Determination of Cortisol Levels in a Small Volume of Saliva of COVID-19-Recovering Patients During Treatment with Psychotropic Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. LC-DAD Conditions
2.3. Preparation of Quality Control (QC) and Standard Solutions
2.4. Collection of Saliva Samples
2.5. Extraction Procedure
2.6. Method Validation
2.6.1. Linearity
2.6.2. Lower Limit of Quantification LLOQ and Limit of Detection
2.6.3. Accuracy and Precision
2.6.4. Selectivity
2.6.5. Absolute Recovery and Extraction Recovery
2.6.6. Matrix Effect
2.6.7. Stability
2.7. Clinical Application
2.8. Statistical Evaluation of the Results
3. Results
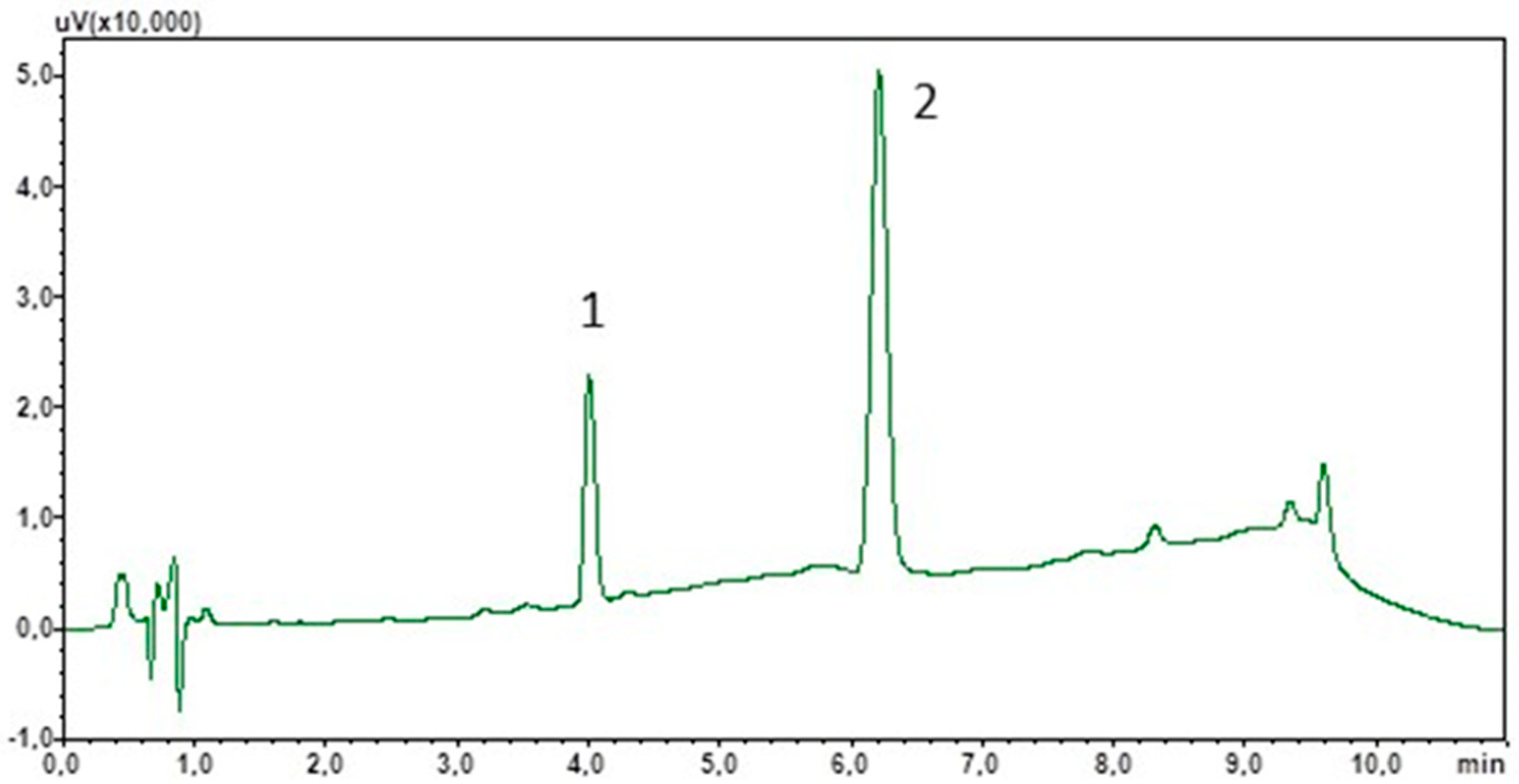
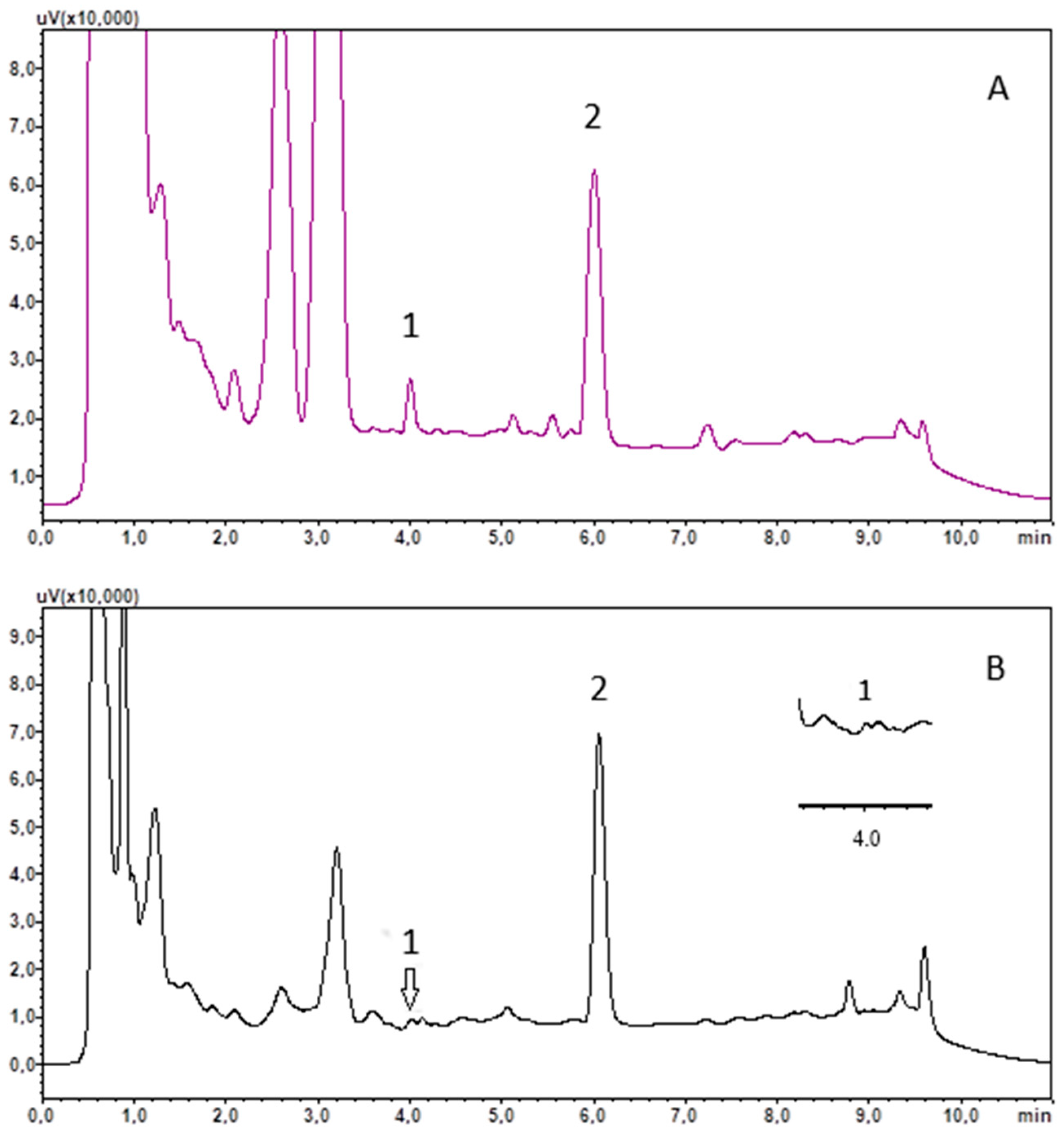
3.1. Chromatographic Analysis
3.2. Solid Phase Extraction
3.3. Method Validation
3.4. Clinical Application
3.5. Statistical Analysis of Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| LC | Liquid chromatography |
| DAD | Diode array detector |
| CV | Coefficient of variation |
| ACTH | Adrenocorticotropic hormone |
| CRH | corticotropin-releasing hormone |
| BD | Bipolar disorder |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| SPE | Solid phase extraction |
| MS | Mass spectrometry |
| IS | Internal standard |
| QC | Quality control |
| EMA | European Medicines Agency |
| LOD | Limit of detection |
| LLOQ | Lower limit of quantification |
References
- Katzung, B.G.; Kruidering-Hall, M.; Tuan, R.; Vanderah, T.W.; Trevor, A.J. Adrenocorticosteroids & Adrenocortical Antagonists. In Katzung & Trevor’s Pharmacology: Examination & Board Review, 13th ed.; Katzung, B.G., Kruidering-Hall, M., Tuan, R., Vanderah, T.W., Trevor, A.J., Eds.; McGraw-Hill: New York, NY, USA, 2018; Available online: https://accessmedicine-1mhmedical-1com-1aqoxlrin0976.han.gumed.edu.pl/content.aspx?bookid=3058§ionid=255306661 (accessed on 23 January 2025).
- Cedernaes, J.; Ramsey, K.; Bass, J. The Role of Circadian Biology in Health and Disease. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill: New York, NY, USA, 2021; Available online: https://accesspharmacy.mhmedical.com/content.aspx?bookid=2129§ionid=192536187 (accessed on 23 January 2025).
- Carroll, T.B.; Aron, D.C.; Findling, J.W.; Tyrrell, J. Glucocorticoids and Adrenal Androgens. In Greenspan’s Basic & Clinical Endocrinology, 10th ed.; Gardner, D.G., Shoback, D., Eds.; McGraw-Hill: New York, NY, USA, 2018; Available online: https://accessmedicine-1mhmedical-1com-1aqoxlrin0976.han.gumed.edu.pl/content.aspx?bookid=2178§ionid=166249274 (accessed on 23 January 2025).
- Butterworth, J.F.; Mackey, D.C.; Wasnick, J.D. Anesthesia for Patients with Neurological & Psychiatric Diseases. In Morgan & Mikhail’s Clinical Anesthesiology, 6th ed.; Butterworth, J.F., Mackey, D.C., Wasnick, J.D., Eds.; McGraw-Hill: New York, NY, USA, 2018; Available online: https://accessmedicine-1mhmedical-1com-1aqoxlrin0976.han.gumed.edu.pl/content.aspx?bookid=2444§ionid=193561247 (accessed on 23 January 2025).
- Holland, J.M.; Schatzberg, A.F.; O’Hara, R.; Marquett, R.M.; Gallagher-Thompson, D. Pretreatment cortisol levels predict posttreatment outcomes among older adults with depression in cognitive behavioural therapy. Psychiatry Res. 2013, 210, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Jabben, N.; Nolen, W.A.; Smit, J.H.; Vreeburg, S.A.; Beekman, A.T.F.; Penninx, B.W.J.H. Co-occurring manic symptomatology influences HPA axis alterations in depression. J. Psych. Res. 2011, 45, 1208–1213. [Google Scholar] [CrossRef]
- DeBattista, C. Antidepressant Agents. In Basic & Clinical Pharmacology, 15th ed.; Katzung, B.G., Vanderah, T.W., Eds.; McGraw-Hill: New York, NY, USA, 2018; Available online: https://accessmedicine-1mhmedical-1com-1aqoxlrin0976.han.gumed.edu.pl/content.aspx?bookid=2988§ionid=250598963 (accessed on 23 January 2025).
- Cherian, K.; Schatzberg, A.F.; Keller, J. HPA axis in psychotic major depression and schizophrenia spectrum disorders: Cortisol, clinical symptomatology, and cognition. Schizophr. Res. 2019, 213, 72–79. [Google Scholar] [CrossRef]
- Touskova, T.P.; Bob, P.; Pec, O.; Mishara, A.; Vanickova, Z.; Raboch, J.; Lysaker, P. Insight and cortisol responses in women with first episode psychosis. Schizophr. Res. 2018, 201, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.F.; Brennan, P.A.; Esterberg, M.; Brasfield, J.; Pearce, B.; Compton, M.T. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J. Abnorm. Psychol. 2010, 119, 401–408. [Google Scholar] [CrossRef]
- Labad, J.; Stojanovic-Pérez, A.; Montalvo, I.; Solé, M.; Cabezas, A.; Ortega, L.; Moreno, I.; Vilella, E.; Martorell, L.; Reynolds, R.M.; et al. Stress biomarkers as predictors of transition to psychosis in at-risk mental states: Roles for cortisol, prolactin and albumin. J. Psych. Res. 2015, 60, 163–169. [Google Scholar] [CrossRef]
- Shah, J.L.; Malla, A.K. Much ado about much: Stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr. Res. 2015, 162, 253–260. [Google Scholar] [CrossRef]
- Chaumette, B.; Kebir, O.; Mam-Lam-Fook, C.; Morvan, Y.; Bourgin, J.; Godsil, B.P.; Plaze, M.; Gaillard, R.; Jay, T.M.; Krebs, M.O. Salivary cortisol in early psychosis: New findings and meta-analysis. Psychoneuroendocrinology 2016, 63, 262–270. [Google Scholar] [CrossRef]
- Nordholm, D.; Rostrup, E.; Mondelli, V.; Randers, L.; Nielsen, M.Ø.; Wulff, S.; Nørbak-Emig, H.; Broberg, B.V.; Krakauer, K.; Dazzan, P.; et al. Multiple measures of HPA axis function in ultra high risk and first-episode schizophrenia patients. Psychoneuroendocrinology 2018, 92, 72–80. [Google Scholar] [CrossRef]
- Corcoran, C.M.; Smith, C.; McLaughlin, D.; Auther, A.; Malaspina, D.; Cornblatt, B. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 2012, 135, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Huber, C.G.; Fröhlich, D.; Borgwardt, S.; Lang, U.E.; Walter, M. Modulation of HPA axis response to social stress in schizophrenia by childhood trauma. Psychoneuroendocrinology 2017, 82, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Streit, F.; Memic, A.; Hasandedić, L.; Rietschel, L.; Frank, J.; Lang, M.; Witt, S.H.; Forstner, A.J.; Degenhardt, F.; Wüst, S.; et al. Perceived stress and hair cortisol: Differences in bipolar disorder and schizophrenia. Psychoneuroendocrinology 2016, 69, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Mokrani, M.-C.; Erb, A.; Danila, V.; Gonzalez Lopera, F.; Jeanjean, L. Dopaminergic, noradrenergic, adrenal, and thyroid abnormalities in psychotic and affective disorders. Front. Psychiatry 2020, 11, 533872. [Google Scholar] [CrossRef]
- Świątkowska-Stodulska, R.; Berlińska, A.; Puchalska-Reglińska, E. Cortisol as an Independent Predictor of Unfavorable Outcomes in Hospitalized COVID-19 Patients. Biomedicines 2022, 10, 1527. [Google Scholar] [CrossRef]
- Ilias, I.; Vassiliou, A.G.; Keskinidou, C.; Vrettou, C.S.; Orfanos, S.; Kotanidou, A.; Dimopoulou, I. Changes in Cortisol Secretion and Corticosteroid Receptors in COVID-19 and Non COVID-19 Critically Ill Patients with Sepsis/Septic Shock and Scope for Treatment. Biomedicines 2023, 11, 1801. [Google Scholar] [CrossRef]
- Tang, N.; Kido, T.; Shi, J.; McCafferty, E.; Ford, J.M.; Dal Bon, K.; Pulliam, L. Blood Markers Show Neural Consequences of LongCOVID-19. Cells 2024, 13, 478. [Google Scholar] [CrossRef]
- Marcus-Perlman, Y.; Tordjman, K.; Greenman, Y.; Limor, R.; Shenkerman, G.; Osher, E.; Stern, N. Low-dose ACTH (1 microg) salivary test: A potential alternative to the classical blood test. Clin. Endocrinol. 2006, 64, 215–218. [Google Scholar] [CrossRef]
- Ney, L.J.; Felmingham, K.L.; Bruno, R.; Matthews, A.; Nichols, D.S. Simultaneous quantification of endocannabinoids, oleoylethanolamide and steroid hormones in human plasma and saliva. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1152, 122252. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Oh, H.; Park, H.R.; Joo, E.Y.; Lee, S.Y. A Sensitive and Specific Liquid Chromatography-Tandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison with Immunoassays. Ann. Lab. Med. 2021, 41, 108–113. [Google Scholar] [CrossRef]
- Matsui, F.; Koh, E.; Yamamoto, K.; Sugimoto, K.; Sin, H.S.; Maeda, Y.; Honma, S.; Namiki, M. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for simultaneous measurement of salivary testosterone and cortisol in healthy men for utilization in the diagnosis of late-onset hypogonadism in males. Endocr. J. 2009, 56, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Montskó, G.; Tarjányi, Z.; Mezősi, E.; Kovács, G.L. A validated method for measurement of serum total, serum free, and salivary cortisol, using high-performance liquid chromatography coupled with high-resolution ESI-TOF mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- McWhinney, B.; Briscoe, S.E.; Ungerer, J.P.; Pretorius, C.J. Measurement of cortisol, cortisone, prednisolone, dexamethasone and 11-deoxycortisol with ultra high performance liquid chromatography-tandem mass spectrometry: Application for plasma, plasma ultrafiltrate, urine and saliva in a routine laboratory. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 2863–2869. [Google Scholar] [CrossRef]
- Antonelli, G.; Ceccato, F.; Artusi, C.; Marinova, M.; Plebani, M. Salivary cortisol and cortisone by LC-MS/MS: Validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin. Chim. Acta 2015, 451, 247–251. [Google Scholar] [CrossRef]
- Antonelli, G.; Padoan, A.; Artusi, C.; Marinova, M.; Zaninotto, M.; Plebani, M. Automated saliva processing for LC-MS/MS: Improving laboratory efficiency in cortisol and cortisone testing. Clin. Biochem. 2016, 49, 518–520. [Google Scholar] [CrossRef]
- Aydin, E.; Drotleff, B.; Noack, H.; Derntl, B.; Lämmerhofer, M. Fast accurate quantification of salivary cortisol and cortisone in a large-scale clinical stress study by micro-UHPLC-ESI-MS/MS using a surrogate calibrant approach. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1182, 122939. [Google Scholar] [CrossRef]
- Jones, R.L.; Owen, L.J.; Adaway, J.E.; Keevil, B.G. Simultaneous analysis of cortisol and cortisone in saliva using XLC-MS/MS for fully automated online solid phase extraction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 881–882, 42–48. [Google Scholar] [CrossRef]
- Žaja, R.; Stipičević, S.; Milošević, M.; Košec, A.; Ajduk, J.; Kelava, I.; Baća, A.Z.; Klarica, M.; Ries, M. Salivary cortisone as potential predictor of occupational exposure to noise and related stress. Arh. Hig. Rada Toksikol. 2023, 74, 232–237. [Google Scholar] [CrossRef]
- Baid, S.K.; Sinaii, N.; Wade, M.; Rubino, D.; Nieman, L.K. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: A comparison of assays to establish hypercortisolism. J. Clin. Endocrinol. Metab. 2007, 92, 3102–3107. [Google Scholar] [CrossRef]
- Laguillier-Morizot, C.; Bonnet-Serrano, F.; Leguy, M.C.; Simeonovic, M.; Sée, C.; Zientek, C.; Soussan, M.; Bouys, L.; Bertherat, J.; Guibourdenche, J. Diagnostic performance of an automated immunoassay for salivary cortisol. Ann. D’endocrinologie 2024, 85, 20–26. [Google Scholar] [CrossRef]
- Kataoka, H.; Matsuura, E.; Mitani, K. Determination of cortisol in human saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Fariha, R.; Jabrah, M.; Hill, C.; Spooner, A.; Deshpande, P.; Tripathi, A. Simultaneous detection of salivary cortisol and cortisone using an automated high-throughput sample preparation method for LC-MS/MS. SLAS Technol. 2022, 27, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Saracino, M.A.; Iacono, C.; Somaini, L.; Gerra, G.; Ghedini, N.; Raggi, M.A. Multi-matrix assay of cortisol, cortisone and corticosterone using a combined MEPS-HPLC procedure. J. Pharm. Biomed. Anal. 2014, 88, 643–648. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 23 January 2025).
- Perry, N.B.; Donzella, B.; Troy, M.F.; Barnes, A.J. Mother and child hair cortisol during the COVID-19 pandemic: Associations among physiological stress, pandemic-related behaviors, and child emotional-behavioral health. Psychoneuroendocrinology 2022, 137, 105656. [Google Scholar] [CrossRef]
- Jopling, E.; Rnic, K.; Tracy, A.; LeMoult, J. Impact of loneliness on diurnal cortisol in youth. Psychoneuroendocrinology 2021, 132, 105345. [Google Scholar] [CrossRef]
- Ibar, C.; Fortuna, F.; Gonzalez, D.; Jamardo, J.; Jacobsen, D.; Pugliese, L.; Giraudo, L.; Ceres, V.; Mendoza, C.; Repetto, E.M.; et al. Evaluation of stress, burnout and hair cortisol levels in health workers at a University Hospital during COVID-19 pandemic. Psychoneuroendocrinology 2021, 128, 105213. [Google Scholar] [CrossRef]
- Šik Novak, K.; Bogataj Jontez, N.; Kenig, S.; Hladnik, M.; Baruca Arbeiter, A.; Bandelj, D.; Černelič Bizjak, M.; Petelin, A.; Mohorko, N.; Jenko Pražnikar, Z. The effect of COVID-19 lockdown on mental health, gut microbiota composition and serum cortisol levels. Stress 2022, 25, 246–257. [Google Scholar] [CrossRef]
- Feeney, J.; Kenny, R.A. Hair cortisol as a risk marker for increased depressive symptoms among older adults during the COVID-19 pandemic. Psychoneuroendocrinology 2022, 143, 105847. [Google Scholar] [CrossRef]
- Ramezani, M.; Simani, L.; Karimialavijeh, E.; Rezaei, O.; Hajiesmaeili, M.; Pakdaman, H. The Role of Anxiety and Cortisol in Outcomes of Patients with COVID-19. Basic Clin. Neurosci. 2020, 11, 179–184. [Google Scholar] [CrossRef]
- Dziurkowska, E.; Wesolowski, M. Multivariate Statistical Analysis as a Supplementary Tool for Interpretation of Variations in Salivary Cortisol Level in Women with Major Depressive Disorder. Sci. World J. 2015, 2015, 987435. [Google Scholar] [CrossRef]
- Dziurkowska, E.; Wesolowski, M.; Dziurkowski, M. Salivary cortisol in women with major depressive disorder under selective serotonin reuptake inhibitors therapy. Arch. Womens Ment. Health 2013, 16, 139–147. [Google Scholar] [CrossRef]
- Jensterle, M.; Herman, R.; Janež, A.; Mahmeed, W.A.; Al-Rasadi, K.; Al-Alawi, K.; Banach, M.; Banerjee, Y.; Ceriello, A.; Cesur, M.; et al. The Relationship between COVID-19 and Hypothalamic-Pituitary-Adrenal Axis: A Large Spectrum from Glucocorticoid Insufficiency to Excess-The CAPISCO International Expert Panel. Int. J. Mol. Sci. 2022, 23, 7326. [Google Scholar] [CrossRef]

| Calibration Curve y = ax + b (n = 4) | |
|---|---|
| Range (ng/mL) | 4–500 |
| Determination coefficient (R2) | 0.9986 ± 0.0009 |
| Slope a ± Δa | 0.0011 ± 0.0001 |
| Intercept b ± Δb | −0.0036 ± 0.0065 |
| LLOQ (ng/mL) | 4.0 |
| LOD (ng/mL) | 2.0 |
| QC (ng/mL) | Intra-Day (CV%) | Inter-Day (CV%) | Extraction Recovery (%) | Absolute Recovery (%) | Matrix Effect (%) | Stability (Difference%) | |
|---|---|---|---|---|---|---|---|
| 8 °C | −21 °C | ||||||
| 4 | 6.16 | 8.13 | 81.22 | 82.10 | 8.34 | −1.28 | −3.05 |
| 15 | 7.87 | 9.81 | 80.29 | 80.68 | 0.49 | −0.47 | −3.42 |
| 200 | 0.85 | 11.97 | 79.12 | 91.25 | 5.35 | −2.52 | −4.89 |
| 350 | 2.80 | 10.86 | 86.55 | 90.63 | 4.71 | −0.21 | −2.31 |
| Concentration of Cortisol (ng/mL) | |||
|---|---|---|---|
| Recoveries | Control | ||
| R1 | 13.33 | C1 | 3.60 |
| R2 | 17.87 | C2 | 6.53 |
| R3 | 8.35 | C3 | 5.65 |
| R4 | 5.58 | C4 | 2.41 |
| R5 | 6.12 | C5 | 2.31 |
| R6 | 14.28 | C6 | 5.56 |
| R7 | 8.78 | C7 | 3.56 |
| R8 | 8.19 | C8 | 7.14 |
| R9 | 3.59 | C9 | 4.22 |
| R10 | 25.56 | C10 | 2.85 |
| R11 | 7.34 | C11 | 3.58 |
| R12 | 18.22 | C12 | 4.63 |
| R13 * | 108.20 | C13 | 2.81 |
| R14 | 8.41 | C14 | 4.17 |
| R15 | 9.74 | C15 | 2.79 |
| R16 | 28.27 | C16 | 3.93 |
| Mean ± SD | 12.24 ± 7.33 | 4.11 ± 1.46 | |
| Median | 8.78 | 3.765 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziurkowska, E.; Guz-Rzeniecka, G.; Dziurkowski, M. Determination of Cortisol Levels in a Small Volume of Saliva of COVID-19-Recovering Patients During Treatment with Psychotropic Drugs. Biomedicines 2025, 13, 697. https://doi.org/10.3390/biomedicines13030697
Dziurkowska E, Guz-Rzeniecka G, Dziurkowski M. Determination of Cortisol Levels in a Small Volume of Saliva of COVID-19-Recovering Patients During Treatment with Psychotropic Drugs. Biomedicines. 2025; 13(3):697. https://doi.org/10.3390/biomedicines13030697
Chicago/Turabian StyleDziurkowska, Ewelina, Grażyna Guz-Rzeniecka, and Maciej Dziurkowski. 2025. "Determination of Cortisol Levels in a Small Volume of Saliva of COVID-19-Recovering Patients During Treatment with Psychotropic Drugs" Biomedicines 13, no. 3: 697. https://doi.org/10.3390/biomedicines13030697
APA StyleDziurkowska, E., Guz-Rzeniecka, G., & Dziurkowski, M. (2025). Determination of Cortisol Levels in a Small Volume of Saliva of COVID-19-Recovering Patients During Treatment with Psychotropic Drugs. Biomedicines, 13(3), 697. https://doi.org/10.3390/biomedicines13030697






