We would like to identify and amend errors in a previously published paper [1]. The authors state that the scientific conclusions are unaffected. This correction was approved by the Academic Editor. The original publication has also been updated.
Error in Figure
In the original publication, a component of Figure 2 (Figure 2d) was missing. The data values in the figure are published and cited in the original manuscript text (first paragraph from ‘secondary outcomes’ section). The corrected Figure 2 appears below.
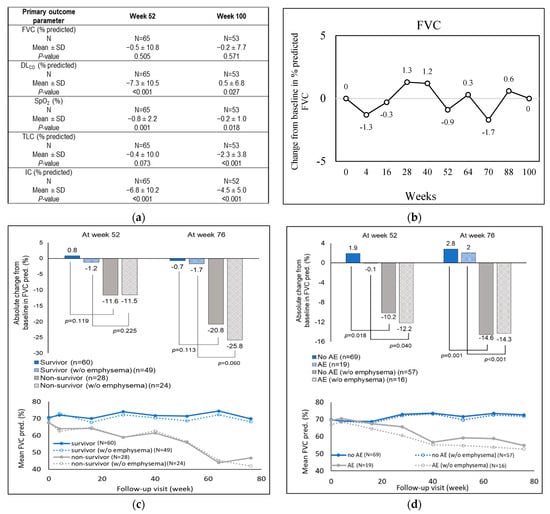
Figure 2.
Changes in baseline of primary outcome parameters across the study period in the treated group. (a) Annual changes from baseline for the primary lung function parameters, (i) FVC, (ii) DLCO, (iii) SpO2, (iv) TLC, and (v) IC, as measured through spirometry. (b) Changes in percent predicted FVC from baseline. (c) Predicted mean and absolute FVC changes in survivors and non-survivors, treated with antifibrotic drugs, and (d) predicted mean and absolute FVC changes in treated patients experiencing versus not experiencing at least one acute exacerbation, stratified by emphysema status. AE, acute exacerbation; DLCO, diffusion of carbon monoxide in lungs; FVC, forced vital capacity; IC, inspiratory capacity; SpO2, oxygen saturation; pred, predicted; TLC, total lung capacity.
Additionally, incorrect values were included in Figure 3c (the week 52 data value of 6 MWT). Correct values are reported in the original manuscript text (Table 6 and second paragraph from ‘secondary outcomes’ section). The corrected Figure 3 appears below.
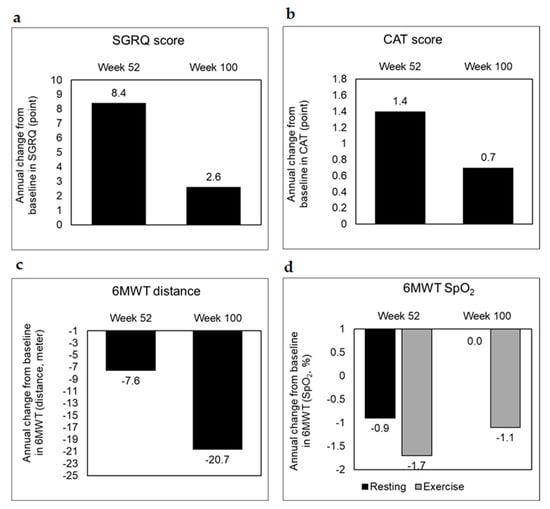
Figure 3.
Secondary outcome trends in the treated group. Secondary outcomes with respect to (a) SGRQ, (b) CAT, and (c,d) 6MWT were scored as annual changes from the baseline at the end of weeks 52 and 100 to assess health-related quality of life, airway obstruction and exercise-related pulmonary function, respectively. SGRQ, St. George’s respiratory questionnaire; CAT, chronic obstructive pulmonary disease assessment test; 6MWT, 6 min walk test.
Error in Table
In the original publication, there was a mistake in Tables 3–5 as published, in that 95% CI was incorrectly written as 95% C.I. There was an extra row in Table 3. The corrected Table 3, Table 4 and Table 5 appear below.

Table 3.
Logistic regression analysis for death between weeks 0–104.

Table 4.
Logistic regression analysis for acute exacerbation/death in treated patients between weeks 0–104.

Table 5.
Logistic regression analysis for acute exacerbation between weeks 53–104.
Text Correction
In the Results Section, Section 3.2.1., Figure 2a was mis-cited in the second sentence of paragraphs 1 and 2 and they should be Figure S1. The corrected sentences appear below.
The mean ± SD of absolute annual change from baseline in FVC was −114.3 ± 441.5 mL at week 52 and −142.5 ± 610.8 mL at week 100 (Figure S1).
There was no significant change from baseline (mean ± SD) for absolute DLCO, which ranged from −2.6 to 0.1 mL/min/mmHg (Figure S1) during the 2-year follow-up.
In the Discussion section, there was an error in the original publication. The “mean ± 1.7%” is updated to “±1.7%” in the first paragraph of the discussion for consistency with the results. The corrected sentence appears below.
Despite significantly compromised baseline functionality, antifibrotic therapy with nintedanib or pirfenidone limited further deteriorations of respiratory functions, especially with respect to annual changes from baseline in percent predicted FVC (±1.7%), without adversely affecting the quality of life.
Modifications in Author Contributions
The corrected Author Contributions appears below.
Conceptualization, S.-L.C., H.-C.W. and D.-W.P.; validation, H.-C.W. and D.-W.P.; investigation, S.-L.C., C.-C.S., C.-F.C., J.-Y.H., K.-C.K., L.-W.H., C.-H.L., W.-F.F., H.-C.W. and D.-W.P.; data curation, S.-L.C., C.-C.S., C.-F.C., J.-Y.H., K.-C.K., L.-W.H., C.-H.L., W.-F.F., H.-C.W. and D.-W.P.; writing—review and editing, S.-L.C.; visualization, S.-L.C., H.-C.W. and D.-W.P. All authors have read and agreed to the published version of the manuscript.
Reference
- Cheng, S.-L.; Sheu, C.-C.; Chian, C.-F.; Hsu, J.-Y.; Kao, K.-C.; Hang, L.-W.; Lin, C.-H.; Fang, W.-F.; Wang, H.-C.; Perng, D.-W. NICEFIT—A Prospective, Non-Interventional, and Multicentric Study for the Management of Idiopathic Pulmonary Fibrosis with Antifibrotic Therapy in Taiwan. Biomedicines 2022, 10, 2362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).