The Molecular Basis of Polycystic Ovary Syndrome and Its Cardiometabolic Correlates: Exploring the Intersection and Its Clinical Implications—A Narrative Review
Abstract
1. Introduction
2. Methodology and Search Criteria
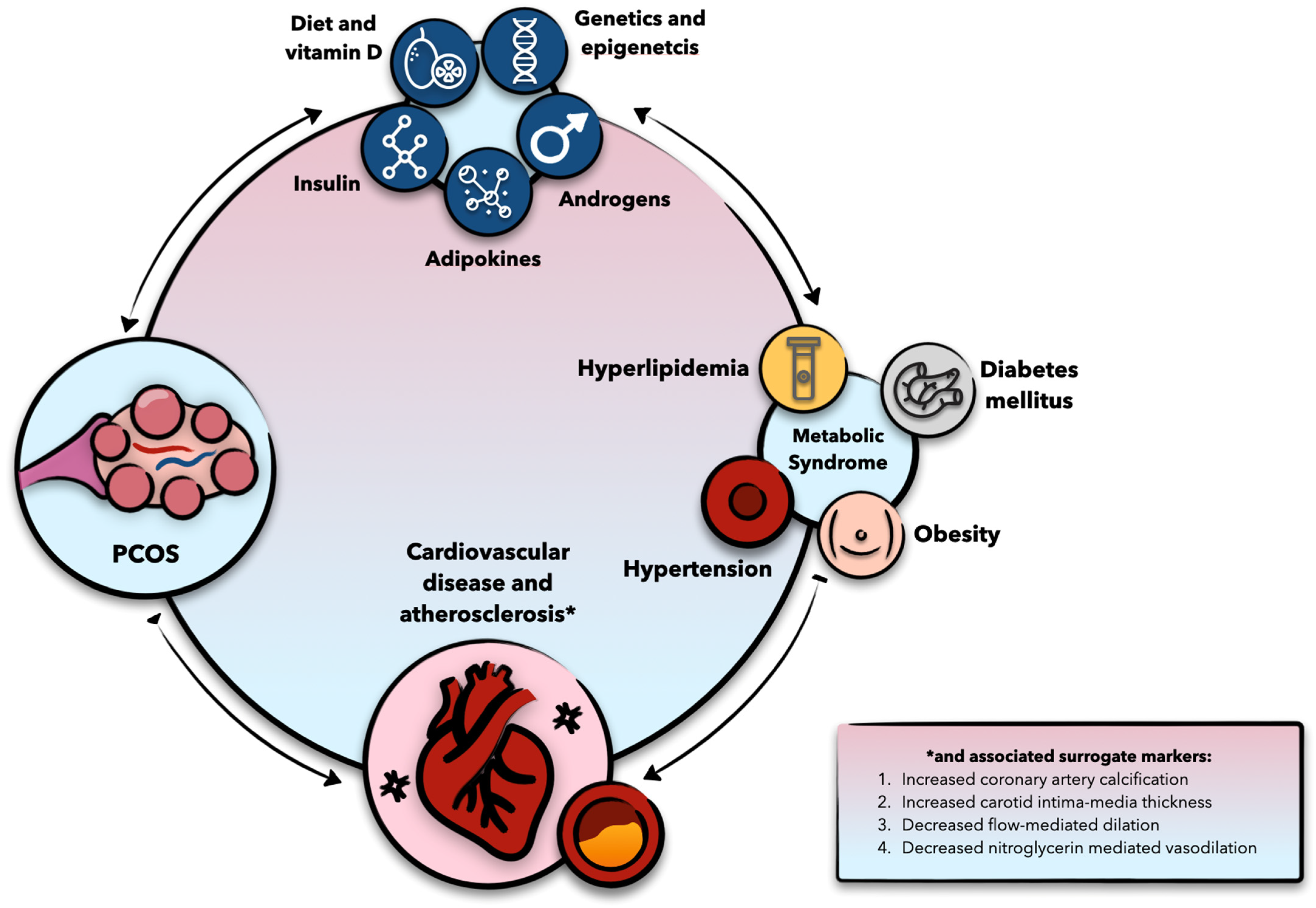
3. PCOS and Cardiometabolic Disease: Epidemiological Insights
4. Molecular Considerations
4.1. Genomics and Epigenomics
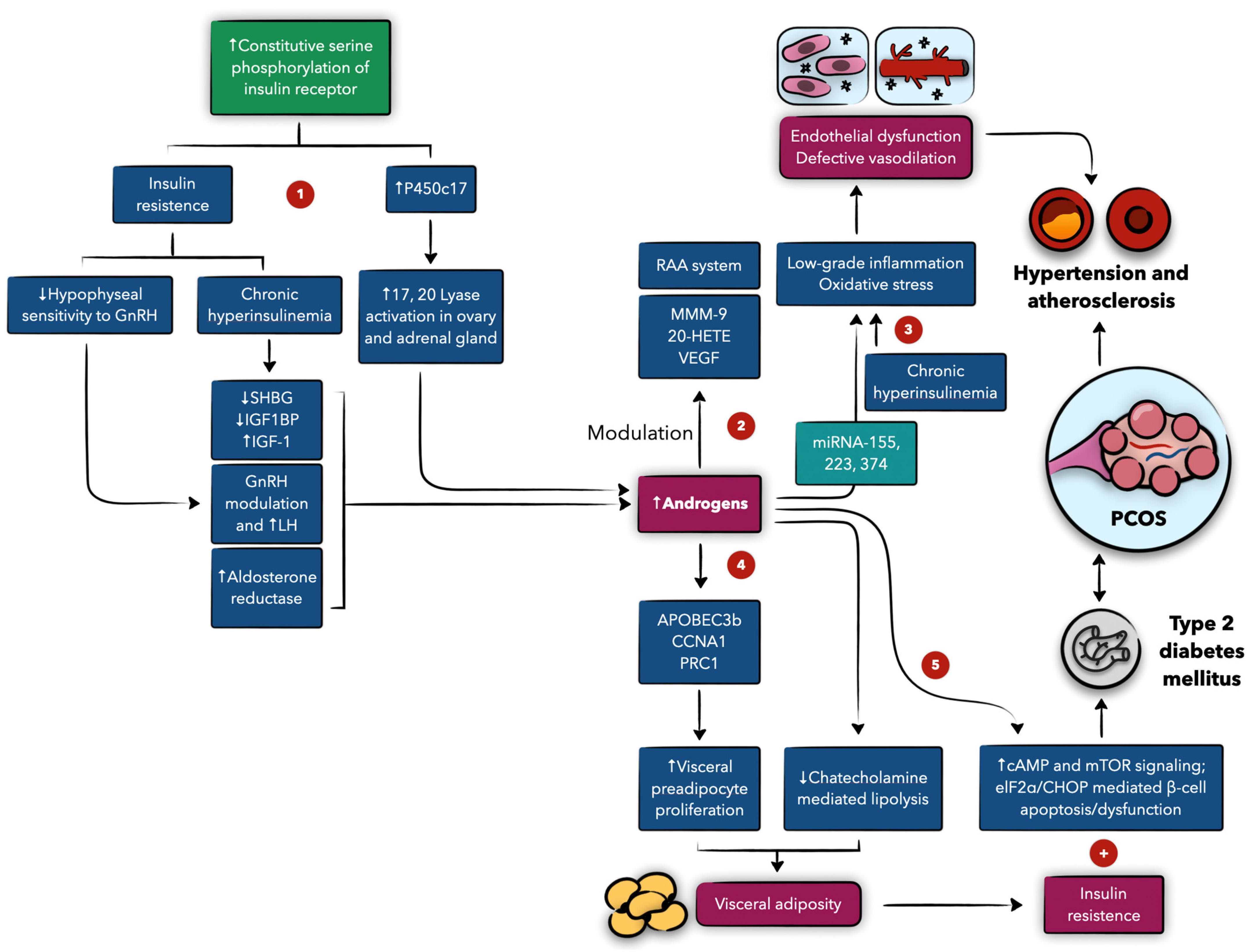
4.2. The Role of Androgens and Insulin, and Their Interplay
4.3. Adipokines
4.4. PCOS and Atherosclerosis
4.5. Angiogenesis
4.6. The Reproductive Microbiome
4.7. The Role of Vitamin D, Oxidative Stress, and Environmental Factors
5. Clinical Implications
5.1. Screening and Diagnostic Implications
5.1.1. Anthropometric Markers
5.1.2. Lifestyle and Behavioral Screening
5.1.3. Metabolic Screening
5.1.4. Atherosclerotic Cardiovascular Disease Risk Stratification
5.1.5. Androgens as Biomarkers
5.1.6. Genetics
5.1.7. Emerging Biomarkers
6. Preventative and Therapeutic Implications
6.1. Behavioral and Lifestyle Modifications
6.2. Therapeutic Implications
6.2.1. Pharmacological Antioxidants
6.2.2. COCs
6.2.3. Metformin
6.2.4. Statins
6.2.5. GLP-1 Agonists
6.2.6. Thiazolidinediones
6.2.7. Surgical Interventions (e.g., Bariatric Surgery)
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Wekker, V.; Van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Roeters van Lennep, J.E.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef] [PubMed]
- van der Ham, K.; Koster, M.P.; Velthuis, B.K.; Budde, R.P.; Fauser, B.C.; Laven, J.S.; Louwers, Y.V. Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 5226. [Google Scholar] [CrossRef]
- Cobin, R.H. Cardiovascular and metabolic risks associated with PCOS. Intern. Emerg. Med. 2013, 8 (Suppl. S1), S61–S64. [Google Scholar] [CrossRef] [PubMed]
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Reddy, S.; Sharma, K.; Johnson, S.; Hardy, G.; Dwivedi, A.K. Polycystic Ovary Syndrome, Insulin Resistance, and Cardiovascular Disease. Curr. Cardiol. Rep. 2024, 26, 483–495. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Wan, Z.; Zhao, J.; Ye, Y.; Sun, Z.; Li, K.; Chen, Y.; Fang, Y.; Zhang, Y.; Lin, J.; Sun, P.; et al. Risk and incidence of cardiovascular disease associated with polycystic ovary syndrome. Eur. J. Prev. Cardiol. 2024, 31, 1560–1570. [Google Scholar] [CrossRef]
- Daan, N.M.; Louwers, Y.V.; Koster, M.P.; Eijkemans, M.J.; de Rijke, Y.B.; Lentjes, E.W.; Fauser, B.C.; Laven, J.S. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: Who is really at risk? Fertil. Steril. 2014, 102, 1444–1451.e1443. [Google Scholar] [CrossRef]
- Ye, W.; Xie, T.; Song, Y.; Zhou, L. The role of androgen and its related signals in PCOS. J. Cell Mol. Med. 2021, 25, 1825–1837. [Google Scholar] [CrossRef]
- AlAshqar, A.; Patzkowsky, K.; Afrin, S.; Wild, R.; Taylor, H.S.; Borahay, M.A. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obstet. Gynecol. Surv. 2019, 74, 661–673. [Google Scholar] [CrossRef]
- Osibogun, O.; Ogunmoroti, O.; Kolade, O.B.; Hays, A.G.; Okunrintemi, V.; Minhas, A.S.; Gulati, M.; Michos, E.D. A Systematic Review and Meta-Analysis of the Association Between Polycystic Ovary Syndrome and Coronary Artery Calcification. J. Womens Health 2022, 31, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ramirez, M.; Mendieta-Azcona, C.; Alvarez-Blasco, F.; Escobar-Morreale, H.F. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum. Reprod. 2007, 22, 3197–3203. [Google Scholar] [CrossRef]
- de Groot, P.C.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.; Helmerhorst, F.M. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 495–500. [Google Scholar] [CrossRef]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Kahsar-Miller, M.D.; Nixon, C.; Boots, L.R.; Go, R.C.; Azziz, R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil. Steril. 2001, 75, 53–58. [Google Scholar] [CrossRef]
- Crespo, R.P.; Bachega, T.; Mendonca, B.B.; Gomes, L.G. An update of genetic basis of PCOS pathogenesis. Arch. Endocrinol. Metab. 2018, 62, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, P.N. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018, 14, e1007813. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhao, H.; He, L.; Shi, Y.; Qin, Y.; Shi, Y.; Li, Z.; You, L.; Zhao, J.; Liu, J.; et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 2011, 43, 55–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, S.; Liu, C.; Zheng, Y.; Li, H.; Gao, F.; Bian, Y.; Liu, X.; Liu, H.; Hu, S.; et al. THADA inhibition in mice protects against type 2 diabetes mellitus by improving pancreatic beta-cell function and preserving beta-cell mass. Nat. Commun. 2023, 14, 1020. [Google Scholar] [CrossRef]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.; Karaderi, T.; Barber, T.M.; McCarthy, M.I.; Franks, S.; et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015, 6, 7502. [Google Scholar] [CrossRef] [PubMed]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef] [PubMed]
- San Millan, J.L.; Corton, M.; Villuendas, G.; Sancho, J.; Peral, B.; Escobar-Morreale, H.F. Association of the polycystic ovary syndrome with genomic variants related to insulin resistance, type 2 diabetes mellitus, and obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2640–2646. [Google Scholar] [CrossRef][Green Version]
- Pan, J.X.; Tan, Y.J.; Wang, F.F.; Hou, N.N.; Xiang, Y.Q.; Zhang, J.Y.; Liu, Y.; Qu, F.; Meng, Q.; Xu, J.; et al. Aberrant expression and DNA methylation of lipid metabolism genes in PCOS: A new insight into its pathogenesis. Clin. Epigenet. 2018, 10, 6. [Google Scholar] [CrossRef]
- Mimouni, N.E.H.; Paiva, I.; Barbotin, A.L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530.e518. [Google Scholar] [CrossRef]
- Kokosar, M.; Benrick, A.; Perfilyev, A.; Fornes, R.; Nilsson, E.; Maliqueo, M.; Behre, C.J.; Sazonova, A.; Ohlsson, C.; Ling, C.; et al. Erratum: Epigenetic and Transcriptional Alterations in Human Adipose Tissue of Polycystic Ovary Syndrome. Sci. Rep. 2016, 6, 25321. [Google Scholar] [CrossRef] [PubMed]
- Echiburu, B.; Milagro, F.; Crisosto, N.; Perez-Bravo, F.; Flores, C.; Arpon, A.; Salas-Perez, F.; Recabarren, S.E.; Sir-Petermann, T.; Maliqueo, M. DNA methylation in promoter regions of genes involved in the reproductive and metabolic function of children born to women with PCOS. Epigenetics 2020, 15, 1178–1194. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Moenter, S.M. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: Implications for a common fertility disorder. Proc. Natl. Acad. Sci. USA 2004, 101, 7129–7134. [Google Scholar] [CrossRef]
- Roland, A.V.; Nunemaker, C.S.; Keller, S.R.; Moenter, S.M. Prenatal androgen exposure programs metabolic dysfunction in female mice. J. Endocrinol. 2010, 207, 213–223. [Google Scholar] [CrossRef]
- Xu, N.; Chua, A.K.; Jiang, H.; Liu, N.A.; Goodarzi, M.O. Early embryonic androgen exposure induces transgenerational epigenetic and metabolic changes. Mol. Endocrinol. 2014, 28, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.M.D.; VanHise, K.; Stachenfeld, N.; Chan, J.L.; Merz, N.B.; Shufelt, C. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil. Steril. 2022, 117, 912–923. [Google Scholar] [CrossRef]
- Singh, R.; Artaza, J.N.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Bhasin, S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003, 144, 5081–5088. [Google Scholar] [CrossRef]
- Barbosa-Desongles, A.; Hernandez, C.; Simo, R.; Selva, D.M. Testosterone induces cell proliferation and cell cycle gene overexpression in human visceral preadipocytes. Am. J. Physiol. Cell Physiol. 2013, 305, C355–C359. [Google Scholar] [CrossRef]
- Dicker, A.; Ryden, M.; Naslund, E.; Muehlen, I.E.; Wiren, M.; Lafontan, M.; Arner, P. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 2004, 47, 420–428. [Google Scholar] [CrossRef]
- Schiffer, L.; Arlt, W.; O’Reilly, M.W. Understanding the Role of Androgen Action in Female Adipose Tissue. Front. Horm. Res. 2019, 53, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Chiu, W.C.; Hsu, M.I.; Chen, Y.J. Effects of androgen on vascular and inflammatory biomarkers in a female hypertensive population. Gynecol. Endocrinol. 2013, 29, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Cheng, J.; Zhang, F.F.; Gotlinger, K.H.; Kelkar, M.; Zhang, Y.; Jat, J.L.; Falck, J.R.; Schwartzman, M.L. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: Role of inhibitor of kappaB Kinase. Hypertension 2011, 57, 788–794. [Google Scholar] [CrossRef]
- Torres Fernandez, E.D.; Huffman, A.M.; Syed, M.; Romero, D.G.; Yanes Cardozo, L.L. Effect of GLP-1 Receptor Agonists in the Cardiometabolic Complications in a Rat Model of Postmenopausal PCOS. Endocrinology 2019, 160, 2787–2799. [Google Scholar] [CrossRef]
- Usselman, C.W.; Yarovinsky, T.O.; Steele, F.E.; Leone, C.A.; Taylor, H.S.; Bender, J.R.; Stachenfeld, N.S. Androgens drive microvascular endothelial dysfunction in women with polycystic ovary syndrome: Role of the endothelin B receptor. J. Physiol. 2019, 597, 2853–2865. [Google Scholar] [CrossRef]
- Abdalla, M.; Deshmukh, H.; Atkin, S.L.; Sathyapalan, T. miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review. Life Sci. 2020, 259, 118174. [Google Scholar] [CrossRef]
- Joffe, H.V.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Rexrode, K.M. Sex hormone-binding globulin and serum testosterone are inversely associated with C-reactive protein levels in postmenopausal women at high risk for cardiovascular disease. Ann. Epidemiol. 2006, 16, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, V.; Zhao, D.; Ouyang, P.; Ying, W.; Vaidya, D.; Ndumele, C.E.; Heckbert, S.R.; Budoff, M.J.; Post, W.S.; Michos, E.D. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Cardiovasc. Comput. Tomogr. 2019, 13, 41–47. [Google Scholar] [CrossRef]
- Alinezhad, A.; Jafari, F. The relationship between components of metabolic syndrome and plasma level of sex hormone-binding globulin. Eur. J. Transl. Myol. 2019, 29, 8196. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.; Al-Rubeaan, K.; Nawaz, S.S.; Aburisheh, K.H.; Alaabdin, A.M.Z.; Tolba, I.A. Serum Sex Hormone Binding Globulin (SHBG) Relation with Different Components of Metabolic Syndrome in Men with Type 2 Diabetes. Horm. Metab. Res. 2018, 50, 138–144. [Google Scholar] [CrossRef]
- Faal, S.; Abedi, P.; Jahanfar, S.; Ndeke, J.M.; Mohaghegh, Z.; Sharifipour, F.; Zahedian, M. Sex hormone binding globulin for prediction of gestational diabetes mellitus in pre-conception and pregnancy: A systematic review. Diabetes Res. Clin. Pract. 2019, 152, 39–52. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Youngren, J.F.; Dunaif, A.; Goldfine, I.D.; Maddux, B.A.; Zhang, B.B.; Evans, J.L. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: Effects of serine kinase inhibitors and IR activators. J. Clin. Endocrinol. Metab. 2002, 87, 4088–4093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, M.R.; Brower, M.A.; Xu, N.; Cui, J.; Mengesha, E.; Chen, Y.D.; Taylor, K.D.; Azziz, R.; Goodarzi, M.O. Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity. PLoS Genet. 2015, 11, e1005455. [Google Scholar] [CrossRef]
- Yalamanchi, S.K.; Sam, S.; Cardenas, M.O.; Holaday, L.W.; Urbanek, M.; Dunaif, A. Association of fibrillin-3 and transcription factor-7-like 2 gene variants with metabolic phenotypes in PCOS. Obesity 2012, 20, 1273–1278. [Google Scholar] [CrossRef]
- Corbould, A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 2007, 192, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Invest. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.K.; Zhou, S.Y.; Liu, J.X.; Pollanen, P.; Sallinen, K.; Makinen, M.; Erkkola, R. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil. Steril. 2003, 80, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Rodriguez, H.; Ohno, S.; Miller, W.L. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: Implications for adrenarche and the polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 1995, 92, 10619–10623. [Google Scholar] [CrossRef]
- Takayama, S.; White, M.F.; Kahn, C.R. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J. Biol. Chem. 1988, 263, 3440–3447. [Google Scholar] [CrossRef]
- Livingstone, C.; Collison, M. Sex steroids and insulin resistance. Clin. Sci. 2002, 102, 151–166. [Google Scholar] [CrossRef]
- Kim, H.H.; DiVall, S.A.; Deneau, R.M.; Wolfe, A. Insulin regulation of GnRH gene expression through MAP kinase signaling pathways. Mol. Cell Endocrinol. 2005, 242, 42–49. [Google Scholar] [CrossRef]
- Adashi, E.Y.; Hsueh, A.J.; Yen, S.S. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology 1981, 108, 1441–1449. [Google Scholar] [CrossRef]
- Brothers, K.J.; Wu, S.; DiVall, S.A.; Messmer, M.R.; Kahn, C.R.; Miller, R.S.; Radovick, S.; Wondisford, F.E.; Wolfe, A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010, 12, 295–305. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef]
- Bergh, C.; Carlsson, B.; Olsson, J.H.; Selleskog, U.; Hillensjo, T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil. Steril. 1993, 59, 323–331. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Kempegowda, P.; Walsh, M.; Taylor, A.E.; Manolopoulos, K.N.; Allwood, J.W.; Semple, R.K.; Hebenstreit, D.; Dunn, W.B.; Tomlinson, J.W.; et al. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 3327–3339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, J.; Shen, S.; Liu, J.; Sun, J.; Gu, T.; Ye, X.; Zhu, D.; Bi, Y. Association of Androgen Excess with Glucose Intolerance in Women with Polycystic Ovary Syndrome. Biomed. Res. Int. 2018, 2018, 6869705. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Akhtar, S.; Sellers, D.J.; Muraleedharan, V.; Channer, K.S.; Jones, T.H. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine 2016, 54, 504–515. [Google Scholar] [CrossRef]
- He, Y.; Wang, C.L. Effects of testosterone on PPARgamma and P450arom expression in polycystic ovary syndrome patients and related mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1549–1553. [Google Scholar] [CrossRef]
- Navarro, G.; Allard, C.; Morford, J.J.; Xu, W.; Liu, S.; Molinas, A.J.; Butcher, S.M.; Fine, N.H.; Blandino-Rosano, M.; Sure, V.N.; et al. Androgen excess in pancreatic beta cells and neurons predisposes female mice to type 2 diabetes. JCI Insight 2018, 3, e98607. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, Z.; Zhao, H.; Chen, X.; Zhang, Y.; Guo, H.; Zhao, Y.; Chen, Z.J. Activation of eIF2alpha signaling cascade is associated with testosterone-induced cell apoptosis in INS-1 cells. Horm. Metab. Res. 2014, 46, 574–580. [Google Scholar] [CrossRef]
- Piltonen, T.T.; Komsi, E.; Morin-Papunen, L.C.; Korhonen, E.; Franks, S.; Jarvelin, M.R.; Arffman, R.K.; Ollila, M.M. AMH as part of the diagnostic PCOS workup in large epidemiological studies. Eur. J. Endocrinol. 2023, 188, 547–554. [Google Scholar] [CrossRef]
- Chang, H.M.; Klausen, C.; Leung, P.C. Antimullerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil. Steril. 2013, 100, 585–592.e581. [Google Scholar] [CrossRef]
- Manti, M.; Fornes, R.; Pironti, G.; McCann Haworth, S.; Zhengbing, Z.; Benrick, A.; Carlstrom, M.; Andersson, D.; Stener-Victorin, E. Maternal androgen excess induces cardiac hypertrophy and left ventricular dysfunction in female mice offspring. Cardiovasc. Res. 2020, 116, 619–632. [Google Scholar] [CrossRef]
- Vyas, A.K.; Hoang, V.; Padmanabhan, V.; Gilbreath, E.; Mietelka, K.A. Prenatal programming: Adverse cardiac programming by gestational testosterone excess. Sci. Rep. 2016, 6, 28335. [Google Scholar] [CrossRef]
- Duran, J.; Lagos, D.; Pavez, M.; Troncoso, M.F.; Ramos, S.; Barrientos, G.; Ibarra, C.; Lavandero, S.; Estrada, M. Ca2+/Calmodulin-Dependent Protein Kinase II and Androgen Signaling Pathways Modulate MEF2 Activity in Testosterone-Induced Cardiac Myocyte Hypertrophy. Front. Pharmacol. 2017, 8, 604. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Azeez, S.H.; Ismail, I.B.; Darogha, S.N. The Effect of Interleukin-6 and Tumor Necrosis Factor-Alpha Gene Polymorphism and Hormone Replacement Therapy on Polycystic Ovary Syndrome. Cell. Mol. Biol. 2022, 67, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Toprak, S.; Ortmann, O.; Buechler, C.; Treeck, O. The Complex Roles of Adipokines in Polycystic Ovary Syndrome and Endometriosis. Biomedicines 2022, 10, 2503. [Google Scholar] [CrossRef]
- Chen, P.; Jia, R.; Liu, Y.; Cao, M.; Zhou, L.; Zhao, Z. Progress of Adipokines in the Female Reproductive System: A Focus on Polycystic Ovary Syndrome. Front. Endocrinol. 2022, 13, 881684. [Google Scholar] [CrossRef]
- Lin, K.; Sun, X.; Wang, X.; Wang, H.; Chen, X. Circulating Adipokine Levels in Nonobese Women with Polycystic Ovary Syndrome and in Nonobese Control Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 537809. [Google Scholar] [CrossRef]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction? Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef]
- Mehrabani, S.; Arab, A.; Karimi, E.; Nouri, M.; Mansourian, M. Blood Circulating Levels of Adipokines in Polycystic Ovary Syndrome Patients: A Systematic Review and Meta-analysis. Reprod. Sci. 2021, 28, 3032–3050. [Google Scholar] [CrossRef]
- Harris, K.; Peters, S.A.E.; Woodward, M. Sex hormones and the risk of myocardial infarction in women and men: A prospective cohort study in the UK Biobank. Biol. Sex. Differ. 2023, 14, 61. [Google Scholar] [CrossRef]
- Zheng, S.H.; Du, D.F.; Li, X.L. Leptin Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and a Meta-Analysis. Reprod. Sci. 2017, 24, 656–670. [Google Scholar] [CrossRef]
- As Habi, A.; Sadeghi, M.; Arab, A.; Hajianfar, H. The association between omentin and diabetes: A systematic review and meta-analysis of observational studies. Diabetes Metab. Syndr. Obes. 2019, 12, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Vilarino-Garcia, T.; Perez-Perez, A.; Santamaria-Lopez, E.; Prados, N.; Fernandez-Sanchez, M.; Sanchez-Margalet, V. Sam68 mediates leptin signaling and action in human granulosa cells: Possible role in leptin resistance in PCOS. Endocr. Connect. 2020, 9, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.S.; Uzun, Y.E.; Kutlu, O.; Pence, H.H.; Ozcelik, F.; Cil, E.O.; Irak, L.; Altun, O.; Ozcan, M.; Ozsoy, N.; et al. The effects of high intensity-interval training on vaspin, adiponectin and leptin levels in women with polycystic ovary syndrome. Arch. Physiol. Biochem. 2022, 128, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Chen, J.; Farhatullah, S.; Adya, R.; Kaur, J.; Heutling, D.; Lewandowski, K.C.; O’Hare, J.P.; Lehnert, H.; Randeva, H.S. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 2009, 58, 1971–1977. [Google Scholar] [CrossRef]
- Fleming, R.; Harborne, L.; MacLaughlin, D.T.; Ling, D.; Norman, J.; Sattar, N.; Seifer, D.B. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil. Steril. 2005, 83, 130–136. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Guan, C.; Zahid, S.; Minhas, A.S.; Ouyang, P.; Vaught, A.; Baker, V.L.; Michos, E.D. Polycystic ovary syndrome: A “risk-enhancing” factor for cardiovascular disease. Fertil. Steril. 2022, 117, 924–935. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef]
- Jabbour, R.; Ott, J.; Eppel, W.; Frigo, P. Carotid intima-media thickness in polycystic ovary syndrome and its association with hormone and lipid profiles. PLoS ONE 2020, 15, e0232299. [Google Scholar] [CrossRef]
- Hughan, K.S.; Tfayli, H.; Warren-Ulanch, J.G.; Barinas-Mitchell, E.; Arslanian, S.A. Early Biomarkers of Subclinical Atherosclerosis in Obese Adolescent Girls with Polycystic Ovary Syndrome. J. Pediatr. 2016, 168, 104–111.e101. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wu, Y.; Ding, M.; Zhu, F. Comprehensive Meta-Analysis of Functional and Structural Markers of Subclinical Atherosclerosis in Women with Polycystic Ovary Syndrome. Angiology 2022, 73, 622–634. [Google Scholar] [CrossRef]
- Vural, B.; Caliskan, E.; Turkoz, E.; Kilic, T.; Demirci, A. Evaluation of metabolic syndrome frequency and premature carotid atherosclerosis in young women with polycystic ovary syndrome. Hum. Reprod. 2005, 20, 2409–2413. [Google Scholar] [CrossRef]
- Shroff, R.; Kerchner, A.; Maifeld, M.; Van Beek, E.J.; Jagasia, D.; Dokras, A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J. Clin. Endocrinol. Metab. 2007, 92, 4609–4614. [Google Scholar] [CrossRef] [PubMed]
- van der Ham, K.; Louwers, Y.V.; Laven, J.S.E. Cardiometabolic biomarkers in women with polycystic ovary syndrome. Fertil. Steril. 2022, 117, 887–896. [Google Scholar] [CrossRef]
- Janez, A.; Herman, R.; Poredos, P.; Mikhailidis, D.P.; Blinc, A.; Sabovic, M.; Studen, K.B.; Jezovnik, M.K.; Schernthaner, G.H.; Anagnostis, P.; et al. Cardiometabolic Risk, Peripheral Arterial Disease and Cardiovascular Events in Polycystic Ovary Syndrome: Time to Implement Systematic Screening and Update the Management. Curr. Vasc. Pharmacol. 2023, 21, 424–432. [Google Scholar] [CrossRef]
- Meun, C.; Franco, O.H.; Dhana, K.; Jaspers, L.; Muka, T.; Louwers, Y.; Ikram, M.A.; Fauser, B.; Kavousi, M.; Laven, J.S.E. High Androgens in Postmenopausal Women and the Risk for Atherosclerosis and Cardiovascular Disease: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2018, 103, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Seifer, D.B.; Tal, R. The Role of Angiogenic Factor Dysregulation in the Pathogenesis of Polycystic Ovarian Syndrome. In Polycystic Ovary Syndrome: Current and Emerging Concepts; Pal, L., Seifer, D.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 449–487. [Google Scholar] [CrossRef]
- Di Pietro, M.; Pascuali, N.; Parborell, F.; Abramovich, D. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction 2018, 155, R199–R209. [Google Scholar] [CrossRef]
- Agrawal, R.; Sladkevicius, P.; Engmann, L.; Conway, G.S.; Payne, N.N.; Bekis, J.; Tan, S.L.; Campbell, S.; Jacobs, H.S. Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum. Reprod. 1998, 13, 651–655. [Google Scholar] [CrossRef]
- Resende, A.V.; Mendes, M.C.; Dias de Moura, M.; Mendonca, H.C.; Gomes Premoli, A.C.; Reis, R.M.; Berezowski, A.T. Doppler study of the uterine arteries and ovarian stroma in patients with polycystic ovary syndrome. Gynecol. Obstet. Investig. 2001, 52, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Almawi, W.Y.; Gammoh, E.; Malalla, Z.H.; Al-Madhi, S.A. Analysis of VEGFA Variants and Changes in VEGF Levels Underscores the Contribution of VEGF to Polycystic Ovary Syndrome. PLoS ONE 2016, 11, e0165636. [Google Scholar] [CrossRef] [PubMed]
- Guruvaiah, P.; Govatati, S.; Reddy, T.V.; Lomada, D.; Deenadayal, M.; Shivaji, S.; Bhanoori, M. The VEGF +405 G>C 5’ untranslated region polymorphism and risk of PCOS: A study in the South Indian Women. J. Assist. Reprod. Genet. 2014, 31, 1383–1389. [Google Scholar] [CrossRef][Green Version]
- Lee, E.J.; Oh, B.; Lee, J.Y.; Kimm, K.; Park, J.M.; Baek, K.H. Association study between single nucleotide polymorphisms in the VEGF gene and polycystic ovary syndrome. Fertil. Steril. 2008, 89, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Monti, M.; Matteucci, C.; Valentino, V.; Cristello, F.; Genazzani, A.R. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol. Endocrinol. 2006, 22, 465–470. [Google Scholar] [CrossRef]
- Raja-Khan, N.; Kunselman, A.R.; Demers, L.M.; Ewens, K.G.; Spielman, R.S.; Legro, R.S. A variant in the fibrillin-3 gene is associated with TGF-beta and inhibin B levels in women with polycystic ovary syndrome. Fertil. Steril. 2010, 94, 2916–2919. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B.; Grazi, R.V.; Malter, H.E. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome: Correlation with ovarian stimulation. Reprod. Biol. Endocrinol. 2014, 12, 82. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Zimering, M.B.; Anderson, R.J.; Ge, L.; Moritz, T.E.; Duckworth, W.C.; Investigators for the VADT. Basic fibroblast growth factor predicts cardiovascular disease occurrence in participants from the veterans affairs diabetes trial. Front. Endocrinol. 2013, 4, 183. [Google Scholar] [CrossRef][Green Version]
- Pardali, E.; Ten Dijke, P. TGFbeta signaling and cardiovascular diseases. Int. J. Biol. Sci. 2012, 8, 195–213. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef] [PubMed]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R. Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Zhou, F.; Li, Y.; Wu, Q.; He, H.; Zhang, Y.; Ma, C.; Ding, J.; Hua, K. Gut and Vaginal Microbiomes in PCOS: Implications for Women’s Health. Front. Endocrinol. 2022, 13, 808508. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Rodriguez Paris, V.; Wong, X.Y.D.; Solon-Biet, S.M.; Edwards, M.C.; Aflatounian, A.; Gilchrist, R.B.; Simpson, S.J.; Handelsman, D.J.; Kaakoush, N.O.; Walters, K.A. The interplay between PCOS pathology and diet on gut microbiota in a mouse model. Gut Microbes 2022, 14, 2085961. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Xi, H.; Chen, L.; Feng, X. Exploration of the Relationship Between Gut Microbiota and Polycystic Ovary Syndrome (PCOS): A Review. Geburtshilfe Frauenheilkd 2020, 80, 161–171. [Google Scholar] [CrossRef]
- Ashraf, S.; Aslam, R.; Bashir, I.; Majeed, I.; Jamshaid, M. Environmental determinants and PCOS symptoms severity: A cross-sectional study. Health Care Women Int. 2022, 43, 98–113. [Google Scholar] [CrossRef]
- Badri-Fariman, M.; Naeini, A.A.; Mirzaei, K.; Moeini, A.; Hosseini, M.; Bagheri, S.E.; Daneshi-Maskooni, M. Association between the food security status and dietary patterns with polycystic ovary syndrome (PCOS) in overweight and obese Iranian women: A case-control study. J. Ovarian Res. 2021, 14, 134. [Google Scholar] [CrossRef]
- Rubin, K.H.; Andersen, M.S.; Abrahamsen, B.; Glintborg, D. Socioeconomic status in Danish women with polycystic ovary syndrome: A register-based cohort study. Acta Obstet. Gynecol. Scand. 2019, 98, 440–450. [Google Scholar] [CrossRef]
- Afrin, S.; AlAshqar, A.; El Sabeh, M.; Miyashita-Ishiwata, M.; Reschke, L.; Brennan, J.T.; Fader, A.; Borahay, M.A. Diet and Nutrition in Gynecological Disorders: A Focus on Clinical Studies. Nutrients 2021, 13, 1747. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Varbiro, S.; Takacs, I.; Tuu, L.; Nas, K.; Sziva, R.E.; Hetthessy, J.R.; Torok, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome—A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef] [PubMed]
- Morgante, G.; Darino, I.; Spano, A.; Luisi, S.; Luddi, A.; Piomboni, P.; Governini, L.; De Leo, V. PCOS Physiopathology and Vitamin D Deficiency: Biological Insights and Perspectives for Treatment. J. Clin. Med. 2022, 11, 4509. [Google Scholar] [CrossRef]
- Simpson, S.; Pal, L.; Seifer, D.B. Emerging Concepts: Role of Vitamin D Deficiency in the Pathogenesis of PCOS. In Polycystic Ovary Syndrome: Current and Emerging Concepts; Pal, L., Seifer, D.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 489–509. [Google Scholar] [CrossRef]
- Hahn, S.; Haselhorst, U.; Tan, S.; Quadbeck, B.; Schmidt, M.; Roesler, S.; Kimmig, R.; Mann, K.; Janssen, O.E. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 577–583. [Google Scholar] [CrossRef]
- Chakraborty, S.; Naskar, T.K.; Basu, B.R. Vitamin D deficiency, insulin resistance, and antimullerian hormone level: A tale of trio in the expression of polycystic ovary syndrome. F&S Sci. 2024, 5, 252–264. [Google Scholar] [CrossRef]
- Wild, R.A.; Carmina, E.; Diamanti-Kandarakis, E.; Dokras, A.; Escobar-Morreale, H.F.; Futterweit, W.; Lobo, R.; Norman, R.J.; Talbott, E.; Dumesic, D.A. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 2010, 95, 2038–2049. [Google Scholar] [CrossRef]
- Blagojevic, I.P.; Eror, T.; Pelivanovic, J.; Jelic, S.; Kotur-Stevuljevic, J.; Ignjatovic, S. Women with Polycystic Ovary Syndrome and Risk of Cardiovascular Disease. J. Med. Biochem. 2017, 36, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef]
- Bajuk Studen, K.; Pfeifer, M. Cardiometabolic risk in polycystic ovary syndrome. Endocr. Connect. 2018, 7, R238–R251. [Google Scholar] [CrossRef]
- Huddleston, H.G.; Dokras, A. Diagnosis and Treatment of Polycystic Ovary Syndrome. JAMA 2022, 327, 274–275. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Taylor, A.E.; Crabtree, N.J.; Hughes, B.A.; Capper, F.; Crowley, R.K.; Stewart, P.M.; Tomlinson, J.W.; Arlt, W. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: The utility of serum androstenedione. J. Clin. Endocrinol. Metab. 2014, 99, 1027–1036. [Google Scholar] [CrossRef]
- Munzker, J.; Hofer, D.; Trummer, C.; Ulbing, M.; Harger, A.; Pieber, T.; Owen, L.; Keevil, B.; Brabant, G.; Lerchbaum, E.; et al. Testosterone to dihydrotestosterone ratio as a new biomarker for an adverse metabolic phenotype in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 653–660. [Google Scholar] [CrossRef]
- Zhu, J.; Pujol-Gualdo, N.; Wittemans, L.B.L.; Lindgren, C.M.; Laisk, T.; Hirschhorn, J.N.; Chan, Y.M. Evidence From Men for Ovary-independent Effects of Genetic Risk Factors for Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e1577–e1587. [Google Scholar] [CrossRef]
- Yilmaz, M.; Bukan, N.; Ersoy, R.; Karakoc, A.; Yetkin, I.; Ayvaz, G.; Cakir, N.; Arslan, M. Glucose intolerance, insulin resistance and cardiovascular risk factors in first degree relatives of women with polycystic ovary syndrome. Hum. Reprod. 2005, 20, 2414–2420. [Google Scholar] [CrossRef][Green Version]
- Shapiro, A.J.; Kushnir, V.; Seifer, D.B. Anti-Mullerian Hormone Signaling: Relevance for Pathophysiology of PCOS and Implications for Novel Therapeutic Approaches to Managing Ovulatory Dysfunction of PCOS. In Polycystic Ovary Syndrome: Current and Emerging Concepts; Pal, L., Seifer, D.B., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 511–525. [Google Scholar] [CrossRef]
- de Kat, A.C.; Verschuren, W.M.; Eijkemans, M.J.; Broekmans, F.J.; van der Schouw, Y.T. Anti-Mullerian Hormone Trajectories Are Associated with Cardiovascular Disease in Women: Results from the Doetinchem Cohort Study. Circulation 2017, 135, 556–565. [Google Scholar] [CrossRef]
- Feldman, R.A.; O’Neill, K.; Butts, S.F.; Dokras, A. Antimullerian hormone levels and cardiometabolic risk in young women with polycystic ovary syndrome. Fertil. Steril. 2017, 107, 276–281. [Google Scholar] [CrossRef]
- Fallahzadeh, A.; Ramezeni Tehrani, F.; Rezaee, M.; Mahboobifard, F.; Amiri, M. Anti-Mullerian hormone and cardiometabolic status: A systematic review. Biomarkers 2023, 28, 486–501. [Google Scholar] [CrossRef]
- Catlin, E.A.; Tonnu, V.C.; Ebb, R.G.; Pacheco, B.A.; Manganaro, T.F.; Ezzell, R.M.; Donahoe, P.K.; Teixeira, J. Mullerian inhibiting substance inhibits branching morphogenesis and induces apoptosis in fetal rat lung. Endocrinology 1997, 138, 790–796. [Google Scholar] [CrossRef][Green Version]
- Lebeurrier, N.; Launay, S.; Macrez, R.; Maubert, E.; Legros, H.; Leclerc, A.; Jamin, S.P.; Picard, J.Y.; Marret, S.; Laudenbach, V.; et al. Anti-Mullerian-hormone-dependent regulation of the brain serine-protease inhibitor neuroserpin. J. Cell Sci. 2008, 121, 3357–3365. [Google Scholar] [CrossRef]
- Huffman, A.M.; Rezq, S.; Basnet, J.; Romero, D.G. Biomarkers in Polycystic Ovary Syndrome. Curr. Opin. Physiol. 2023, 36, 100717. [Google Scholar] [CrossRef]
- Blankenberg, S.; Tiret, L.; Bickel, C.; Peetz, D.; Cambien, F.; Meyer, J.; Rupprecht, H.J.; AtheroGene, I. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002, 106, 24–30. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 103. [Google Scholar] [CrossRef]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Glueck, C.J.; Papanna, R.; Wang, P.; Goldenberg, N.; Sieve-Smith, L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism 2003, 52, 908–915. [Google Scholar] [CrossRef]
- Karakas, S.E. Reactive Hypoglycemia: A Trigger for Nutrient-Induced Endocrine and Metabolic Responses in Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 7252. [Google Scholar] [CrossRef]
- Dietz de Loos, A.L.P.; Jiskoot, G.; Timman, R.; Beerthuizen, A.; Busschbach, J.J.V.; Laven, J.S.E. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod. Biomed. Online 2021, 43, 298–309. [Google Scholar] [CrossRef]
- Deshmukh, H.; Papageorgiou, M.; Wells, L.; Akbar, S.; Strudwick, T.; Deshmukh, K.; Vitale, S.G.; Rigby, A.; Vince, R.V.; Reid, M.; et al. The Effect of a Very-Low-Calorie Diet (VLCD) vs. a Moderate Energy Deficit Diet in Obese Women with Polycystic Ovary Syndrome (PCOS)—A Randomised Controlled Trial. Nutrients 2023, 15, 3872. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Khalid, K.; Apparow, S.; Mushaddik, I.L.; Anuar, A.; Rizvi, S.A.A.; Habib, A. Effects of Ketogenic Diet on Reproductive Hormones in Women with Polycystic Ovary Syndrome. J. Endocr. Soc. 2023, 7, bvad112. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Moustakli, E.; Zachariou, A.; Tsirka, G.; Tsiampali, C.; Palapela, N.; Sofikitis, N.; Dimitriadis, F. The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants 2023, 12, 1490. [Google Scholar] [CrossRef]
- Zuo, T.; Zhu, M.; Xu, W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxid. Med. Cell Longev. 2016, 2016, 8589318. [Google Scholar] [CrossRef]
- Gurusinghe, D.; Gill, S.; Almario, R.U.; Lee, J.; Horn, W.F.; Keim, N.L.; Kim, K.; Karakas, S.E. In polycystic ovary syndrome, adrenal steroids are regulated differently in the morning versus in response to nutrient intake. Fertil. Steril. 2010, 93, 1192–1199. [Google Scholar] [CrossRef]
- Tiboni, G.M.; Bucciarelli, T.; Giampietro, F.; Sulpizio, M.; Di Ilio, C. Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. Int. J. Immunopathol. Pharmacol. 2004, 17, 389–393. [Google Scholar] [CrossRef]
- Tay, C.T.; Loxton, D.; Bahri Khomami, M.; Teede, H.; Harrison, C.L.; Joham, A.E. High prevalence of medical conditions and unhealthy lifestyle behaviours in women with PCOS during preconception: Findings from the Australian Longitudinal Study on Women’s Health. Hum. Reprod. 2023, 38, 2267–2276. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramirez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Karamali, M.; Gholizadeh, M. The effects of coenzyme Q10 supplementation on metabolic profiles and parameters of mental health in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2022, 38, 45–49. [Google Scholar] [CrossRef]
- Pallotti, F.; Bergamini, C.; Lamperti, C.; Fato, R. The Roles of Coenzyme Q in Disease: Direct and Indirect Involvement in Cellular Functions. Int. J. Mol. Sci. 2021, 23, 128. [Google Scholar] [CrossRef]
- Yalle-Vasquez, S.; Osco-Rosales, K.; Nieto-Gutierrez, W.; Benites-Zapata, V.; Perez-Lopez, F.R.; Alarcon-Ruiz, C.A. Vitamin E supplementation improves testosterone, glucose- and lipid-related metabolism in women with polycystic ovary syndrome: A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2022, 38, 548–557. [Google Scholar] [CrossRef]
- Gray, B.; Swick, J.; Ronnenberg, A.G. Vitamin E and adiponectin: Proposed mechanism for vitamin E-induced improvement in insulin sensitivity. Nutr. Rev. 2011, 69, 155–161. [Google Scholar] [CrossRef]
- Panidis, D.; Balaris, C.; Farmakiotis, D.; Rousso, D.; Kourtis, A.; Balaris, V.; Katsikis, I.; Zournatzi, V.; Diamanti-Kandarakis, E. Serum parathyroid hormone concentrations are increased in women with polycystic ovary syndrome. Clin. Chem. 2005, 51, 1691–1697. [Google Scholar] [CrossRef]
- Nowak, A.; Wojtowicz, M.; Baranski, K.; Galczynska, D.; Daniluk, J.; Pluta, D. The correlation of vitamin D level with body mass index in women with PCOS. Ginekol. Pol. 2023, 94, 883–888. [Google Scholar] [CrossRef]
- Subramanian, A.; Harmon, Q.E.; Bernardi, L.A.; Carnethon, M.R.; Marsh, E.E.; Baird, D.D.; Jukic, A.M.Z. Association between serum 25-hydroxyvitamin D and antimullerian hormone levels in a cohort of African-American women. Fertil. Steril. 2024, 121, 642–650. [Google Scholar] [CrossRef]
- Lejman-Larysz, K.; Golara, A.; Baranowska, M.; Kozlowski, M.; Guzik, P.; Szydlowska, I.; Nawrocka-Rutkowska, J.; Sowinska-Przepiera, E.; Cymbaluk-Ploska, A.; Brodowska, A. Influence of Vitamin D on the Incidence of Metabolic Syndrome and Hormonal Balance in Patients with Polycystic Ovary Syndrome. Nutrients 2023, 15, 2952. [Google Scholar] [CrossRef]
- Zhao, J.F.; Li, B.X.; Zhang, Q. Vitamin D improves levels of hormonal, oxidative stress and inflammatory parameters in polycystic ovary syndrome: A meta-analysis study. Ann. Palliat. Med. 2021, 10, 169–183. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Bassiouni, Y.A.; Hasan, I.H.; Al-Amin, M.A.; Al-Ajmi, H.N.; Mohamad, R.A. Vitamin D attenuates pro-inflammatory TNF-alpha cytokine expression by inhibiting NF-small ka, CyrillicB/p65 signaling in hypertrophied rat hearts. J. Physiol. Biochem. 2015, 71, 289–299. [Google Scholar] [CrossRef]
- Fitz, V.; Graca, S.; Mahalingaiah, S.; Liu, J.; Lai, L.; Butt, A.; Armour, M.; Rao, V.; Naidoo, D.; Maunder, A.; et al. Inositol for Polycystic Ovary Syndrome: A systematic review and meta-analysis to inform the 2023 update of the International Evidence-Based PCOS Guidelines. J. Clin. Endocrinol. Metab. 2024, 109, 1630–1655. [Google Scholar] [CrossRef]
- Lagana, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef]
- Sairally, B.Z.F.; Dhillon-Smith, R.K.; Jethwani, G.; Latthe, P. Myoinositol or D-chiro-inositol for PCOS symptoms in adolescents: A narrative review. J. Pediatr. Endocrinol. Metab. 2024, 37, 91–101. [Google Scholar] [CrossRef]
- Teede, H.; Tassone, E.C.; Piltonen, T.; Malhotra, J.; Mol, B.W.; Pena, A.; Witchel, S.F.; Joham, A.; McAllister, V.; Romualdi, D.; et al. Effect of the combined oral contraceptive pill and/or metformin in the management of polycystic ovary syndrome: A systematic review with meta-analyses. Clin. Endocrinol. 2019, 91, 479–489. [Google Scholar] [CrossRef]
- Dardzinska, J.A.; Rachon, D.; Kuligowska-Jakubowska, M.; Aleksandrowicz-Wrona, E.; Ploszynski, A.; Wyrzykowski, B.; Lysiak-Szydlowska, W. Effects of metformin or an oral contraceptive containing cyproterone acetate on serum c-reactive protein, interleukin-6 and soluble vascular cell adhesion molecule-1 concentrations in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2014, 122, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Shah, N.; Deshmukh, H.; Sahebkar, A.; Ostlundh, L.; Al-Rifai, R.H.; Atkin, S.L.; Sathyapalan, T. Impact of pharmacological interventions on biochemical hyperandrogenemia in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomised controlled trials. Arch. Gynecol. Obstet. 2023, 307, 1347–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Chen, Z.; Feng, W.J.; Long, S.L.; Mo, Z.C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin. Chim. Acta 2019, 499, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, K.; Davidson, K.; Kochman, L.; Cherry, T.; Kopin, L.; Guzick, D.S. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J. Clin. Endocrinol. Metab. 2008, 93, 4299–4306. [Google Scholar] [CrossRef]
- Yousuf, S.D.; Ganie, M.A.; Urwat, U.; Andrabi, S.M.; Zargar, M.A.; Dar, M.A.; Manzoor-Ul-Rehman, M.; Mudassar, S.; Rashid, F. Oral contraceptive pill (OCP) treatment alters the gene expression of intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor-alpha (TNF-alpha), monocyte chemoattractant protein-1 (MCP-1) and plasminogen activator inhibitor-1 (PAI-1) in polycystic ovary syndrome (PCOS) women compared to drug-naive PCOS women. BMC Womens Health 2023, 23, 68. [Google Scholar] [CrossRef]
- Melin, J.; Forslund, M.; Alesi, S.; Piltonen, T.; Romualdi, D.; Spritzer, P.M.; Tay, C.T.; Pena, A.; Witchel, S.F.; Mousa, A.; et al. The impact of metformin with or without lifestyle modification versus placebo on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Endocrinol. 2023, 189, S37–S63. [Google Scholar] [CrossRef]
- Malin, S.K.; Kashyap, S.R. Effects of metformin on weight loss: Potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 323–329. [Google Scholar] [CrossRef]
- Melin, J.M.; Forslund, M.; Alesi, S.J.; Piltonen, T.; Romualdi, D.; Spritzer, P.M.; Tay, C.T.; Pena, A.S.; Witchel, S.F.; Mousa, A.; et al. Effects of different insulin sensitisers in the management of polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Endocrinol. 2024, 100, 149–163. [Google Scholar] [CrossRef]
- Morin-Papunen, L.; Rantala, A.S.; Unkila-Kallio, L.; Tiitinen, A.; Hippelainen, M.; Perheentupa, A.; Tinkanen, H.; Bloigu, R.; Puukka, K.; Ruokonen, A.; et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): A multicenter, double-blind, placebo-controlled randomized trial. J. Clin. Endocrinol. Metab. 2012, 97, 1492–1500. [Google Scholar] [CrossRef]
- Mazzieri, A.; Basta, G.; Calafiore, R.; Luca, G. GLP-1 RAs and SGLT2i: Two antidiabetic agents associated with immune and inflammation modulatory properties through the common AMPK pathway. Front. Immunol. 2023, 14, 1163288. [Google Scholar] [CrossRef]
- Kim, Y.D.; Park, K.G.; Lee, Y.S.; Park, Y.Y.; Kim, D.K.; Nedumaran, B.; Jang, W.G.; Cho, W.J.; Ha, J.; Lee, I.K.; et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008, 57, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Wu, F.; Shi, D.; Sun, H.; Wang, F.; Xu, C. Metformin: Update on mechanisms of action on liver diseases. Front. Nutr. 2023, 10, 1327814. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.H.; Russo, M.; Dinicola, S.; Forte, G.; Unfer, V. Questioning PCOS phenotypes for reclassification and tailored therapy. Trends Endocrinol. Metab. 2023, 34, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhu, Y. Efficacy of simvastatin plus metformin for polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 257, 19–24. [Google Scholar] [CrossRef]
- Chen, L.L.; Zheng, J.H. Effects of atorvastatin on the insulin resistance in women of polycystic ovary syndrome: A systematic review and meta-analysis. Medicine 2021, 100, e26289. [Google Scholar] [CrossRef]
- Hao, S.L.; Zhang, C.L.; Meng, X.Y. Comparison of different drug for reducing testosterone levels in women with polycystic ovary syndrome: A systematic review and network meta-analysis. Medicine 2023, 102, e35152. [Google Scholar] [CrossRef]
- Xiong, T.; Fraison, E.; Kolibianaki, E.; Costello, M.F.; Venetis, C.; Kostova, E.B. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst. Rev. 2023, 7, CD008565. [Google Scholar] [CrossRef]
- Carmina, E.; Longo, R.A. Semaglutide Treatment of Excessive Body Weight in Obese PCOS Patients Unresponsive to Lifestyle Programs. J. Clin. Med. 2023, 12, 5921. [Google Scholar] [CrossRef]
- Duah, J.; Seifer, D.B. Medical therapy to treat obesity and optimize fertility in women of reproductive age: A narrative review. Reprod. Biol. Endocrinol. 2025, 23, 2. [Google Scholar] [CrossRef]
- Szczesnowicz, A.; Szeliga, A.; Niwczyk, O.; Bala, G.; Meczekalski, B. Do GLP-1 Analogs Have a Place in the Treatment of PCOS? New Insights and Promising Therapies. J. Clin. Med. 2023, 12, 5915. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, A.V.; Fernandez, C.J.; Jacob, K. Efficacy and Cardiovascular Safety of Thiazolidinediones. Curr. Drug Saf. 2021, 16, 233–249. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, C.; Zhang, J.; He, B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: A network meta-analysis. Reprod. Health 2021, 18, 171. [Google Scholar] [CrossRef]
- Aroda, V.R.; Ciaraldi, T.P.; Burke, P.; Mudaliar, S.; Clopton, P.; Phillips, S.; Chang, R.J.; Henry, R.R. Metabolic and hormonal changes induced by pioglitazone in polycystic ovary syndrome: A randomized, placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2009, 94, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Lewin, Z.; Vitek, W.S.; O’Malley, W.; Astapova, O. Resolution of Hyperandrogenism, Insulin Resistance and Acanthosis Nigricans (HAIR-AN) Syndrome After Sleeve Gastrectomy. JCEM Case Rep. 2023, 1, luac030. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, N.M.; Sadegh, G.H.M.; Farsi, Y.; Besharat, S.; Hajimirzaie, S.H.; Abbasi, M. The impact of sleeve gastrectomy on polycystic ovarian syndrome: A single-center 1-year cohort study. Ir. J. Med. Sci. 2024, 193, 721–724. [Google Scholar] [CrossRef]
- Wang, X.T.; Hou, Y.S.; Zhao, H.L.; Wang, J.; Guo, C.H.; Guan, J.; Lv, Z.G.; Ma, P.; Han, J.L. Effect of laparoscopic sleeve gastrectomy on related variables of obesity complicated with polycystic ovary syndrome. World J. Gastrointest. Surg. 2023, 15, 2423–2429. [Google Scholar] [CrossRef]
- Samarasinghe, S.N.S.; Woods, C.; Miras, A.D. Bariatric Surgery in Women with Polycystic Ovary Syndrome. Metabolism 2024, 151, 155745. [Google Scholar] [CrossRef]
- Akalestou, E.; Miras, A.D.; Rutter, G.A.; le Roux, C.W. Mechanisms of Weight Loss After Obesity Surgery. Endocr. Rev. 2022, 43, 19–34. [Google Scholar] [CrossRef]
- Svane, M.S.; Jorgensen, N.B.; Bojsen-Moller, K.N.; Dirksen, C.; Nielsen, S.; Kristiansen, V.B.; Torang, S.; Wewer Albrechtsen, N.J.; Rehfeld, J.F.; Hartmann, B.; et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. 2016, 40, 1699–1706. [Google Scholar] [CrossRef]
| Gene | Role |
|---|---|
| INSL4, INSL6 | Insulin signaling |
| MAPRE1 | Adipocyte function, lipid metabolism |
| PLZF | Cardiac remodeling |
| THADA | Insulin secretion |
| KCNH7 | Potassium voltage-gated channel |
| FIGN | Microtubule severing |
| GRB14 | Insulin receptor signaling |
| PON1 | LDL metabolism |
| IGF2 | Encodes insulin-like growth factor 2 |
| LEPR, LEP | Associated with leptin |
| ADIPOR2 | Associated with adiponectin |
| Adipokine | Changes in PCOS | Effects in the Context of PCOS |
|---|---|---|
| Chemerin | Increased | Increases insulin resistance; increases insulin secretion in the uterus and stromal cells |
| Leptin | Increased | Stimulates insulin secretion from adipose tissue; in PCOS, impairs aromatase expression in granulosa cells thereby increasing circulating androgen levels |
| Omentin | Decreased | Has protective effects on cardiovascular health |
| Adiponectin | Decreased | Enhances tissue sensitivity to insulin; promotes lipid oxidation via AMP-activated protein kinase (AMPK) signaling |
| Resistin | Increased | Upregulates 17-α-hydroxylase, increasing androgen production; induces macrophage-mediated production of proinflammatory cytokines |
| Medication | Mechanism of Action | Effects |
|---|---|---|
| Antioxidants | ||
| CoQ10 | Stabilizes cell membranes, prevents mitochondrial dysfunction and oxidative stress |
|
| Vitamin E | Upregulates endogenous ligands involved in activating peroxisome proliferator activated receptor gamma (PPAR-γ) |
|
| Vitamin D | Inhibits nuclear factor kappa B (NF-κB), reducing production of free radical and proinflammatory cytokines |
|
| Inositols | Involved in cell signaling pathways to transport glucose transporter-4 (GLUT4) to the plasma membrane for glucose uptake, and regulates maturation and proliferation of granulosa cells and aromatase synthesis |
|
| Combined oral contraceptives | Increase SHBG and luteinizing hormone (LH) production, reducing serum androgens |
|
| Metformin | Activates the AMPK pathway to prevent gluconeogenesis, improve hyperglycemia, triglyceridemia, and activate T regulatory cell proliferation |
|
| Statins | Inhibit 3-hydroxy-3methylglutaryl coenzyme A reductase and thereby decreasing lipid production |
|
| Glucagon-like peptide-1 (GLP-1) agonists | Agonist of GLP-1 receptors, heightening satiety, reducing appetite, and regulating appetite by acting on L cells of the small intestine |
|
| Thiazolidinediones | Agonist of PPAR-γ, increasing peroxisomes and disposal of insulin dependent glucose, while decreasing hepatic glucose output |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahabamunuge, J.; Sekula, N.M.; Lepore, C.; Kudrimoti, M.; Upadhyay, A.; Alshowaikh, K.; Li, H.J.; Seifer, D.B.; AlAshqar, A. The Molecular Basis of Polycystic Ovary Syndrome and Its Cardiometabolic Correlates: Exploring the Intersection and Its Clinical Implications—A Narrative Review. Biomedicines 2025, 13, 709. https://doi.org/10.3390/biomedicines13030709
Mahabamunuge J, Sekula NM, Lepore C, Kudrimoti M, Upadhyay A, Alshowaikh K, Li HJ, Seifer DB, AlAshqar A. The Molecular Basis of Polycystic Ovary Syndrome and Its Cardiometabolic Correlates: Exploring the Intersection and Its Clinical Implications—A Narrative Review. Biomedicines. 2025; 13(3):709. https://doi.org/10.3390/biomedicines13030709
Chicago/Turabian StyleMahabamunuge, Jasmin, Nicole M. Sekula, Christina Lepore, Meghana Kudrimoti, Animesh Upadhyay, Khadija Alshowaikh, Howard J. Li, David B. Seifer, and Abdelrahman AlAshqar. 2025. "The Molecular Basis of Polycystic Ovary Syndrome and Its Cardiometabolic Correlates: Exploring the Intersection and Its Clinical Implications—A Narrative Review" Biomedicines 13, no. 3: 709. https://doi.org/10.3390/biomedicines13030709
APA StyleMahabamunuge, J., Sekula, N. M., Lepore, C., Kudrimoti, M., Upadhyay, A., Alshowaikh, K., Li, H. J., Seifer, D. B., & AlAshqar, A. (2025). The Molecular Basis of Polycystic Ovary Syndrome and Its Cardiometabolic Correlates: Exploring the Intersection and Its Clinical Implications—A Narrative Review. Biomedicines, 13(3), 709. https://doi.org/10.3390/biomedicines13030709






