Biomarker-Based Nomogram to Predict Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Tissue Specimens and RNA Isolation
2.3. Whole-Genome Gene Expression Microarray: Biomarker Discovery Phase
2.4. Reverse-Transcription Quantitative PCR (RT-qPCR): Biomarker Validation Phase
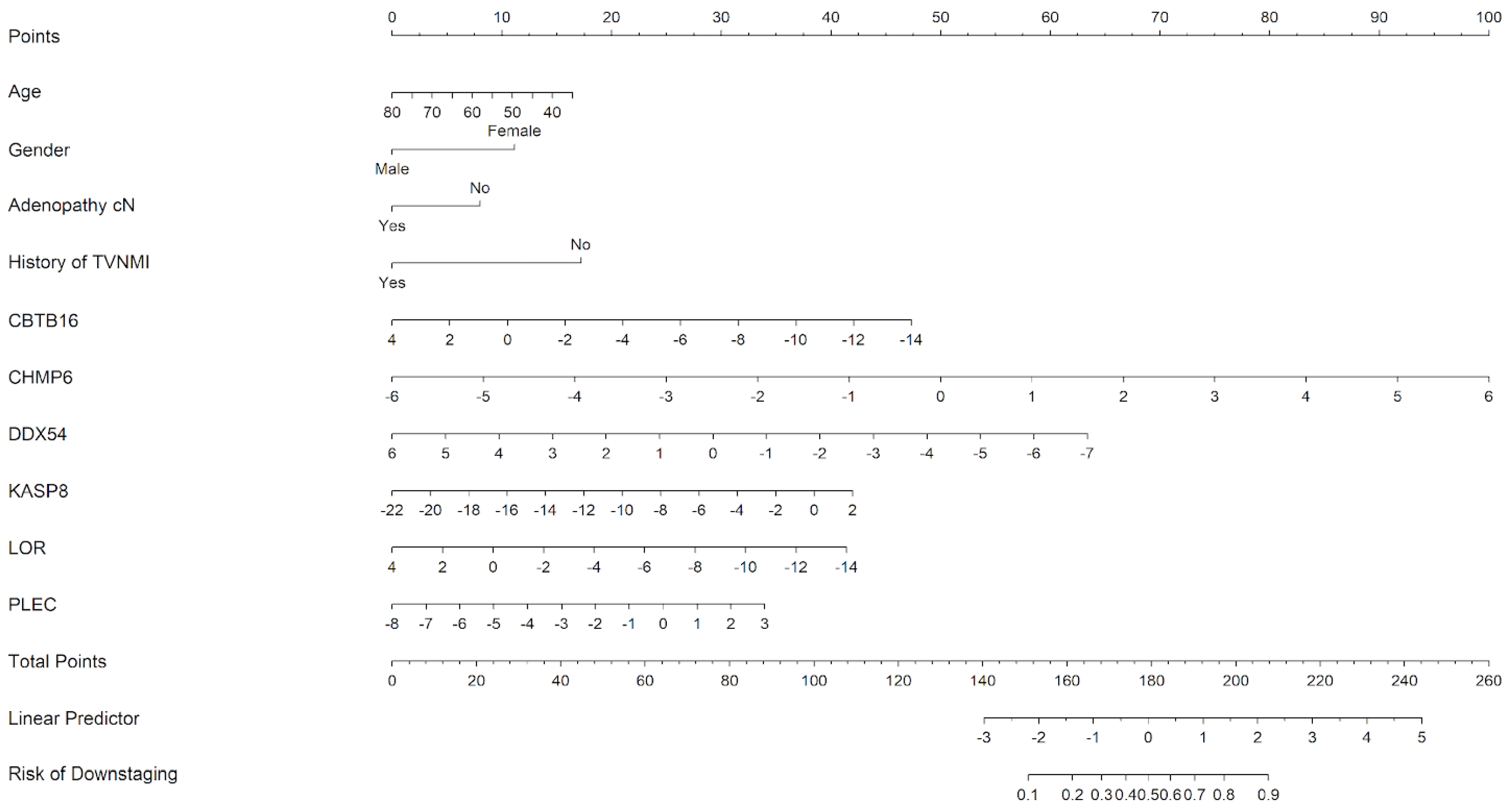
2.5. Downstaging Predictors
2.6. Survival Analysis
3. Results
3.1. Clinicopathological Features of the Cohort
3.2. Biomarker Discovery Phase
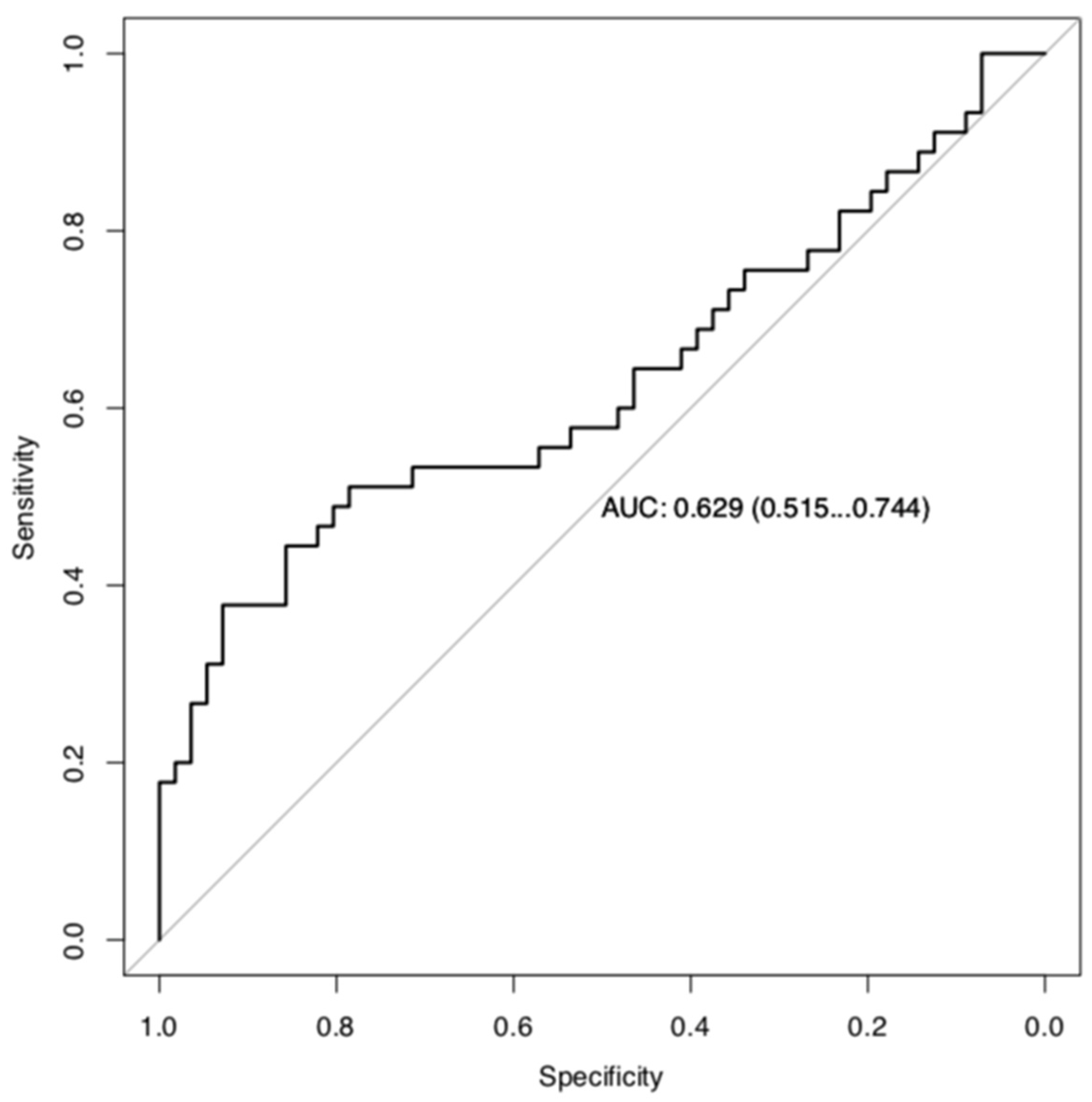
3.3. Classifier Development Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAC | Neoadjuvant chemotherapy |
| RC | Radical cystectomy |
| TURB | Transurethral resection of the bladder |
| AIC | Akaike information criterion |
| RCTs | Randomized controlled trials |
| MIBC | Muscle-invasive bladder cancer |
| FFPE | Formalin-fixed paraffin-embedded |
| CI | Confidence interval |
| HR | Hazard ratio |
| CG | Cisplatin + gemcitabine |
| ddMVAC | Dose-dense methotrexate + vinblastine + doxorubicin + cisplatin |
| EAU | European Association of Urology |
| CT | Computerized tomography |
| RNA | Ribonucleic acid |
| RT-qPCR | Real-time quantitative reverse-transcription polymerase chain reaction |
| RS | Risk score |
| FDR | False Discovery Rate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-Analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 2005, 48, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Pfister, C.; Gravis, G.; Fléchon, A.; Soulié, M.; Guy, L.; Laguerre, B.; Mottet, N.; Joly, F.; Allory, Y.; Harter, V.; et al. Randomized Phase III Trial of Dose-dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin, or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients with Muscle-invasive Bladder Cancer. Analysis of the GETUG/AFU V05 VESPER Trial Secondary Endpoints: Chemotherapy Toxicity and Pathological Responses. Eur. Urol. 2021, 79, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, F.; Sternberg, C.N. Neoadjuvant and adjuvant chemotherapy in muscle-invasive bladder cancer. Eur. Urol. 2009, 55, 348–358. [Google Scholar] [CrossRef]
- Milowsky, M.I.; Rumble, R.B.; Booth, C.M.; Gilligan, T.; Eapen, L.J.; Hauke, R.J.; Boumansour, P.; Lee, C.T. Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2016, 34, 1945–1952. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Y.; Wang, H.; Dong, B.; Yan, W.P.; Lin, Y.; Shi, Q.; Chen, K.N. Possible prediction of the response of esophageal squamous cell carcinoma to neoadjuvant chemotherapy based on gene expression profiling. Oncotarget 2016, 7, 4531–4541. [Google Scholar] [CrossRef]
- Dayde, D.; Tanaka, I.; Jain, R.; Tai, M.C.; Taguchi, A. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int. J. Mol. Sci. 2017, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Pang, Z.; Zhao, Z. A gene signature predicts response to neoadjuvant chemotherapy in triple-negative breast cancer patients. Biosci. Rep. 2019, 39, BSR20190414. [Google Scholar] [CrossRef]
- Sella, T.; Gelber, S.I.; Poorvu, P.D.; Kim, H.J.; Dominici, L.; Guzman-Arocho, Y.D.; Collins, L.; Ruddy, K.J.; Tamimi, R.M.; Peppercorn, J.M.; et al. Response to neoadjuvant chemotherapy and the 21-gene Breast Recurrence Score test in young women with estrogen receptor-positive early breast cancer. Breast Cancer Res. Treat. 2021, 186, 157–165. [Google Scholar] [CrossRef]
- Bownes, R.J.; Turnbull, A.K.; Martinez-Perez, C.; Cameron, D.A.; Sims, A.H.; Oikonomidou, O. On-treatment biomarkers can improve prediction of response to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res. 2019, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Takata, R.; Katagiri, T.; Kanehira, M.; Shuin, T.; Miki, T.; Namiki, M.; Kohri, K.; Tsunoda, T.; Fujioka, T.; Nakamura, Y. Validation study of the prediction system for clinical response of M-VAC neoadjuvant chemotherapy. Cancer Sci. 2007, 98, 113–117. [Google Scholar] [CrossRef]
- Kato, Y.; Zembutsu, H.; Takata, R.; Miya, F.; Tsunoda, T.; Obara, W.; Fujioka, T.; Nakamura, Y. Predicting response of bladder cancers to gemcitabine and carboplatin neoadjuvant chemotherapy through genome-wide gene expression profiling. Exp. Ther. Med. 2011, 2, 47–5613. [Google Scholar] [CrossRef] [PubMed]
- Plimack, E.R.; Dunbrack, R.L.; Brennan, T.A.; Andrake, M.D.; Zhou, Y.; Serebriiskii, I.G.; Slifker, M.; Alpaugh, K.; Dulaimi, E.; Palma, N.; et al. Defects in DNA Repair Genes Predict Response to Neoadjuvant Cisplatin-based Chemotherapy in Muscle-invasive Bladder Cancer. Eur. Urol. 2015, 68, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef]
- Alfred Witjes, J.; Max Bruins, H.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef]
- Mengual, L.; Burset, M.; Ribal, M.J.; Ars, E.; Marín-Aguilera, M.; Fernández, M.; Ingelmo-Torres, M.; Villavicencio, H.; Alcaraz, A. Gene expression signature in urine for diagnosing and assessing aggressiveness of bladder urothelial carcinoma. Clin. Cancer Res. 2010, 16, 2624–2633. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Huang, Y.; Liu, S.; Zhang, J.; Ding, H.; Yang, J.; Chen, Z. A six-gene prognostic model predicts overall survival in bladder cancer patients. Cancer Cell Int. 2019, 19, 229. [Google Scholar] [CrossRef]
- Buttigliero, C.; Tucci, M.; Vignani, F.; Scagliotti, G.V.; Di Maio, M. Molecular biomarkers to predict response to neoadjuvant chemotherapy for bladder cancer. Cancer Treat. Rev. 2017, 54, 1–9. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Newton, K.; Wickliffe, K.E.; Maltzman, A.; Dugger, D.L.; Reja, R.; Zhang, Y.; Roose-Girma, M.; Modrusan, Z.; Sagolla, M.S.; Webster, J.D.; et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature 2019, 575, 679–682. [Google Scholar] [CrossRef]
- Cai, J.; Ye, Q.; Luo, S.; Zhuang, Z.; He, K.; Zhuo, Z.J.; Wan, X.; Cheng, J. CASP8 -652 6N insertion/deletion polymorphism and overall cancer risk: Evidence from 49 studies. Oncotarget 2017, 8, 56780–56790. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Aftabi, S.; Moazeni-Roodi, A.; Sarani, H.; Wiechec, E.; Ghavami, S. Association of CASP8 polymorphisms and cancer susceptibility: A meta-analysis. Eur. J. Pharmacol. 2020, 881, 173201. [Google Scholar] [CrossRef]
- Liu, S.; Garcia-Marques, F.; Zhang, C.A.; Lee, J.J.; Nolley, R.; Shen, M.; Hsu, E.-C.; Aslan, M.; Koul, K.; Pitteri, S.J.; et al. Discovery of CASP8 as a potential biomarker for high-risk prostate cancer through a high-multiplex immunoassay. Sci. Rep. 2021, 11, 7612. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, N.; Zhang, G.; Chen, M. CASP4 and CASP8 as newly defined autophagy-pyroptosis-related genes associated with clinical and prognostic features of renal cell carcinoma. J. Cancer Res. Ther. 2022, 18, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Tortorelli, G.A.; Torricelli, C.; Carron, J.; Costa, E.F.D.; Lopes-Aguiar, L.; Carvalho, B.F.; Rinck-Junior, J.A.; Mariano, F.V.; Altemani, A.M.A.M.; Lima, C.S.P.; et al. CASP8 (rs3834129) and CASP3 (rs4647601) polymorphisms in oropharynx cancer risk, tumor cell differentiation, and prognosis in a cohort of the Brazilian population. Mol. Biol. Rep. 2019, 46, 6557–6563. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Liu, S.; Huang, Y.; Chen, B.; Wang, D. Polymorphisms in the CASP8 gene and the risk of epithelial ovarian cancer. Gynecol. Oncol. 2011, 122, 554–559. [Google Scholar] [CrossRef]
- Peng, F.; Zhu, F.; Cao, B.; Peng, L. Multidimensional Analysis of PANoptosis-Related Molecule CASP8: Prognostic Significance, Immune Microenvironment Effect, and Therapeutic Implications in Hepatocellular Carcinoma. Genet. Res. 2023, 2023, 2406193. [Google Scholar] [CrossRef]
- Palanca Suela, S.; Esteban Cardeñosa, E.; Barragán González, E.; de Juan Jiménez, I.; Chirivella González, I.; Segura Huerta, A.; Guillén Ponce, C.; Martínez de Dueñas, E.; Montalar Salcedo, J.; Castel Sánchez, V.; et al. CASP8 D302H polymorphism delays the age of onset of breast cancer in BRCA1 and BRCA2 carriers. Breast Cancer Res. Treat. 2010, 119, 87–93. [Google Scholar] [CrossRef]
- Tvrdík, D.; Skálová, H.; Dundr, P.; Povýšil, C.; Velenská, Z.; Berková, A.; Staněk, L.; Petruželka, L. Apoptosis—Associated genes and their role in predicting responses to neoadjuvant breast cancer treatment. Med. Sci. Monit. 2012, 18, BR60–BR67. [Google Scholar] [CrossRef]
- Kelley, S.T.; Coppola, D.; Yeatman, T.; Marcet, J. Tumor response to neoadjuvant chemoradiation therapy for rectal adenocarcinoma is mediated by p53-dependent and caspase 8-dependent apoptotic pathways. Clin. Color. Cancer 2005, 5, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Haruta, M.; Sugawara, W.; Sasaki, F.; Ohira, M.; Matsunaga, T.; Yamaoka, H.; Horie, H.; Ohnuma, N.; Nakagawara, A.; et al. The methylation status of RASSF1A promoter predicts responsiveness to chemotherapy and eventual cure in hepatoblastoma patients. Int. J. Cancer 2008, 123, 1117–1125. [Google Scholar] [CrossRef]
- Gundesli, H.; Kori, M.; Arga, K.Y. The Versatility of Plectin in Cancer: A Pan-Cancer Analysis on Potential Diagnostic and Prognostic Impacts of Plectin Isoforms. OMICS 2023, 27, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.M.; Brinton, L.T.; Kelly, K.A. Plectin in Cancer: From Biomarker to Therapeutic Target. Cells 2021, 10, 2246. [Google Scholar] [CrossRef]
- Prekovic, S.; Chalkiadakis, T.; Roest, M.; Roden, D.; Lutz, C.; Schuurman, K.; Opdam, M.; Hoekman, L.; Abbott, N.; Tesselaar, T.; et al. Luminal breast cancer identity is determined by loss of glucocorticoid receptor activity. EMBO Mol. Med. 2023, 15, e17737. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Q.; Sherrod, A.E.; Hurth, K.M. ZBTB16: A new biomarker for primitive neuroectodermal tumor element/Ewing sarcoma. Pathol. Res. Pract. 2019, 215, 152536. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Li, F.; Unger, P.D.; Katerji, H.; Yang, Q.; McMahon, L.; Burstein, D.E. ZBTB16: A novel sensitive and specific biomarker for yolk sac tumor. Mod. Pathol. 2016, 29, 591–598. [Google Scholar] [CrossRef]


| Discovery Phase (n = 34) | Validation Phase (n = 115) | |
|---|---|---|
| Age at diagnosis (years) | 63.16 | 61.86 |
| Gender (male/female) | 31/3 | 104/11 |
| NMIBC history (%) | 20.5 | 27.8 |
| Associated CIS (%) | 17.6 | 7.8 |
| Downstaging after neoadjuvant chemotherapy (%) | 47 | 44.3 |
| Pathologic stage | ||
| T0 | 7 (20.5) | 32 (27.8) |
| Cis | 2 (0.05) | 9 (7.8) |
| Ta | 3(0.08) | 2 (1.7) |
| T1 | 4(11.7) | 8 (6.9) |
| T2 | 7(20.5) | 18 (15.6) |
| T3 | 8(23.5) | 24 (20.8) |
| T4 | 3(8.8) | 22 (19.1) |
| Characteristic | HR 1 | 95% CI 1 | p-Value |
|---|---|---|---|
| Age | 0.97 | 0.94, 1.00 | 0.084 |
| Adenopathy | |||
| No | - | - | |
| Yes | 0.54 | 0.29, 1.00 | 0.051 |
| Sex | |||
| Female | - | - | |
| Male | 0.42 | 0.14, 1.25 | 0.12 |
| History of NMIBC | |||
| No | - | - | |
| Yes | 0.27 | 0.11, 0.63 | 0.003 |
| CBTB16 | 0.82 | 0.68, 0.98 | 0.031 |
| CHMP6 | 1.90 | 1.34, 2.71 | <0.001 |
| DDX54 | 0.69 | 0.53, 0.88 | 0.004 |
| KASP8 | 1.14 | 1.05, 1.25 | 0.003 |
| LOR | 0.84 | 0.70, 1.00 | 0.048 |
| PLEC | 1.27 | 0.96, 1.68 | 0.10 |
| Univariate HR (95% CI) | p | |
|---|---|---|
| FN1 | 0.85 (0.73–1) | 0.05 |
| VASP | 0.85 (0.71–1.01) | 0.06 |
| CEP63 | 0.83 (0.70–0.99) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, M.; Lozano, J.J.; Ingelmo-Torres, M.; Domenech, M.; Fernández Ramón, C.; Witjes, J.A.; van der Heijden, A.G.; Requena, M.J.; Coy, A.; Calderon, R.; et al. Biomarker-Based Nomogram to Predict Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer. Biomedicines 2025, 13, 740. https://doi.org/10.3390/biomedicines13030740
Pérez M, Lozano JJ, Ingelmo-Torres M, Domenech M, Fernández Ramón C, Witjes JA, van der Heijden AG, Requena MJ, Coy A, Calderon R, et al. Biomarker-Based Nomogram to Predict Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer. Biomedicines. 2025; 13(3):740. https://doi.org/10.3390/biomedicines13030740
Chicago/Turabian StylePérez, Meritxell, Juan José Lozano, Mercedes Ingelmo-Torres, Montserrat Domenech, Caterina Fernández Ramón, J. Alfred Witjes, Antoine G. van der Heijden, Maria José Requena, Antonio Coy, Ricard Calderon, and et al. 2025. "Biomarker-Based Nomogram to Predict Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer" Biomedicines 13, no. 3: 740. https://doi.org/10.3390/biomedicines13030740
APA StylePérez, M., Lozano, J. J., Ingelmo-Torres, M., Domenech, M., Fernández Ramón, C., Witjes, J. A., van der Heijden, A. G., Requena, M. J., Coy, A., Calderon, R., Mellado, B., Alcaraz, A., Vilaseca, A., & Ribal, M. J. (2025). Biomarker-Based Nomogram to Predict Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer. Biomedicines, 13(3), 740. https://doi.org/10.3390/biomedicines13030740








