Testosterone Supplementation: A Potential Therapeutic Strategy for Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
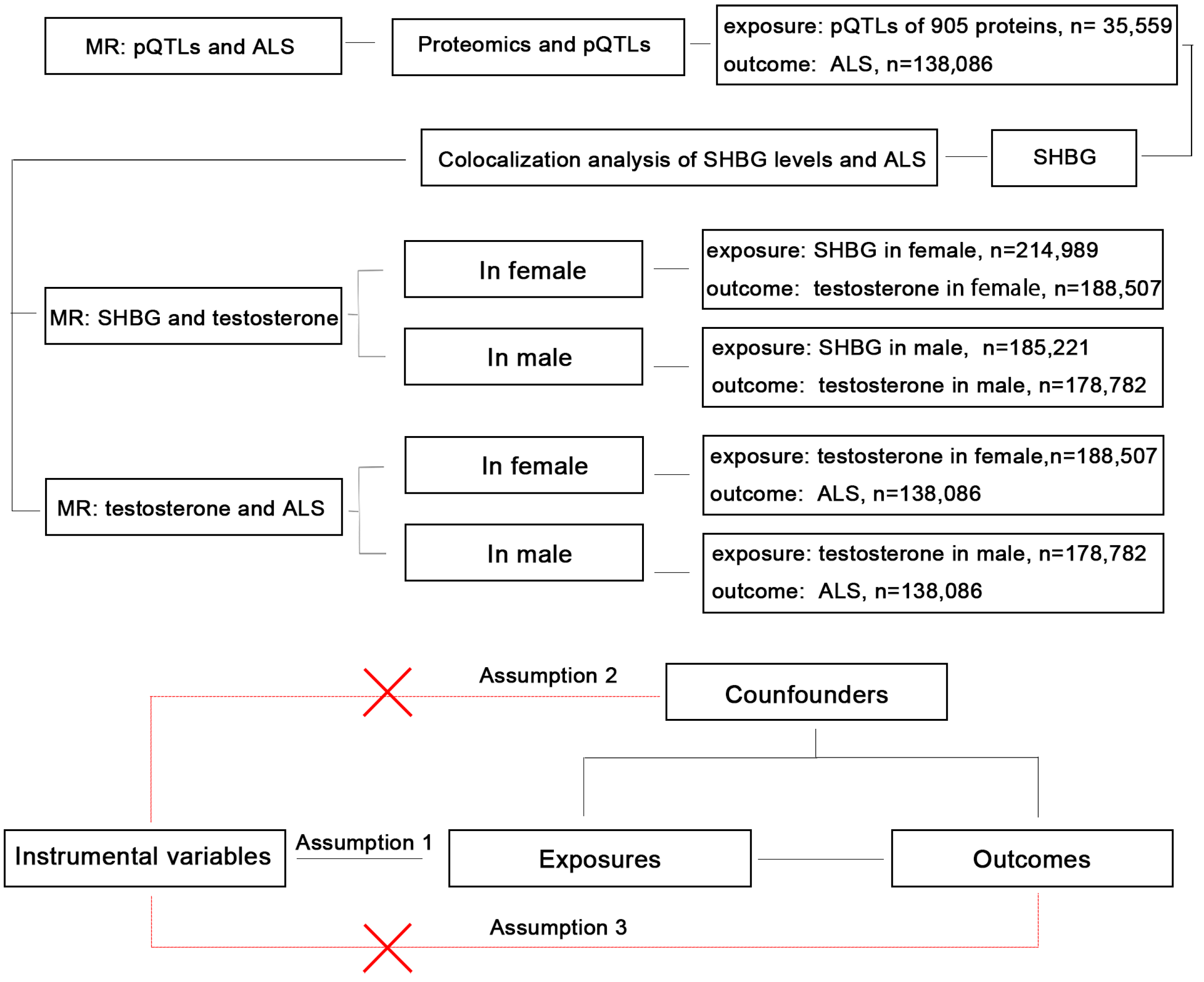
2. Materials and Methods
2.1. Study Design
2.2. Proteomics and pQTL Data Sources
2.3. GWAS Data Sources
2.4. MR Analysis
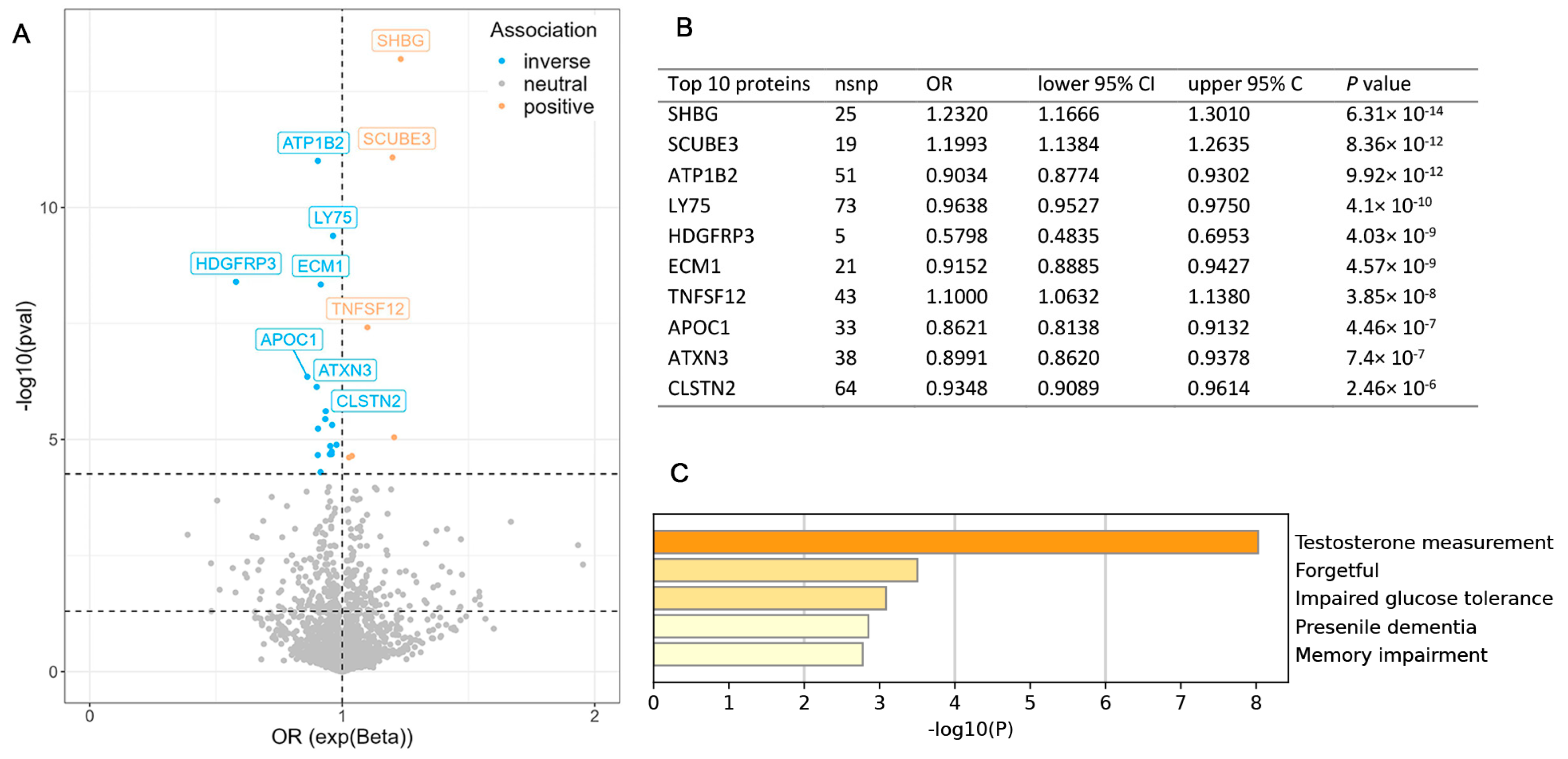
2.5. Functional Enrichment Analysis
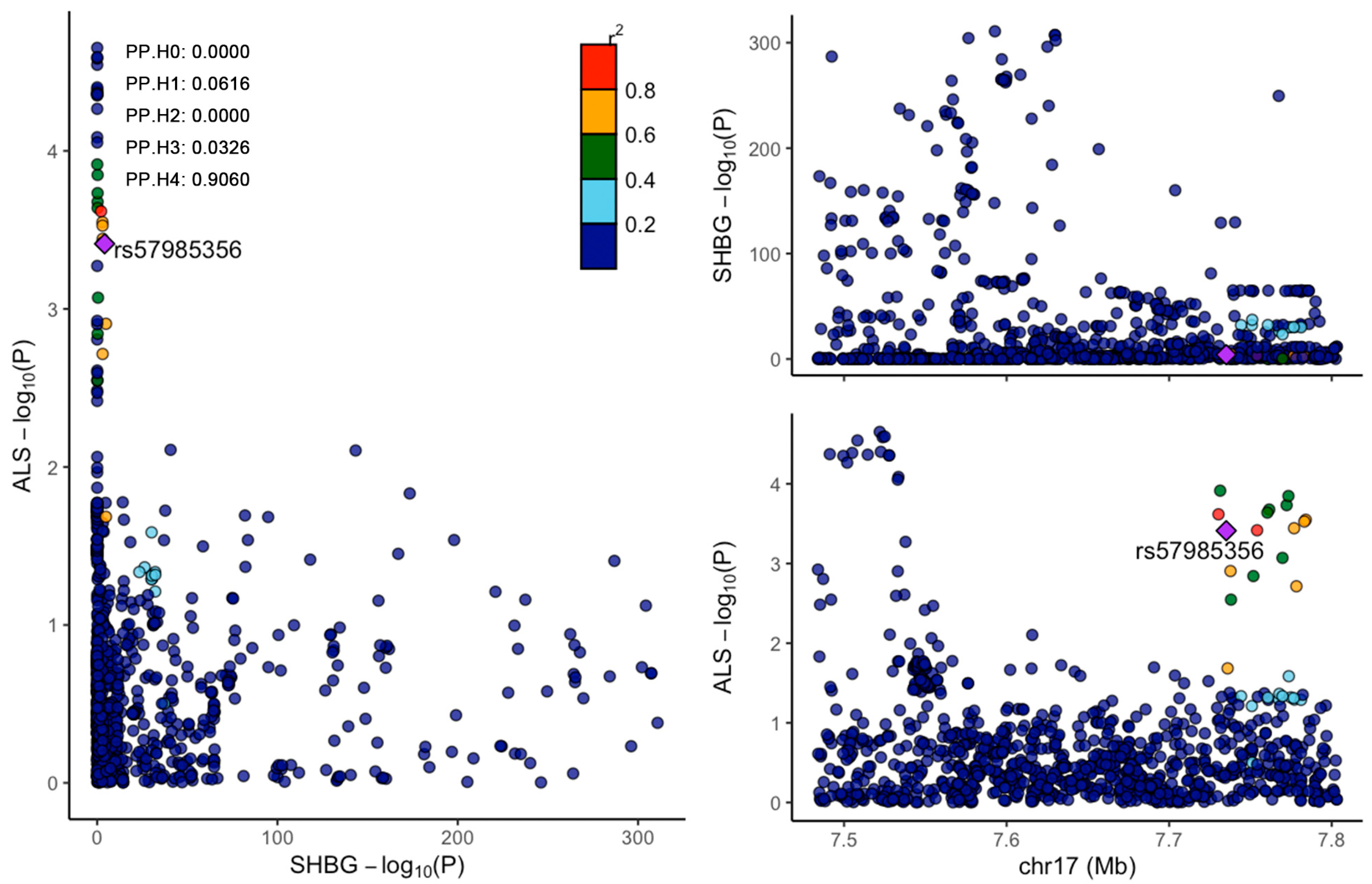
2.6. Colocalization Analysis
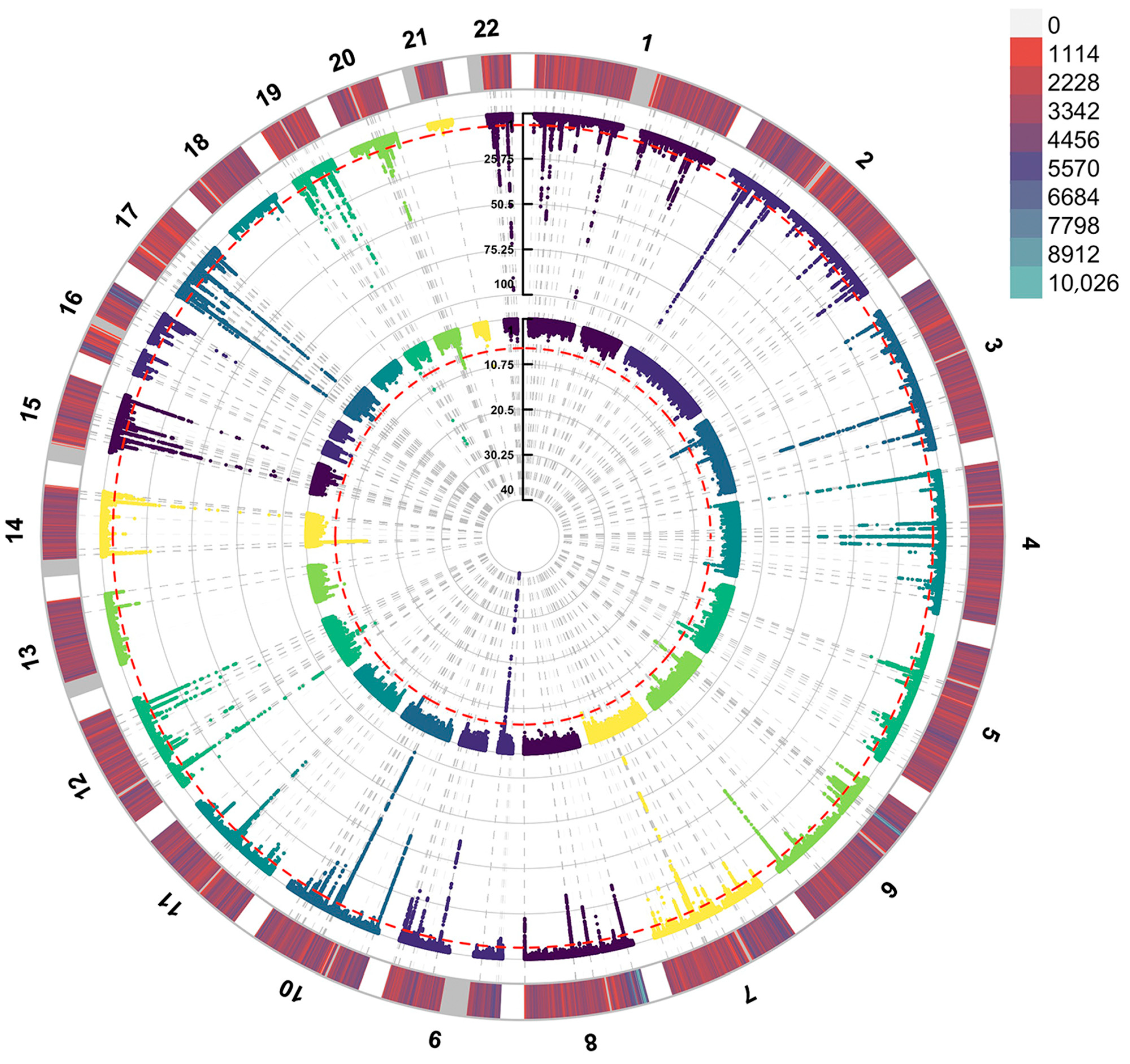
2.7. Manhattan Plot
3. Results
3.1. MR Analysis Between Proteomics and ALS
3.2. Colocalization Analysis of SHBG and ALS
3.3. MR Analysis Between SHBG and Bioavailable Testosterone in Males and Females
3.4. MR Analysis Between Bioavailable Testosterone and ALS in Males and Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzeplaeff, L.; Wilfling, S.; Requardt, M.V.; Herdick, M. Current State and Future Directions in the Therapy of ALS. Cells 2023, 12, 1523. [Google Scholar] [CrossRef]
- Ilieva, H.; Vullaganti, M.; Kwan, J. Advances in molecular pathology, diagnosis, and treatment of amyotrophic lateral sclerosis. BMJ 2023, 383, e075037. [Google Scholar] [CrossRef]
- Khamaysa, M.; Pradat, P.F. Status of ALS Treatment, Insights into Therapeutic Challenges and Dilemmas. J. Pers. Med. 2022, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Haberland, V.; Baird, D.; Walker, V.; Haycock, P.C.; Hurle, M.R.; Gutteridge, A.; Erola, P.; Liu, Y.; Luo, S.; et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat. Genet. 2020, 52, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Birney, E. Mendelian Randomization. Cold Spring Harb. Perspect. Med. 2022, 12, a041302. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, J.; Parker, B.L. Proteome-wide Systems Genetics to Identify Functional Regulators of Complex Traits. Cell Syst. 2021, 12, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef]
- Ou, Y.N.; Yang, L.; Wu, B.S.; Tan, L.; Yu, J.T. Causal effects of serum sex hormone binding protein levels on the risk of amyotrophic lateral sclerosis: A mendelian randomization study. Ann. Transl. Med. 2022, 10, 1054. [Google Scholar] [CrossRef]
- Ferkingstad, E.; Sulem, P.; Atlason, B.A.; Sveinbjornsson, G.; Magnusson, M.I.; Styrmisdottir, E.L.; Gunnarsdottir, K.; Helgason, A.; Oddsson, A.; Halldorsson, B.V.; et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 2021, 53, 1712–1721. [Google Scholar] [CrossRef]
- Nounu, A.; Kar, S.P.; Relton, C.L.; Richmond, R.C. Sex steroid hormones and risk of breast cancer: A two-sample Mendelian randomization study. Breast Cancer Res. 2022, 24, 66. [Google Scholar] [CrossRef]
- Ruth, K.S.; Day, F.R.; Tyrrell, J.; Thompson, D.J.; Wood, A.R.; Mahajan, A.; Beaumont, R.N.; Wittemans, L.; Martin, S.; Busch, A.S.; et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020, 26, 252–258. [Google Scholar] [CrossRef]
- van Rheenen, W.; van der Spek, R.A.A.; Bakker, M.K.; van Vugt, J.; Hop, P.J.; Zwamborn, R.A.J.; de Klein, N.; Westra, H.J.; Bakker, O.B.; Deelen, P.; et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat. Genet. 2021, 53, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Verdonck, L.; Kaufman, J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clin. Endocrinol. Metab. 1999, 84, 3666–3672. [Google Scholar] [CrossRef]
- Chung, M.C.; Gombar, S.; Shi, R.Z. Implementation of Automated Calculation of Free and Bioavailable Testosterone in Epic Beaker Laboratory Information System. J. Pathol. Inform. 2017, 8, 28. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Lawlor, D.A. Commentary: Two-sample Mendelian randomization: Opportunities and challenges. Int. J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Giambartolomei, C.; Vukcevic, D.; Schadt, E.E.; Franke, L.; Hingorani, A.D.; Wallace, C.; Plagnol, V. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014, 10, e1004383. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 2020, 16, e1008720. [Google Scholar] [CrossRef]
- Wallace, C. A more accurate method for colocalisation analysis allowing for multiple causal variants. PLoS Genet. 2021, 17, e1009440. [Google Scholar] [CrossRef]
- Liu, B.; Gloudemans, M.J.; Rao, A.S.; Ingelsson, E.; Montgomery, S.B. Abundant associations with gene expression complicate GWAS follow-up. Nat. Genet. 2019, 51, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Sex hormone binding globulin and incident Alzheimer’s disease in elderly men and women. Neurobiol. Aging 2010, 31, 1758–1765. [Google Scholar] [CrossRef]
- Xu, W.; Su, B.J.; Shen, X.N.; Bi, Y.L.; Tan, C.C.; Li, J.Q.; Cao, X.P.; Dong, Q.; Tan, L.; Alzheimer’s Disease Neuroimaging, I.; et al. Plasma sex hormone-binding globulin predicts neurodegeneration and clinical progression in prodromal Alzheimer’s disease. Aging 2020, 12, 14528–14541. [Google Scholar] [CrossRef] [PubMed]
- Nitkowska, M.; Tomasiuk, R.; Czyzyk, M.; Friedman, A. Prolactin and sex hormones levels in males with Parkinson’s disease. Acta Neurol. Scand. 2015, 131, 411–416. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Rizzi, L.; Bresciani, E.; Omeljaniuk, R.J.; Torsello, A. Androgen Therapy in Neurodegenerative Diseases. J. Endocr. Soc. 2020, 4, bvaa120. [Google Scholar] [CrossRef]
- Carteri, R.B.; Kopczynski, A.; Rodolphi, M.S.; Strogulski, N.R.; Sartor, M.; Feldmann, M.; De Bastiani, M.A.; Duval Wannmacher, C.M.; de Franceschi, I.D.; Hansel, G.; et al. Testosterone Administration after Traumatic Brain Injury Reduces Mitochondrial Dysfunction and Neurodegeneration. J. Neurotrauma 2019, 36, 2246–2259. [Google Scholar] [CrossRef]
- Gurer, B.; Kertmen, H.; Kasim, E.; Yilmaz, E.R.; Kanat, B.H.; Sargon, M.F.; Arikok, A.T.; Erguder, B.I.; Sekerci, Z. Neuroprotective effects of testosterone on ischemia/reperfusion injury of the rabbit spinal cord. Injury 2015, 46, 240–248. [Google Scholar] [CrossRef]
- Breza, M.; Koutsis, G. Kennedy’s disease (spinal and bulbar muscular atrophy): A clinically oriented review of a rare disease. J. Neurol. 2019, 266, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Stipa, G.; Ancidoni, A.; Vanacore, N.; Bellomo, G. Raw Water and ALS: A Unifying Hypothesis for the Environmental Agents Involved in ALS. Ann. Neurosci. 2023, 30, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Mehta, P.; Larson, T.; Pioro, E.P.; Horton, D.K. Reproductive History and Age of Onset for Women Diagnosed with Amyotrophic Lateral Sclerosis: Data from the National ALS Registry: 2010–2018. Neuroepidemiology 2021, 55, 416–424. [Google Scholar] [CrossRef]
- Militello, A.; Vitello, G.; Lunetta, C.; Toscano, A.; Maiorana, G.; Piccoli, T.; La Bella, V. The serum level of free testosterone is reduced in amyotrophic lateral sclerosis. J. Neurol. Sci. 2002, 195, 67–70. [Google Scholar] [CrossRef]
- Sawal, N.; Kaur, J.; Kaur, K.; Gombar, S. Dihydrotestosterone in Amyotrophic lateral sclerosis-The missing link? Brain Behav. 2020, 10, e01645. [Google Scholar] [CrossRef] [PubMed]
- Vivekananda, U.; Manjalay, Z.R.; Ganesalingam, J.; Simms, J.; Shaw, C.E.; Leigh, P.N.; Turner, M.R.; Al-Chalabi, A. Low index-to-ring finger length ratio in sporadic ALS supports prenatally defined motor neuronal vulnerability. J. Neurol. Neurosurg. Psychiatry 2011, 82, 635–637. [Google Scholar] [CrossRef]
- Pfeiffer, R.M.; Mayer, B.; Kuncl, R.W.; Check, D.P.; Cahoon, E.K.; Rivera, D.R.; Freedman, D.M. Identifying potential targets for prevention and treatment of amyotrophic lateral sclerosis based on a screen of medicare prescription drugs. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 235–245. [Google Scholar] [CrossRef]
- Handa, R.J.; Stadelman, H.L.; Resko, J.A. Effect of estrogen on androgen receptor dynamics in female rat pituitary. Endocrinology 1987, 121, 84–89. [Google Scholar] [CrossRef]
- Altintas, U.B.; Seo, J.H.; Giambartolomei, C.; Ozturan, D.; Fortunato, B.J.; Nelson, G.M.; Goldman, S.R.; Adelman, K.; Hach, F.; Freedman, M.L.; et al. Decoding the epigenetics and chromatin loop dynamics of androgen receptor-mediated transcription. Nat. Commun. 2024, 15, 9494. [Google Scholar] [CrossRef]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 2014, 35, 42–57. [Google Scholar] [CrossRef]
- Lara, A.; Esperante, I.; Meyer, M.; Liere, P.; Di Giorgio, N.; Schumacher, M.; Guennoun, R.; Gargiulo-Monachelli, G.; De Nicola, A.F.; Gonzalez Deniselle, M.C. Neuroprotective Effects of Testosterone in Male Wobbler Mouse, a Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2021, 58, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- McLeod, V.M.; Lau, C.L.; Chiam, M.D.F.; Rupasinghe, T.W.; Roessner, U.; Djouma, E.; Boon, W.C.; Turner, B.J. Androgen receptor antagonism accelerates disease onset in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2019, 176, 2111–2130. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, T.; Polanco, M.J.; Scaramuzzino, C.; Rocchi, A.; Milioto, C.; Emionite, L.; Ognio, E.; Sambataro, F.; Galbiati, M.; Poletti, A.; et al. Androgens affect muscle, motor neuron, and survival in a mouse model of SOD1-related amyotrophic lateral sclerosis. Neurobiol. Aging 2014, 35, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Tyagi, R.K.; Song, C.S.; Lavrovsky, Y.; Ahn, S.C.; Oh, T.S.; Chatterjee, B. Androgen receptor: Structural domains and functional dynamics after ligand-receptor interaction. Ann. N. Y. Acad. Sci. 2001, 949, 44–57. [Google Scholar] [CrossRef]
- Pham, J.; Keon, M.; Brennan, S.; Saksena, N. Connecting RNA-Modifying Similarities of TDP-43, FUS, and SOD1 with MicroRNA Dysregulation Amidst A Renewed Network Perspective of Amyotrophic Lateral Sclerosis Proteinopathy. Int. J. Mol. Sci. 2020, 21, 3464. [Google Scholar] [CrossRef]
- Daigle, J.G.; Lanson, N.A., Jr.; Smith, R.B.; Casci, I.; Maltare, A.; Monaghan, J.; Nichols, C.D.; Kryndushkin, D.; Shewmaker, F.; Pandey, U.B. RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations. Hum. Mol. Genet. 2013, 22, 1193–1205. [Google Scholar] [CrossRef]
- Hallegger, M.; Chakrabarti, A.M.; Lee, F.C.Y.; Lee, B.L.; Amalietti, A.G.; Odeh, H.M.; Copley, K.E.; Rubien, J.D.; Portz, B.; Kuret, K.; et al. TDP-43 condensation properties specify its RNA-binding and regulatory repertoire. Cell 2021, 184, 4680–4696 e4622. [Google Scholar] [CrossRef]


| Trait | Accession Number | Population | Sample Size | Year | Reference |
|---|---|---|---|---|---|
| SHBG levels in females | ieu-b-4870 | European | 214,989 | 2022 | Rebecca Richmond [10] |
| SHBG levels in males | sieu-b-4871 | European | 185,221 | 2022 | Rebecca Richmond [10] |
| Bioavailable testosterone levels in females | ebi-a-GCST90012102 | European | 188,507 | 2020 | Ruth KS [11] |
| Bioavailable testosterone levels in males | ebi-a-GCST90012103 | European | 178,782 | 2020 | Ruth KS [11] |
| ALS | ebi-a-GCST90027163 | European | 138,086 | 2021 | van Rheenen W [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Xiao, W.; Liu, X.; Li, H.; Huang, T.; Fan, D. Testosterone Supplementation: A Potential Therapeutic Strategy for Amyotrophic Lateral Sclerosis. Biomedicines 2025, 13, 622. https://doi.org/10.3390/biomedicines13030622
Yang W, Xiao W, Liu X, Li H, Huang T, Fan D. Testosterone Supplementation: A Potential Therapeutic Strategy for Amyotrophic Lateral Sclerosis. Biomedicines. 2025; 13(3):622. https://doi.org/10.3390/biomedicines13030622
Chicago/Turabian StyleYang, Wenzhi, Wendi Xiao, Xiangyi Liu, Hui Li, Tao Huang, and Dongsheng Fan. 2025. "Testosterone Supplementation: A Potential Therapeutic Strategy for Amyotrophic Lateral Sclerosis" Biomedicines 13, no. 3: 622. https://doi.org/10.3390/biomedicines13030622
APA StyleYang, W., Xiao, W., Liu, X., Li, H., Huang, T., & Fan, D. (2025). Testosterone Supplementation: A Potential Therapeutic Strategy for Amyotrophic Lateral Sclerosis. Biomedicines, 13(3), 622. https://doi.org/10.3390/biomedicines13030622








