components and manifestations determined by cardiovascular MR and MDCT imaging. World J. Radiol. 2010, 2, 1. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Markousis-Mavrogenis, G.; Bacopoulou, F.; Chrousos, G.P. Cardiovascular magnetic resonance imaging as an adjunct to the evaluation of cardiovascular involvement in diabetes mellitus. J. Pers. Med. 2023, 13, 724. [Google Scholar] [CrossRef]
- Xue, H.; Davies, R.H.; Brown, L.A.; Knott, K.D.; Kotecha, T.; Fontana, M.; Plein, S.; Moon, J.C.; Kellman, P. Automated inline analysis of myocardial perfusion MRI with deep learning. Radiol. Artif. Intell. 2020, 2, e200009. [Google Scholar] [CrossRef]
- Mavrogeni, S.I.; Bacopoulou, F.; Markousis-Mavrogenis, G.; Giannakopoulou, A.; Kariki, O.; Vartela, V.; Kolovou, G.; Charmandari, E.; Chrousos, G. Cardiovascular magnetic resonance as pathophysiologic tool in diabetes mellitus. Front. Endocrinol. 2021, 12, 672302. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, W.; Dai, X.; Yu, L.; Lan, Z.; Ding, X.; Zhang, J. Microvascular myocardial ischemia in patients with diabetes without obstructive coronary stenosis and its association with angina. Korean J. Radiol. 2023, 24, 1081. [Google Scholar] [CrossRef]
- Penna, C.; Andreadou, I.; Aragno, M.; Beauloye, C.; Bertrand, L.; Lazou, A.; Falcão-Pires, I.; Bell, R.; Zuurbier, C.J.; Pagliaro, P.; et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia–reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br. J. Pharmacol. 2020, 177, 5312–5335. [Google Scholar] [CrossRef]
- Dia, M.; Paccalet, A.; Pillot, B.; Leon, C.; Ovize, M.; Crola Da Silva, C.; Bochaton, T.; Paillard, M. Myocardial ischemia-reperfusion and diabetes: Lessons learned from bedside to bench. Front. Cardiovasc. Med. 2021, 8, 660698. [Google Scholar] [CrossRef] [PubMed]
- Sharrack, N.; Knott, K.D.; Gulsin, G.S.; Kotecha, T.; Brown, L.A.; Yeo, J.L.; Porcari, A.; Adam, R.D.; Thirunavukarasu, S.; Chowdhary, A.; et al. Metformin associates with higher myocardial perfusion reserve and survival in type 2 diabetes mellitus patients. Sci. Rep. 2024, 14, 27280. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, A.; Salerno, N.; Sabatino, J.; Scatteia, A.; Bisaccia, G.; De Rosa, S.; Dellegrottaglie, S.; Bucciarelli-Ducci, C.; Torella, D.; Leo, I. Underlying mechanisms and cardioprotective effects of SGLT2i and GLP-1Ra: Insights from cardiovascular magnetic resonance. Cardiovasc. Diabetol. 2024, 23, 94. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kumar, B.; Ashique, S.; Yasmin, S.; Venkatesan, K.; Islam, A.; Ghosh, S.; Sahu, A.; Bhui, U.; Ansari, M.Y. A critical review on SGLT2 inhibitors for diabetes mellitus, renal health, and cardiovascular conditions. Diabetes Res. Clin. Pract. 2025, 205, 112050. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K. Myocardial tissue imaging with cardiovascular magnetic resonance. J. Cardiol. 2022, 80, 377–385. [Google Scholar] [CrossRef]
- Karur, G.R.; Aneja, A.; Stojanovska, J.; Hanneman, K.; Latchamsetty, R.; Kersting, D.; Rajiah, P.S. Imaging of cardiac fibrosis: An update, from the AJR special series on imaging of fibrosis. Am. J. Roentgenol. 2024, 222, e2329870. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT00091637?term=NCT00091637&rank=1 (accessed on 22 January 2025).
- Available online: https://clinicaltrials.gov/study/NCT04134078?intr=Nitric%20oxide%20inhalation&rank=7 (accessed on 16 January 2025).
- Kharbanda, R.K. Cardiac conditioning: A review of evolving strategies to reduce ischaemia-reperfusion injury. Heart 2010, 96, 1179–1186. [Google Scholar] [CrossRef]
- Basso, C.; Thiene, G. The pathophysiology of myocardial reperfusion: A pathologist’s perspective. Heart 2006, 92, 1559–1562. [Google Scholar] [CrossRef]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment–elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef]
- Nozari, Y.; Eshraghi, A.; Talasaz, A.H.; Bahremand, M.; Salamzadeh, J.; Salarifar, M.; Pourhosseini, H.; Jalali, A.; Mortazavi, S.H. Protection from reperfusion injury with intracoronary N-acetylcysteine in patients with STEMI undergoing primary percutaneous coronary intervention in a cardiac tertiary center. Am. J. Cardiovasc. Drugs 2018, 18, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Campbell, A.M.; Lu, Y.; An, L.; Alpert, J.S.; Chen, Q.M. N-Acetylcysteine for cardiac protection during coronary artery reperfusion: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2021, 8, 752939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Cheng, Y.; Liu, D.Z.; Liu, M.; Cui, H.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J. Nanobiotechnol. 2019, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Yingzhong, C.; Lin, C.; Chunbin, W. Clinical effects of cyclosporine A on reperfusion injury in myocardial infarction: A meta-analysis of randomized controlled trials. Springerplus 2016, 5, 1117. [Google Scholar] [CrossRef]
- Leboube, S.; Camboulives, L.; Bochaton, T.; Amaz, C.; Bergerot, C.; Altman, M.; Loppinet, T.; Cherpaz, M.; Monsec, T.; Sportouch, C.; et al. What underlies sex differences in heart failure onset within the first year after a first myocardial infarction? Front. Cardiovasc. Med. 2024, 10, 1290375. [Google Scholar] [CrossRef]
- Ottani, F.; Latini, R.; Staszewsky, L.; La Vecchia, L.; Locuratolo, N.; Sicuro, M.; Masson, S.; Barlera, S.; Milani, V.; Lombardi, M.; et al. Cyclosporine A in reperfused myocardial infarction: The multicenter, controlled, open-label CYCLE trial. J. Am. Coll. Cardiol. 2016, 67, 365–374. [Google Scholar] [CrossRef]
- Lim, G.B. Lack of benefit of cyclosporine to attenuate reperfusion injury after PCI. Nat. Rev. Cardiol. 2015, 12, 621. [Google Scholar] [CrossRef]
- Bernink, F.J.P.; Timmers, L.; Beek, A.M.; Diamant, M.; Roos, S.T.; Van Rossum, A.C.; Appelman, Y. Progression in attenuating myocardial reperfusion injury: An overview. Int. J. Cardiol. 2014, 170, 261–269. [Google Scholar] [CrossRef]
- Fathil, M.F.M.; Md Arshad, M.K.; Gopinath, S.C.B.; Hashim, U.; Adzhri, R.; Ayub, R.M.; Ruslinda, A.; M. Nuzaihan, M.N.; Azman, A.; Zaki, M.; et al. Diagnostics on acute myocardial infarction: Cardiac troponin biomarkers. Biosens. Bioelectron. 2015, 70, 209–220. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Theofilis, P.; Pamporis, K.; Sagris, M.; Vlachakis, P.K.; Koufakis, T.; Antoniadis, A.P.; Fragakis, N. GLP-1 receptor agonists and myocardial perfusion: Bridging mechanisms to clinical outcomes. Int. J. Mol. Sci. 2025, 26, 3050. [Google Scholar] [CrossRef]
- Chen, L.; Weng, Y.; Qing, A.; Li, J.; Yang, P.; Ye, L.; Zhu, T. Protective effect of remote ischemic preconditioning against myocardial ischemia-reperfusion injury in rats and mice: A systematic review and meta-analysis. Rev. Cardiovasc. Med. 2022, 23, 413. [Google Scholar] [CrossRef] [PubMed]
- Haller, P.M.; Vargas, K.G.; Haller, M.C.; Piackova, E.; Wojta, J.; Gyöngyösi, M.; Gersh, B.J.; Kiss, A.; Podesser, B.K.; Huber, K. Remote ischaemic conditioning for myocardial infarction or elective PCI: Systematic review and meta-analyses of randomised trials. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 82–92. [Google Scholar] [CrossRef]
- Liu, H.; Fu, L.; Sun, X.; Peng, W.; Chen, Z.; Li, Y. Remote ischemic conditioning improves myocardial parameters and clinical outcomes during primary percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Oncotarget 2017, 9, 8653–8663. [Google Scholar] [CrossRef]
- Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Jangaard, N.; Hosbond, S.; Diederichsen, A.C.; Thygesen, K.; Mickley, H. Clinical Characteristics and Outcomes of Patients with Myocardial Infarction, Myocardial Injury, and Nonelevated Troponins. Am. J. Med. 2016, 129, 446.e5–446.e21. [Google Scholar] [CrossRef]
- Martins, J.T.; Li, D.J.; Baskin, L.B.; Jialal, I.; Keffer, J.H. Comparison of Cardiac Troponin I and Lactate Dehydrogenase Isoenzymes for the Late Diagnosis of Myocardial Injury. Am. J. Clin. Pathol. 1996, 106, 705–708. [Google Scholar] [CrossRef]
- Povlsen, J.A.; Løfgren, B.; Dalgas, C.; Jespersen, N.R.; Johnsen, J.; Bøtker, H.E. Frequent biomarker analysis in the isolated perfused heart reveals two distinct phases of reperfusion injury. Int. J. Cardiol. 2014, 171, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, H.; Schulz-Menger, J. Cardiovascular Magnetic Resonance T2-weighted Imaging of Myocardial Edema in Acute Myocardial Infarction. Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 63–68. [Google Scholar] [CrossRef]
- Tada, Y.; Yang, P.C. Myocardial Edema on T2-Weighted MRI. Circ. Res. 2017, 121, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, P.D.V.; Broderick, T.M.; Williams, E.S.; Davis, C.; Dillon, J.C.; Armstrong, W.F.; Fineberg, N.; Ryan, T.; Feigenbaum, H. Early recovery of regional left ventricular function after reperfusion in acute myocardial infarction assessed by serial two-dimensional echocardiography. Am. J. Cardiol. 1989, 63, 641–646. [Google Scholar] [CrossRef]
- Kobets, A.V.; Kopytsia, M.P.; Tytarenko, N.V.; Rodionova, Y.V. Role of speckle tracking echocardiography in patients with myocardial infarction. Pathologia 2021, 18, 117–124. [Google Scholar] [CrossRef]
- Wang, Q.; Zuurbier, C.J.; Huhn, R.; Torregroza, C.; Hollmann, M.W.; Preckel, B.; Brom, C.E.v.D.; Weber, N.C. Pharmacological Cardioprotection against Ischemia Reperfusion Injury—The Search for a Clinical Effective Therapy. Cells 2023, 12, 1432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef]
- Grothusen, C.; Hagemann, A.; Attmann, T.; Braesen, J.; Broch, O.; Cremer, J.; Schoettler, J. Impact of an Interleukin-1 Receptor Antagonist and Erythropoietin on Experimental Myocardial Ischemia/Reperfusion Injury. Sci. World J. 2012, 2012, 737585. [Google Scholar] [CrossRef] [PubMed]
- Rout, A.; Tantry, U.S.; Novakovic, M.; Sukhi, A.; Gurbel, P.A. Targeted pharmacotherapy for ischemia reperfusion injury in acute myocardial infarction. Expert Opin. Pharmacother. 2020, 21, 1851–1865. [Google Scholar] [CrossRef]
- McLeod, S.L.; Iansavichene, A.; Cheskes, S. Remote ischemic perconditioning to reduce reperfusion injury during acute ST-segment-elevation myocardial infarction: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e005522. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. Preconditioning and postconditioning: New strategies for cardioprotection. Diabetes Obes. Metab. 2008, 10, 451–459. [Google Scholar] [CrossRef]
- El Farissi, M.; Keulards, D.C.J.; Zelis, J.M.; van ’t Veer, M.; Zimmermann, F.M.; Pijls, N.H.J.; Otterspoor, L.C. Hypothermia for Reduction of Myocardial Reperfusion Injury in Acute Myocardial Infarction: Closing the Translational Gap. Circ. Cardiovasc. Interv. 2021, 14, e010326. [Google Scholar] [CrossRef]
- Yang, D.; Guo, S.; Zhang, T.; Li, H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett. 2009, 583, 2500–2506. [Google Scholar] [CrossRef]
- Du, Q. Metabolic modulators in cardioprotection: A focus on trimetazidine. Arch. Pharm. 2024, 74, 679–688. [Google Scholar] [CrossRef]
- Deng, R.M.; Zhou, J. The role of PI3K/AKT signaling pathway in myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2023, 123, 110714. [Google Scholar] [CrossRef]
- Contessotto, P.; Pandit, A. Therapies to prevent post-infarction remodelling: From repair to regeneration. Biomaterials 2021, 275, 120906. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shi, C.; Qin, Y.; Wang, S.; Pan, H.; Chen, M.; Yu, X.; Lou, Y.; Fan, G. Network pharmacology-based investigation and experimental exploration of the antiapoptotic mechanism of colchicine on myocardial ischemia reperfusion injury. Front. Pharmacol. 2021, 12, 804030. [Google Scholar] [CrossRef]
- He, Z.; Liu, L.; Han, D.; Gao, K.; Dong, L.; Bu, D.; Huo, P.; Wang, Z.; Deng, W.; Liu, J.; et al. POINT: A web-based platform for pharmacological investigation enhanced by multi-omics networks and knowledge graphs. arXiv 2025, arXiv:2503.07203. [Google Scholar] [CrossRef]
- Huang, S.; Chen, M.; Yu, H.; Lin, K.; Guo, Y.; Zhu, P. Co-expression of tissue kallikrein 1 and tissue inhibitor of matrix metalloproteinase 1 improves myocardial ischemia-reperfusion injury by promoting angiogenesis and inhibiting oxidative stress. Mol. Med. Rep. 2021, 23, 166. [Google Scholar] [CrossRef]
- Takawale, A.; Fan, D.; Basu, R.; Shen, M.; Parajuli, N.; Wang, W.; Wang, X.; Oudit, G.Y.; Kassiri, Z. Myocardial recovery from ischemia–reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ. Heart Fail. 2014, 7, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Lavu, M.; Gundewar, S.; Lefer, D.J. Gene therapy for ischemic heart disease. J. Mol. Cell. Cardiol. 2011, 50, 742–750. [Google Scholar] [CrossRef]
- Henning, R.J. Therapeutic Angiogenesis: Angiogenic Growth Factors for Ischemic Heart Disease. Future Cardiol. 2016, 12, 585–599. [Google Scholar] [CrossRef]
- Chatterjee, S.; Stewart, A.S.; Bish, L.T.; Jayasankar, V.; Kim, E.M.; Pirolli, T.; Burdick, J.; Woo, Y.J.; Gardner, T.J.; Sweeney, H.L. Viral Gene Transfer of the Antiapoptotic Factor Bcl-2 Protects Against Chronic Postischemic Heart Failure. Circulation 2002, 106, I212–I217. [Google Scholar] [CrossRef]
- Chen, C.; Ponnusamy, M.; Liu, C.; Gao, J.; Wang, K.; Li, P. MicroRNA as a Therapeutic Target in Cardiac Remodeling. Biomed. Res. Int. 2017, 2017, 1–25. [Google Scholar] [CrossRef]
- Wang, W.E.; Chen, X.; Houser, S.R.; Zeng, C. Potential of cardiac stem/progenitor cells and induced pluripotent stem cells for cardiac repair in ischaemic heart disease. Clin. Sci. 2013, 125, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Zhao, Q.; Zhang, Y.C.; Cheng, L.; Liu, M.; Shi, J.; Yang, Y.Z.; Pan, C.; Ge, J.; Phillips, M. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul. Pept. 2004, 117, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Stastna, M.; Abraham, M.R.; Van Eyk, J.E. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009, 583, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Taheri, B.; Soleimani, M.; Fekri Aval, S.; Esmaeili, E.; Bazi, Z.; Zarghami, N. Induced pluripotent stem cell-derived extracellular vesicles: A novel approach for cell-free regenerative medicine. J. Cell. Physiol. 2019, 234, 8455–8464. [Google Scholar] [CrossRef]
- Liu, N.B.; Wu, M.; Chen, C.; Fujino, M.; Huang, J.S.; Zhu, P.; Li, X.K. Novel molecular targets participating in myocardial ischemia-reperfusion injury and cardioprotection. Cardiol. Res. Pract. 2019, 2019, 6935147. [Google Scholar] [CrossRef] [PubMed]
- Vander Heide, R.S.; Steenbergen, C. Cardioprotection and Myocardial Reperfusion. Circ. Res. 2013, 113, 464–477. [Google Scholar] [CrossRef]
- Wang, R.-S.; Maron, B.A.; Loscalzo, J. Multiomics Network Medicine Approaches to Precision Medicine and Therapeutics in Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Aimo, A.; Padoan, A.; Passino, C.; Fortunato, A.; Galli, C.; Plebani, M. Advancements and challenges in high-sensitivity cardiac troponin assays: Diagnostic, pathophysiological, and clinical perspectives: On behalf of the Italian Study Group on Cardiac Biomarkers. Clin. Chem. Lab. Med. 2025, 63, 1260–1278. [Google Scholar] [CrossRef]

| Animal Model | Method of Inducing MI/Reperfusion Injury | Mechanisms Studied | Therapeutic Agents/Interventions | Key Findings | References |
|---|---|---|---|---|---|
| Rat (Wistar/SD) | LAD ligation (30–45 min ischemia + reperfusion) | Oxidative stress, apoptosis, inflammation | N-acetylcysteine, melatonin, cyclosporine A | Reduced infarct size, improved cardiac function | [53] |
| Mouse (C57BL/6) | Temporary LAD occlusion (30 min) + reperfusion | Mitochondrial dysfunction, necroptosis | MitoQ, necrostatin-1, ischemic preconditioning | Decreased ROS, inhibited necroptosis, cardioprotection | [54] |
| Rabbit | Coronary artery occlusion (30 min) + reperfusion | Endothelial dysfunction, calcium overload | Nitric oxide donors, verapamil | Improved endothelial function, reduced calcium overload | [55] |
| Pig | LAD occlusion (60 min) + reperfusion | Microvascular obstruction, no-reflow phenomenon | Adenosine, therapeutic hypothermia | Reduced no-reflow area, improved myocardial perfusion | [56] |
| Dog | Coronary occlusion + reperfusion (variable duration) | Complement activation, inflammation | Pexelizumab (anti-C5 antibody) | Decreased complement-mediated injury | [57] |
| Guinea pig | LAD ligation + reperfusion | Arrhythmias, calcium handling | Beta-blockers, calcium channel blockers | Reduced arrhythmias, stabilized calcium homeostasis | [58] |
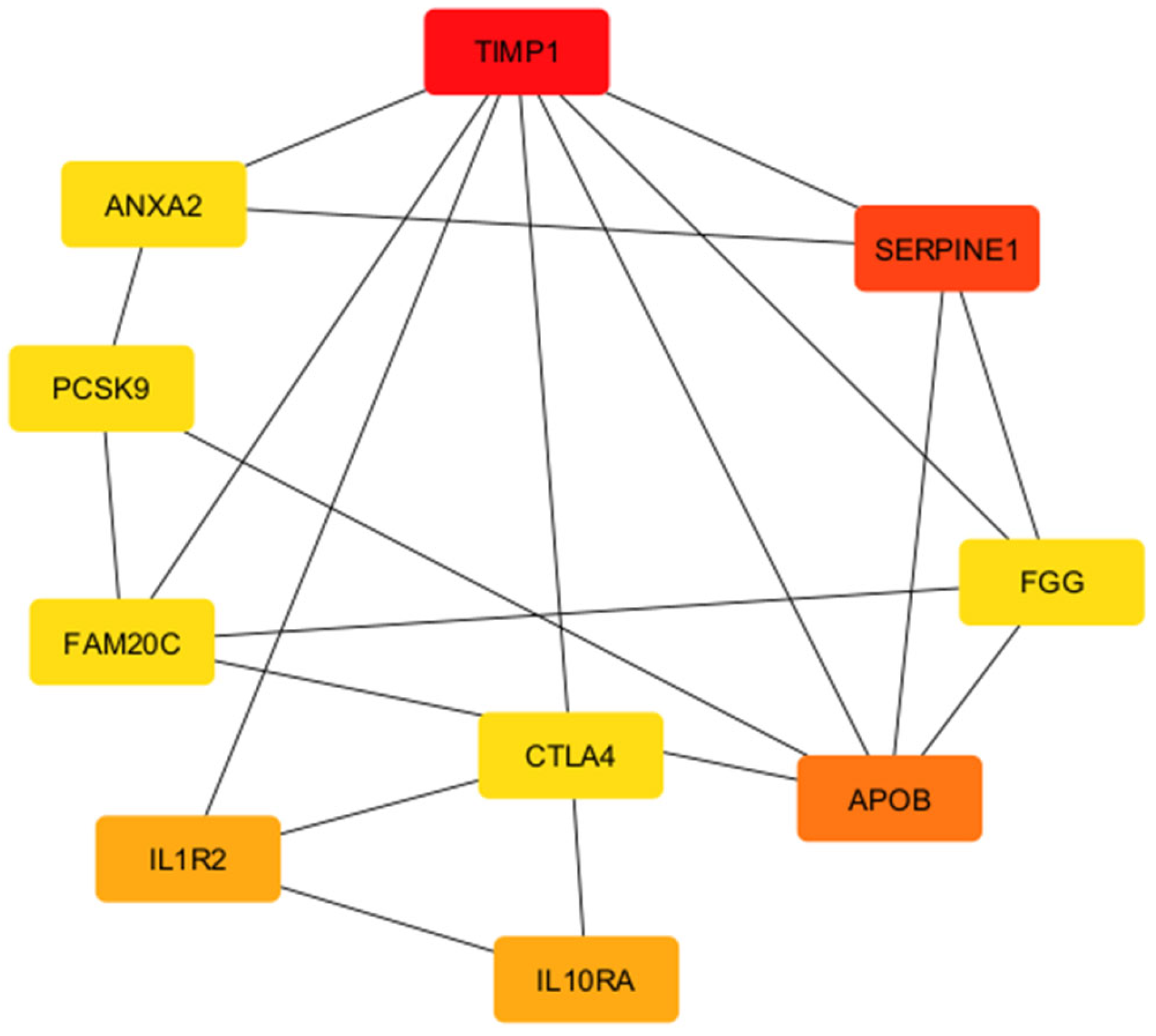
| Rank | Name | Score |
|---|---|---|
| 1 | TIMP1 | 10 |
| 2 | SERPINE1 | 8 |
| 3 | APOB | 7 |
| 4 | IL1R2 | 5 |
| 4 | IL10RA | 5 |
| 6 | CTLA4 | 4 |
| 6 | FGG | 4 |
| 6 | FAM20C | 4 |
| 6 | PCSK9 | 4 |
| 6 | ANXA2 | 4 |
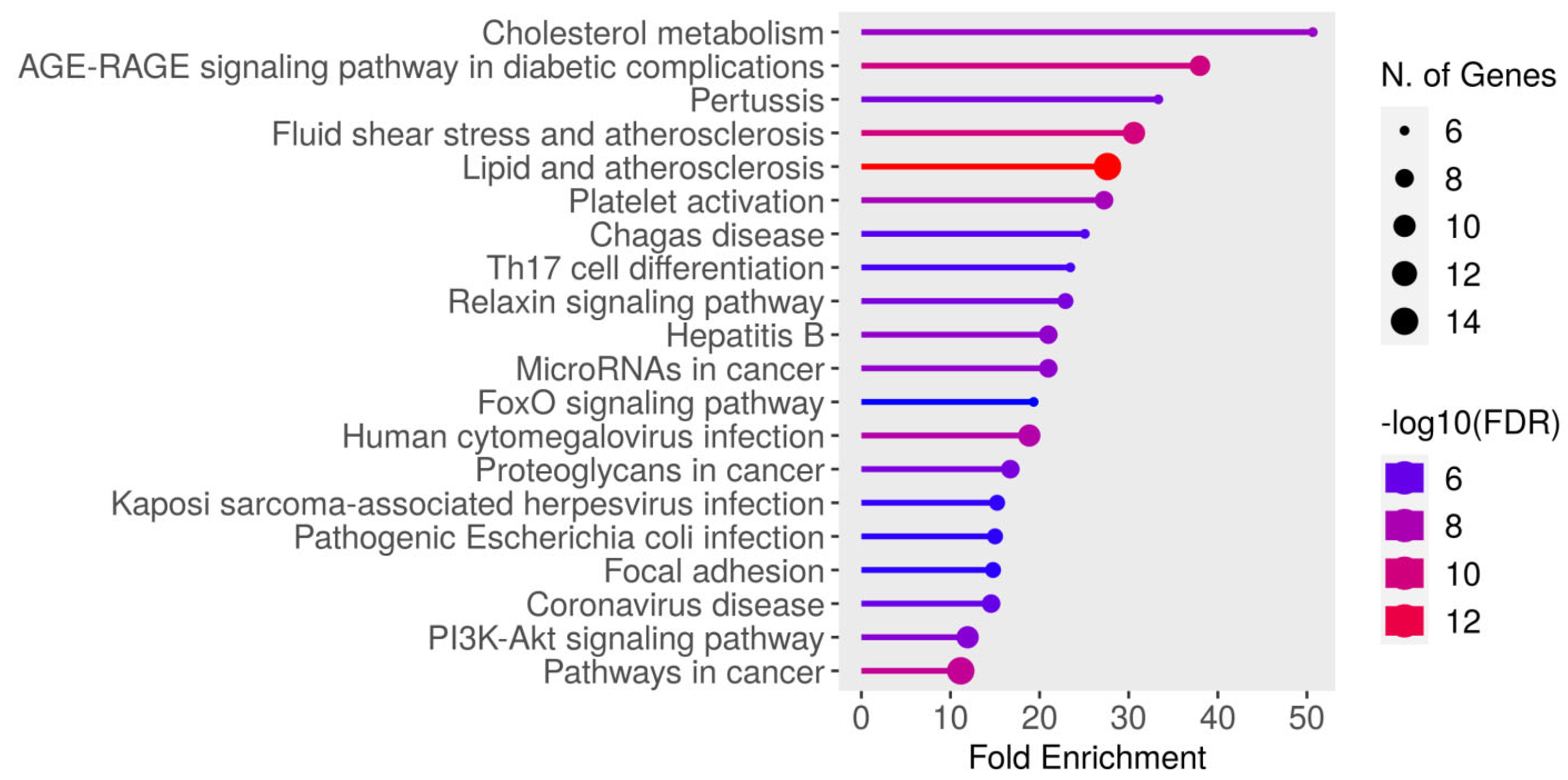
| Enrichment FDR | No. of Genes | Pathway Genes | Fold Enrichment | Pathways |
|---|---|---|---|---|
| 5.7 × 10−8 | 6 | 50 | 50.7 | Cholesterol metabolism (https://www.kegg.jp/kegg-bin/show_pathway?hsa04979) (accessed on 17 April 2025) |
| 2.0 × 10−5 | 4 | 41 | 41.2 | Bladder cancer (https://www.kegg.jp/kegg-bin/show_pathway?hsa05219) (accessed on 17 April 2025) |
| 2.3 × 10−4 | 3 | 31 | 40.9 | Antifolate resistance (https://www.kegg.jp/kegg-bin/show_pathway?hsa01523) (accessed on 17 April 2025) |
| 2.9 × 10−3 | 2 | 22 | 38.4 | Arginine biosynthesis (https://www.kegg.jp/kegg-bin/show_pathway?hsa00220) (accessed on 17 April 2025) |
| 1.3 × 10−10 | 9 | 100 | 38 | AGE–RAGE signaling pathway in diabetic complications (https://www.kegg.jp/kegg-bin/show_pathway?hsa04933) (accessed on 17 April 2025) |
| 3.5 × 10−5 | 4 | 49 | 34.5 | Malaria (https://www.kegg.jp/kegg-bin/show_pathway?hsa05144) (accessed on 17 April 2025) |
| 3.2 × 10−4 | 3 | 37 | 34.2 | African trypanosomiasis (https://www.kegg.jp/kegg-bin/show_pathway?hsa05143) (accessed on 17 April 2025) |
| 4.0 × 10−7 | 6 | 76 | 33.3 | Pertussis (https://www.kegg.jp/kegg-bin/show_pathway?hsa05133) (accessed on 17 April 2025) |
| 9.3 × 10−11 | 10 | 138 | 30.6 | Fluid shear stress and atherosclerosis (https://www.kegg.jp/kegg-bin/show_pathway?hsa05418) (accessed on 17 April 2025) |
| 4.7 × 10−3 | 2 | 29 | 29.1 | Linoleic acid metabolism (https://www.kegg.jp/kegg-bin/show_pathway?hsa00591) (accessed on 17 April 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, J.; Sah, A.K.; Choudhary, R.K.; Elshaikh, R.H.; Bhui, U.; Chowdhury, S.; Abbas, A.M.; Shalabi, M.G.; Siddique, N.A.; Alshammari, R.R.; et al. Network Pharmacology Approaches to Myocardial Infarction Reperfusion Injury: Exploring Mechanisms, Pathophysiology, and Novel Therapies. Biomedicines 2025, 13, 1532. https://doi.org/10.3390/biomedicines13071532
Das J, Sah AK, Choudhary RK, Elshaikh RH, Bhui U, Chowdhury S, Abbas AM, Shalabi MG, Siddique NA, Alshammari RR, et al. Network Pharmacology Approaches to Myocardial Infarction Reperfusion Injury: Exploring Mechanisms, Pathophysiology, and Novel Therapies. Biomedicines. 2025; 13(7):1532. https://doi.org/10.3390/biomedicines13071532
Chicago/Turabian StyleDas, Joy, Ashok Kumar Sah, Ranjay Kumar Choudhary, Rabab H. Elshaikh, Utpal Bhui, Shreya Chowdhury, Anass M. Abbas, Manar G. Shalabi, Nadeem Ahmad Siddique, Raji Rubayyi Alshammari, and et al. 2025. "Network Pharmacology Approaches to Myocardial Infarction Reperfusion Injury: Exploring Mechanisms, Pathophysiology, and Novel Therapies" Biomedicines 13, no. 7: 1532. https://doi.org/10.3390/biomedicines13071532
APA StyleDas, J., Sah, A. K., Choudhary, R. K., Elshaikh, R. H., Bhui, U., Chowdhury, S., Abbas, A. M., Shalabi, M. G., Siddique, N. A., Alshammari, R. R., Trivedi, N., Ali Buwaiqi, K. S., Al Ghenaimi, S., & Prabhakar, P. K. (2025). Network Pharmacology Approaches to Myocardial Infarction Reperfusion Injury: Exploring Mechanisms, Pathophysiology, and Novel Therapies. Biomedicines, 13(7), 1532. https://doi.org/10.3390/biomedicines13071532










