The Association of Toll-like Receptor-9 Gene Single-Nucleotide Polymorphism and AK155(IL-26) Serum Levels with Chronic Obstructive Pulmonary Disease Exacerbation Risk: A Case-Controlled Study with Bioinformatics Analysis
Abstract
1. Introduction
1.1. Problem
1.2. Study Aim
2. Subjects and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Study Participants
2.4. Blood Sample Collection
2.4.1. Molecular Analysis
DNA Extraction and Genotyping
Assessment of Genomic DNA Concentration and Purity
TLR9 SNP TaqMan Genotyping Assay
Quantification of Serum AK155(IL-26)
2.5. In Silico Database(s) Search and Bioinformatics Analysis
2.5.1. In Silico Identification of Immune Cells
2.5.2. PICKLE (Protein InteraCtion KnowLedgebasE)
2.5.3. Gene–Gene Interactions and Pathways by Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Study Participants’ Clinico-Pathological and Demographic Details
3.2. COPD and Control Groups TLR9 rs5743836
3.3. TLR9 rs5743836 and Disease Severity
3.4. Association Between TLR9 rs5743836 Genotypes and Different Patient Clinico-Pathological Parameters (Table 3)
| (A) | ||||
|---|---|---|---|---|
| TLR9 rs5743836 in Exacerbated COPD Patients (n = 48) | Test Value | |||
| TT (n = 11) | TC (n = 27) | CC (n = 10) | ||
| Sex | ||||
| Female | 1 (9.1%) | 10 (37.0%) | 1 (10.0%) | 4.771 |
| Male | 10 (90.9%) | 17 (63.0%) | 9 (90.0%) | |
| Age (years) | 50.27 ± 5.31 | 51.74 ± 10.04 | 49.90 ± 10.27 | 1.059 |
| SI | ||||
| Mild | 0 (0.0%) | 1 (3.7%) | 1 (10.0%) | 2.897 |
| Moderate | 0 (0.0%) | 2 (7.4%) | 1 (10.0%) | |
| Severe | 10 (90.9%) | 20 (74.1%) | 7 (70.0%) | |
| Passive | 1 (9.1%) | 4 (14.8%) | 1 (10.0%) | |
| BMI [kg/m2] | 28.00 ± 5.7C | 33.11 ± 2.9B | 36.40 ± 0.8A | 15.859 * |
| PH | 7.37 ± 0.04 | 7.37 ± 0.05 | 7.36 ± 0.05 | 0.358 |
| PCO2 (mmHg) | 43.45 ± 9.63 | 45.52 ± 9.28 | 47.20 ± 11.18 | 0.390 |
| PO2 (mmHg) | 82.36 ± 8.48 | 82.74 ± 8.53 | 82.10 ± 10.18 | 0.021 |
| HCO3 (mmol/L) | 24.96 ± 1.59 | 25.07 ± 1.52 | 25.40 ± 1.78 | 0.220 |
| S O2% | 90.36 ± 2.01 | 90.27 ± 2.58 | 89.60 ± 2.22 | 0.342 |
| FEV1% | 37.82 ± 5.4A | 31.37 ± 4.7B | 23.602.4C | 26.261 * |
| FVC% | 49.45 ± 8.20 | 47.37 ± 7.18 | 50.80 ± 11.01 | 0.704 |
| FEV1/FVC | 67.94 ± 2.3A | 64.07 ± 4.0A | 59.39 ± 4.8B | 12.710 * |
| VC% | 51.82 ± 7.31 | 50.81 ± 6.40 | 52.90 ± 10.58 | 0.288 |
| FEF% | 41.18 ± 11.08 | 40.04 ± 10.86 | 42.60 ± 6.26 | 0.240 |
| WBCs (103/mm3) | 10.08 ± 5.12 | 10.27 ± 5.04 | 10.38 ± 5.42 | 0.009 |
| RBCs (103/mm3) | 5.23 ± 0.38 | 5.14 ± 0.46 | 5.00 ± 0.58 | 0.638 |
| Hb (gm/dL) | 15.34 ± 1.57 | 14.84 ± 1.56 | 14.87 ± 2.02 | 0.364 |
| HCT% | 47.98 ± 4.59 | 46.25 ± 4.93 | 44.80 ± 5.61 | 1.070 |
| MCV (µm3) | 88.52 ± 3.05 | 87.95 ± 3.20 | 87.93 ± 2.57 | 0.150 |
| MCHC (gm/dL) | 32.15 ± 1.88 | 32.67 ± 1.76 | 32.70 ± 1.06 | 0.432 |
| PLT (103/mm3) | 305.82 ± 60.81 | 308.81 ± 48.12 | 314.10 ± 43.54 | 0.073 |
| CRP (gm/L) | 8.54 ± 2.23C | 10.98 ± 2.05B | 17.19 ± 4.86A | 25.754 * |
| AK155(IL-26) (pg/mL) | 31.09 ± 5.68C | 42.11 ± 4.40B | 49.60 ± 1.58A | 49.141 * |
| (B) | ||||
| TLR9 rs5743836 in Stable COPD Patients (n = 48) | Test Value | |||
| TT (n = 11) | TC (n = 27) | CC (n = 10) | ||
| Sex | ||||
| Female | 2 (10.0%) | 5 (21.7%) | 1 (20.0%) | 1.106 |
| Male | 18 (90.0%) | 18 (78.3%) | 4 (80.0%) | |
| Age (years) | 50.55 ± 11.02 | 51.78 ± 7.32 | 50.40 ± 10.57 | 0.397 |
| SI | ||||
| Mild | 2 (10.0%) | 3 (13.0%) | 1 (20.0%) | 7.605 |
| Moderate | 5 (25.0%) | 3 (13.0%) | 1 (20.0%) | |
| Severe | 9 (45.0%) | 17 (73.9%) | 2 (40.0%) | |
| Passive | 4 (20.0%) | 0 (0.0%) | 1 (20.0%) | |
| BMI [kg/m2] | 20.10 ± 1.1C | 27.17 ± 3.7B | 33.80 ± 2.7A | 60.664 * |
| PH | 7.42 ± 0.03 | 7.41 ± 0.03 | 7.41 ± 0.02 | 0.244 |
| PCO2 (mmHg) | 36.99 ± 1.25 | 37.64 ± 2.08 | 37.94 ± 3.41 | 0.814 |
| PO2 (mmHg) | 82.10 ± 9.35 | 83.26 ± 7.31 | 80.60 ± 13.72 | 0.217 |
| HCO3 (mmol/L) | 24.09 ± 1.53 | 24.22 ± 0.77 | 23.84 ± 1.41 | 0.218 |
| S O2% | 93.10 ± 2.98 | 93.90 ± 2.40 | 92.18 ± 1.33 | 1.128 |
| FEV1% | 59.60 ± 8.4A | 40.74 ± 7.1B | 31.00 ± 6.8C | 45.911 * |
| FVC% | 60.93 ± 5.47 | 59.39 ± 12.84 | 59.68 ± 6.05 | 0.135 |
| FEV1/FVC | 63.15 ± 8.5A | 43.78 ± 6.3B | 37.00 ± 3.4C | 50.515 * |
| VC% | 24.50 ± 7.78 | 31.09 ± 10.60 | 31.00 ± 11.34 | 2.739 |
| FEF% | 30.75 ± 8.87 | 26.62 ± 8.10 | 32.33 ± 17.21 | 0.876 |
| WBCs (103/mm3) | 6.05 ± 1.13 | 6.65 ± 1.09 | 6.44 ± 1.21 | 1.514 |
| RBCs (103/mm3) | 5.14 ± 0.37 | 5.25 ± 0.35 | 5.18 ± 0.26 | 0.604 |
| Hb (gm/dL) | 13.81 ± 1.21 | 13.98 ± 0.78 | 14.98 ± 0.47 | 2.983 |
| HCT% | 44.83 ± 3.00 | 46.35 ± 3.16 | 45.36 ± 2.29 | 1.369 |
| MCV (µm3) | 88.21 ± 3.23 | 87.53 ± 6.39 | 85.56 ± 2.94 | 0.562 |
| MCHC (gm/dL) | 33.47 ± 1.42 | 32.63 ± 1.32 | 32.82 ± 1.36 | 2.022 |
| PLT (103/mm3) | 292.20 ± 63.13 | 266.00 ± 61.94 | 264.60 ± 48.17 | 1.085 |
| CRP (gm/L) | 3.57 ± 0.47 | 6.27 ± 1.52 | 8.56 ± 0.57 | 53.957 * |
| AK155(IL-26) (pg/mL) | 15.21 ± 2.42 | 25.22 ± 3.69 | 32.40 ± 1.52 | 90.995 * |
3.5. Serum Levels of AK155(IL-26) in COPD Patients, Exacerbated, Stable Groups and Its Correlation with Different Clinical Characteristics
3.6. Diagnostic/Prognostic Utility of AK155(IL-26)
3.7. In Silico Data Processing and Bioinformatics Analysis Accessed 5 November 2024
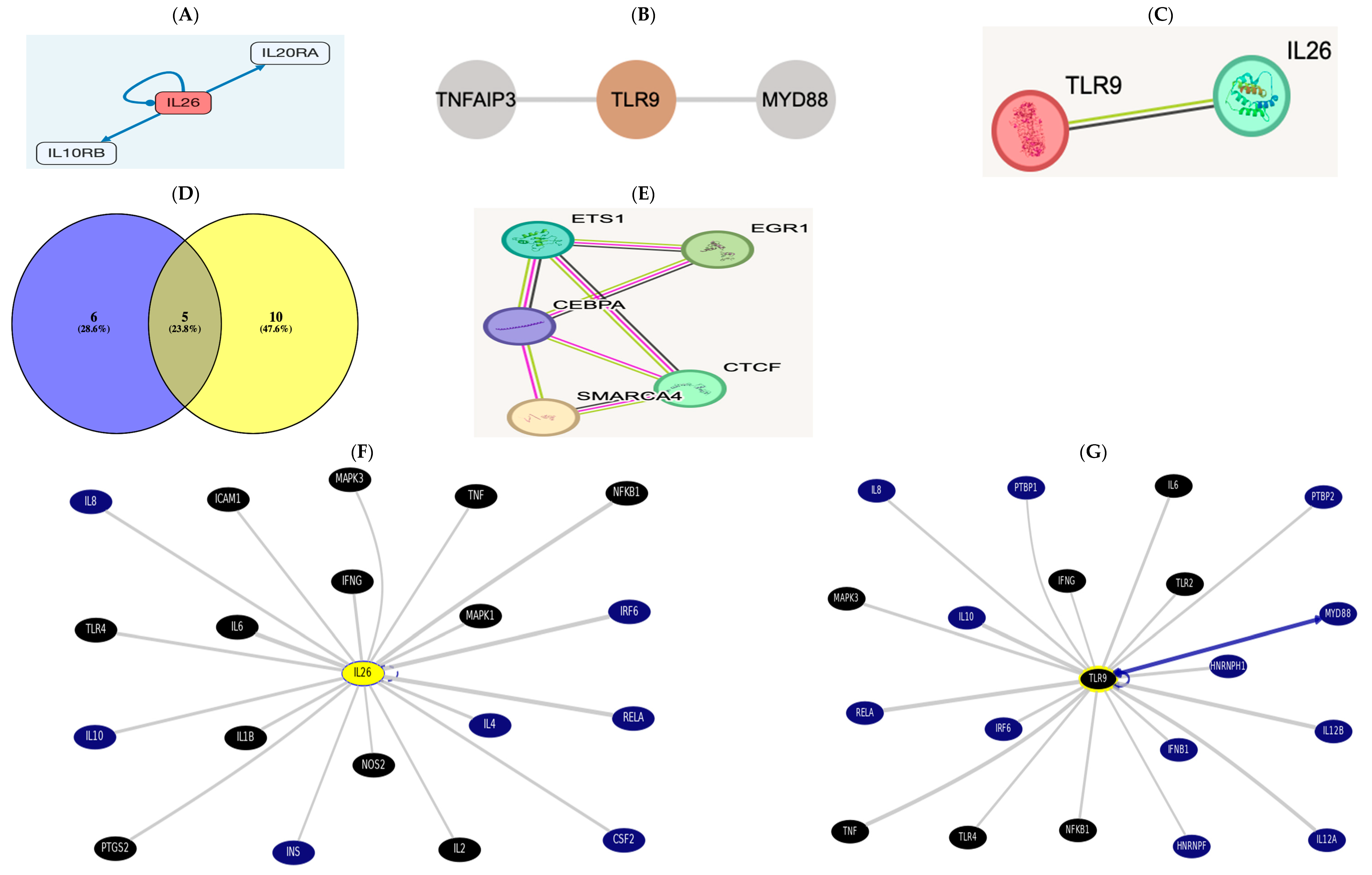
3.7.1. Immune Cell Infiltration Functional Enrichment Analysis
3.7.2. Gene-to-Protein, Gene-to-Gene, or Protein-to-Protein Interaction (PPI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AK155(IL-26) | Interleukin-26. |
| BMI | Body mass index. |
| CD | Cluster of Differentiation. |
| COPD | Chronic obstructive pulmonary disease. |
| CpG | Cytosine-phosphate guanine. |
| CRP | C-Reactive Protein. |
| ECOPD | Exacerbated chronic obstructive pulmonary disease. |
| FEF% | Forced Expiratory Flow Rate. |
| FEV1/FVC | Forced Expiratory Volume in the first second of expiration/Forced Vital Capacity. |
| FEV1% | Forced Expiratory Volume in the first second of expiration. |
| FVC% | Forced Vital Capacity. |
| GWAS | Genome-Wide Association Studies. |
| Hb | Hemoglobin. |
| HCO3 | Bicarbonate. |
| HCT | Hematocrit. |
| JAK | Janus kinase. |
| JAK-STAT | Janus Kinase Signal Transducer Activator of Transcription. |
| MCHC | Mean Corpuscular Hemoglobin Concentration. |
| MCV | Mean Corpuscular Volume. |
| MyD88 | Myeloid differentiation primary response gene 88. |
| NF-kB | Nuclear factor Kappa Beta. |
| NK | Natural Killer. |
| PCO2 | Partial Pressure of CO2. |
| PDCs | Plasmacytoid dendritic cells. |
| PLT | Platelets. |
| PO2 | Partial Pressure of O2. |
| RBCs | Red Blood Cells. |
| RT-PCR | Reverse Transcriptase-Polymerase Chain Reaction. |
| SI | Smoking index. |
| S O2 | O2 saturation. |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-Corona Virus-2. |
| SNP | Single-Nucleotide Polymorphism. |
| Th-17 | T helper-17. |
| TLR9 | Toll Like Receptor-9. |
| TyK | Tyrosine Kinase. |
| VC% | Vital Capacity. |
References
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Sidletskaya, K.; Vitkina, T.; Denisenko, Y. The role of toll-like receptors 2 and 4 in the pathogenesis of chronic obstructive pulmonary disease. Int. J. Chron. Obstr. Pulm. Dis. 2020, 15, 1481–1493. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B.; Martínez-Espinosa, I.; Pérez-Padilla, R. Mechanisms of Lung Damage and Development of COPD Due to Household Biomass-Smoke Exposure: Inflammation, Oxidative Stress, MicroRNAs, and Gene Polymorphisms. Cells 2022, 12, 67. [Google Scholar] [CrossRef]
- Speletas, M.; Merentiti, V.; Kostikas, K.; Liadaki, K.; Minas, M.; Gourgoulianis, K.; Germenis, A.E. Association of TLR4-T399I polymorphism with chronic obstructive pulmonary disease in smokers. Clin. Dev. Immunol. 2009, 2009, 260286. [Google Scholar] [CrossRef]
- Pomerenke, A.; Lea, S.R.; Herrick, S.; Lindsay, M.A.; Singh, D. Characterization of TLR-induced inflammatory responses in COPD and control lung tissue explants. Int. J. Chron. Obstr. Pulm. Dis. 2016, 11, 2409–2417. [Google Scholar] [CrossRef]
- El Mesallamy, H.O.; Rashed, W.M.; Hamdy, N.M.; Hamdy, N. High-dose methotrexate in Egyptian pediatric acute lymphoblastic leukemia: The impact of ABCG2 C421A genetic polymorphism on plasma levels, what is next? J. Cancer Res. Clin. Oncol. 2014, 140, 1359–1365. [Google Scholar] [CrossRef]
- Ding, X.; Lin, Q.; Zhao, J.; Fu, Y.; Zheng, Y.; Mo, R.; Zhang, L.; Zhang, B.; Chen, J.; Xie, T.; et al. Synonymous mutations in TLR2 and TLR9 genes decrease COPD susceptibility in the Chinese Han population. Pulmonology 2022, 26, 46. [Google Scholar] [CrossRef]
- Youssef, S.S.; Hamdy, N.M. SOCS1 and pattern recognition receptors: TLR9 and RIG-I; novel haplotype associations in Egyptian fibrotic/cirrhotic patients with HCV genotype 4. Arch. Virol. 2017, 162, 3347–3354. [Google Scholar] [CrossRef]
- Hansbro, P.M.; Haw, T.J.; Starkey, M.R.; Miyake, K. Toll-like receptors in COPD. Eur. Respir. J. 2017, 49, 1700739. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Liang, J.; Wang, J.; Feng, S.; Zhang, G. Association between toll-like receptors 9 (TLR9) gene polymorphism and risk of pulmonary tuberculosis: Meta-analysis. BMC Pulm. Med. 2015, 15, 57. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Mollaki, V.; Georgiadis, T.; Tassidou, A.; Ioannou, M.; Daniil, Z.; Koutsokera, A.; A Papathanassiou, A.; Zintzaras, E.; Vassilopoulos, G. Polymorphisms and haplotypes in TLR9 and MYD88 are associated with the development of Hodgkin’s lymphoma: A candidate-gene association study. J. Hum. Genet. 2009, 54, 655–659. [Google Scholar] [CrossRef]
- Velasco, W.V.; Khosravi, N.; Castro-Pando, S.; Torres-Garza, N.; Grimaldo, M.T.; Krishna, A.; Clowers, M.J.; Umer, M.; Tariq Amir, S.; Del Bosque, D.; et al. Toll-like receptors 2, 4, and 9 modulate promoting effect of COPD-like airway inflammation on K-ras- driven lung cancer through activation of the MyD88/NF-ĸB pathway in the airway epithelium. Front. Immunol. 2023, 14, 1118721. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, Y.; Feng, J. Association of toll-like receptors polymorphisms with COPD risk in Chinese population. Front. Genet. 2022, 13, 955810. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Kruzel, R.L.; Wrona, C.T.; Mammen, M.J.; Sethi, S. Impaired Innate COPD Alveolar Macrophage Responses and Toll-Like Receptor-9 Polymorphisms. PLoS ONE 2015, 10, e0134209. [Google Scholar] [CrossRef]
- Hamdy, N.M. Relationship between pro-anti-inflammatory cytokines, T-cell activation and CA 125 in obese patients with heart failure. Med. Sci. Monit. 2011, 17, CR174–CR179. [Google Scholar] [CrossRef]
- Lin, Y.; Sang, L.; Wang, J.; Chen, Y.; Lai, J.; Zhu, X.; Yang, Y.; Zhang, Z.; Liu, Y.; Wen, S.; et al. Analysis of Airway Thickening and Serum Cytokines in COPD Patients with Frequent Exacerbations: A Heart of the Matter. Int. J. Chron. Obstr. Pulm. Dis. 2023, 18, 2353–2364. [Google Scholar] [CrossRef]
- Ali, N.A.; Hamdy, N.M.; Gibriel, A.A.; El Mesallamy, H.O. Investigation of the relationship between CTLA4 and the tumor suppressor RASSF1A and the possible mediating role of STAT4 in a cohort of Egyptian patients infected with hepatitis C virus with and without hepatocellular carcinoma. Arch. Virol. 2021, 166, 1643–1651. [Google Scholar] [CrossRef]
- Bartziokas, K.; Fouka, E.; Loukides, S.; Steiropoulos, P.; Bakakos, P.; Papaioannou, A.I. AK155(IL-26) in the Lung and Its Role in COPD Inflammation. J. Pers. Med. 2022, 12, 1685. [Google Scholar] [CrossRef]
- Yew-Booth, L.; Birrell, M.A.; Lau, M.S.; Baker, K.; Jones, V.; Kilty, I.; Belvisi, M.G. JAK- STAT pathway activation in COPD. Eur. Respir. J. 2015, 46, 843–845. [Google Scholar] [CrossRef]
- Cardenas, E.I.; Robertson, J.; Misaghian, S.; Brown, J.; Wang, M.; Stengelin, M.; Sigal, G.; Wohlstadter, J.; Gisslén, M.; Lindén, A. Systemic increase in IL-26 is associated with severe COVID-19 and comorbid obstructive lung disease. Front. Immunol. 2024, 15, 1434186. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, S.; Che, K.F.; Linden, A. Interleukin-26: An emerging player in host defense and inflammation. J. Innate Immun. 2016, 8, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.L.; Khader, S.A. Bringing in the cavalry: IL-26 mediates neutrophil recruitment to the lungs. Am. J. Respir. Crit. Care Med. 2014, 190, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Che, K.F.; Bozinovski, S.; Ji, J.; Gregory, J.A.; Kumlien Georén, S.; Adner, M.; Cardell, L.O.; Linden, A. Recombinant human IL-26 facilitates the innate immune response to endotoxin in the bronchoalveolar space of mice in vivo. PLoS ONE 2017, 12, e0188909. [Google Scholar] [CrossRef]
- Che, K.F.; Tufvesson, E.; Tengvall, S.; Lappi-Blanco, E.; Kaarteenaho, R.; Levänen, B.; Ekberg, M.; Brauner, A.; Wheelock, Å.M.; Bjermer, L.; et al. The neutrophil-mobilizing cytokine interleukin-26 in the airways of long-term tobacco smokers. Clin. Sci. 2018, 132, 959–983. [Google Scholar] [CrossRef]
- Cardenas, E.I.; Che, K.F.; Konradsen, J.R.; Bao, A.; Lindén, A. AK155(IL-26) in asthma and COPD. Expert. Rev. Respir. Med. 2022, 16, 293–301. [Google Scholar] [CrossRef]
- Badway, M.S.; Hamed, A.F.; Yousef, F.M. Prevalence of chronic obstructive pulmonary disease (COPD) in Qena Governorate. Egypt. J. Chest Dis. Tuberc. 2016, 65, 29–34. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2024 report: GOLD executive summary. Eur. Respir. J. 2024, 49, 1700214. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Csabai, L.; Fazekas, D.; Kadlecsik, T.; Szalay-Bekő, M.; Bohár, B.; Madgwick, M.; Módos, D.; Ölbei, M.; Gul, L.; Sudhakar, P.; et al. SignaLink3: A multi-layered resource to uncover tissue-specific signaling networks. Nucleic Acids Res. 2021, 50, gkab909. [Google Scholar] [CrossRef]
- Oliveros, J.C.; Venny, C. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 5 February 2025).
- Xiong, X.F.; Zhu, M.; Wu, H.X.; Fan, L.L.; Cheng, D.Y. Immunophenotype in acute exacerbation of chronic obstructive pulmonary disease: A cross-sectional study. Respir. Res. 2022, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. How toll-like receptors signal: What we know and what we don’t know. Curr. Opin. Immunol. 2006, 18, 3–9. [Google Scholar] [CrossRef]
- Schneberger, D.; Caldwell, S.; Kanthan, R.; Singh, B. Expression of Toll-like receptor 9 in mouse and human lungs. J. Anat. 2013, 222, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Alhabeeb, Z.A.; AL-Hasnawi, A.T.; Huda Hadi Al-Hasnawy, H.H. Role of Toll Like Receptor-9 Gene Polymorphism among Patients with Exacerbation Chronic Obstructive Pulmonary Disease in Holy Karbala, Iraq. Indian J. Public Health Res. Dev. 2019, 10, 6. [Google Scholar] [CrossRef]
- Foronjy, R.F.; Salathe, M.A.; Dabo, A.J.; Baumlin, N.; Cummins, N.; Eden, E.; Geraghty, P. TLR9 expression is required for the development of cigarette smoke-induced emphysema in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L154–L166. [Google Scholar] [CrossRef]
- Noguchi, E.; Nishimura, F.; Fukai, H.; Kim, J.; Ichikawa, K.; Shibasaki, M.; Arinami, T. An association study of asthma and total serum immunoglobin E levels for Toll-like receptor polymorphisms in a Japanese population. Clin. Exp. Allergy 2004, 34, 177–183. [Google Scholar] [CrossRef]
- Carvalho, A.; Pasqualotto, A.C.; Pitzurra, L.; Romani, L.; Denning, D.W.; Rodrigues, F. Polymorphisms in toll- like receptor genes and susceptibility to pulmonary aspergillosis. J. Infect. Dis. 2008, 197, 618–621. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Lu, K.-C.; Lee, H.-S.; Huang, S.-M.; Lin, Y.-F.; Wu, C.-C.; Salter, D.M.; Su, S.-L. Role of the functional Toll-like receptor-9 promoter polymorphism (-T1237T/C) in increased risk of end-stage renal disease: A case control study. PLoS ONE 2013, 8, e58444. [Google Scholar]
- Ng, M.T.H.; Van’t Hof, R.; Crockett, J.C.; Hope, M.E.; Berry, S.; Thomson, J.; McLean, M.H.; McColl, K.E.; El-Omar, E.M.; Hold, G.L. Increase in NF-kB binding affinity of the variant C allele of the toll-like receptor 9 -1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect. Immun. 2010, 78, 1345–1352. [Google Scholar] [CrossRef]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Schurz, H.; Daya, M.; Möller, M.; Hoal, E.G.; Salie, M. TLR1, 2, 4, 6 and 9 variants associated with tuberculosis susceptibility: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0139711. [Google Scholar] [CrossRef] [PubMed]
- Nadigel, J.; Préfontaine, D.; Baglole, C.J.; Maltais, F.; Bourbeau, J.; Eidelman, D.H.; Hamid, Q. Cigarette smoke increases TLR4 and TLR9 expression and induces cytokine production from CD8(+) T cells in chronic obstructive pulmonary disease. Respir. Res. 2011, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Isajevs, S.; Taivans, I.; Svirina, D.; Strazda, G.; Kopeika, U. Patterns of inflammatory responses in large and small airways in smokers with and without chronic obstructive pulmonary disease. Respiration 2011, 81, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Fujii, M.; Tsukumo, S.; Maekawa, Y.; Kishihara, K.; Kimoto, Y.; Horiuchi, T.; Hisaeda, H.; Akira, S.; Kagami, S.; et al. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann. Rheum. Dis. 2007, 66, 905–909. [Google Scholar] [CrossRef]
- Elmaagacli, A.H.; Koldehoff, M.; Beelen, D.W. Improved outcome of hematopoietic SCT in patients with homozygous gene variant of Toll-like receptor 9. Bone Marrow Transplant. 2009, 44, 295–302. [Google Scholar] [CrossRef]
- Pabst, S.; Bradler, O.; Gillissen, A.; Nickenig, G.; Skowasch, D.; Grohe, C. Toll-like receptor-9 polymorphisms in sarcoidosis and chronic obstructive pulmonary disease. Adv. Exp. Med. Biol. 2013, 756, 239–245. [Google Scholar]
- Lazarus, R.; Klimecki, W.T.; Raby, B.A.; Vercelli, D.; Palmer, L.J.; Kwiatkowski, D.J.; Silverman, E.K.; Martinez, F.; Weiss, S.T. Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): Frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case-control disease association studies. Genomics 2003, 81, 85–91. [Google Scholar] [CrossRef]
- Lundgren, R.; Andelid, K.; Ezerskyte, M.; Pournaras, N.; Brundin, B.; Che, K.F.; Linden, A. Systemic interleukin-26 relates to the risk of exacerbations in smokers with COPD and chronic bronchitis. Eur. Respir. J. 2021, 58 (Suppl. 65), PA3829. [Google Scholar] [CrossRef]
- Savchenko, L.; Mykytiuk, M.; Cinato, M.; Tronchere, H.; Kunduzova, O.; Kaidashev, I. AK155(IL-26) in the induced sputum is associated with the level of systemic inflammation, lung functions and body weight in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2569–2575. [Google Scholar] [CrossRef]
- Zuo, L.; Lucas, K.; Fortuna, C.A.; Chuang, C.-C.; Best, T.M. Molecular Regulation of Toll-like Receptors in Asthma and COPD. Front. Physiol. 2015, 6, 312. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Phipps, J.C.; Aronoff, D.M.; Curtis, J.L.; Goel, D.; O’Brien, E. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect. Immun. 2010, 78, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Di Domizio, J.; Voo, K.S.; Friedrich, H.C.; Chamilos, G.; Ganguly, D.; Conrad, C.; Gregorio, J.; Le Roy, D.; Roger, T.; et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat. Immunol. 2015, 16, 970–979. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Metzke, D.; Guckelberger, O.; Pascher, A.; Grötzinger, C.; Przesdzing, I.; Dörffel, Y.; Schmitz, J.; Thomas, S. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn’s disease and ulcerative colitis. Clin. Exp. Immunol. 2011, 166, 46–54. [Google Scholar] [CrossRef]
- Mokhtar, E.R.; Elmadbouly, A.A.; Abo Elkheir, O.I.; Mansour, M.N.; El Attar, S.; Heiba, M.A.; Mohamed, M.N.; Elhakeem, H.; Gad, L.A.; Abdelrahman, H.M.; et al. Peripheral Blood B-Cell Subsets Frequency and Distribution and the BSF-2(IL-6) to CSIF:TGIF(IL-10) Ratio as Severity-Associated Signatures in Primary Open-Angle Glaucoma: A Case-Controlled Study. Biomedicines 2024, 12, 485. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, Y.F.; Lin, Y.J.; Huang, K.C.; Chen, H.Y.; Hamdy, N.M.; Huang, T.C.; Chang, H.Y.; Shieh, T.M.; Huang, Y.J.; et al. Effect of Vitamin D Supplementation on Primary Dysmenorrhea: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2023, 15, 2830. [Google Scholar] [CrossRef]


| Groups (n) | COPD Group (n) | |||||
|---|---|---|---|---|---|---|
| Parameters | COPD (96) | Control (48) | p-Value | Stable (n = 48) | Exacerbation (48) | p-Value |
| Sex | ||||||
| Female | 20 (20.8%) | 13 (27.1%) | NS | 8 (16.7%) | 12 (25.0%) | NS |
| Male | 76 (79.2%) | 35 (72.9%) | 40 (83.3%) | 36 (75.0%) | ||
| Age (years) | ||||||
| Mean ± SD | 50.68 ± 10.12 | 49.19 ± 7.80 | NS | 48.50 ± 9.24 | 51.85 ± 9.28 | NS |
| Range | 36–84 | 38–70 | 36–75 | 42–84 | ||
| Exacerbation Severity; n (%) | ||||||
| Mild/Moderate/Sever | – | – | – | (22,11,15) (45.8%, 22.9%, 31.3%) | (18/12/18) (37.5%/25%/37.5%) | – |
| Treatment; n (%) | ||||||
| Long-acting B2 agonist (LABA) | 22 (45.8%) | – | ||||
| LABA + long-acting muscarinic receptor antagonist (LAMA) + inhaled corticosteroids (ICS) + Azithromycin | 11 (22.9%) | – | ||||
| LABA + LAMA + ICS+ Roflumilast | 15 (31.25%) | – | ||||
| Short-acting B2 agonist (SABA) + short–acting muscarinic receptor antagonist (SAMA) + ICS+ Levofloxacin | – | 8 (16.7%) | ||||
| SABA + SAMA + ICS + Ceftriaxone | – | 10 (20.8%) | ||||
| SABA + SAMA + Oral Corticosteroids | – | 12 (25%) | ||||
| SABA + SAMA + Oral Oxygen | – | 9 (18.75) | ||||
| SABA + SAMA + IV Corticosteroids + Oral Oxygen + Non-invasive mechanical Ventilation (NIV) | – | 9 (18.75) | ||||
| Smoking index (SI) | ||||||
| Mild | 8 (8.3%) | 9 (18.8%) | 0.001 | 6 (12.5%) | 2 (4.2%) | NS |
| Moderate | 12 (12.5%) | 3 (6.3%) | 9 (18.8%) | 3 (6.3%) | ||
| Severe | 65 (67.7%) | 6 (12.5%) | 28 (58.3%) | 37 (77.1%) | ||
| Passive | 11 (11.5%) | 30 (62.5%) | 5 (10.4%) | 6 (12.5%) | ||
| BMI [kg/m2] | ||||||
| Mean ± SD | 28.77 ± 6.23 | 24.46 ± 1.76 | 0.001 | 24.92 ± 5.33 | 32.63 ± 4.45 | 0.001 |
| Range | 18–38 | 21–28 | 18–36 | 20–38 | ||
| PH | ||||||
| Mean ± SD | 7.39 ± 0.04 | 7.42 ± 0.02 | 0.001 | 7.41 ± 0.03 | 7.37 ± 0.05 | 0.001 |
| Range | 7.28–7.47 | 7.4–7.45 | 7.36–7.47 | 7.28–7.43 | ||
| PCO2 (mmHg) | ||||||
| Mean ± SD | 41.40 ± 8.00 | 38.92 ± 2.53 | 0.038 | 37.40 ± 1.95 | 45.40 ± 9.64 | 0.001 |
| Range | 33.7–68 | 35–43 | 33.7–43 | 36–68 | ||
| PO2 (mmHg) | ||||||
| Mean ± SD | 82.51 ± 8.69 | 87.06 ± 2.79 | 0.001 | 82.50 ± 8.78 | 82.52 ± 8.69 | NS |
| Range | 57–93 | 83–92 | 57–92 | 65–93 | ||
| HCO3 (mmol/L) | ||||||
| Mean ± SD | 24.62 ± 1.47 | 24.19 ± 0.81 | NS | 24.12 ± 1.19 | 25.12 ± 1.56 | 0.001 |
| Range | 21.9–29 | 23.1–25.5 | 21.9–26.9 | 22–29 | ||
| S O2% | ||||||
| Mean ± SD | 91.77 ± 2.96 | 97.23 ± 1.46 | 0.001 | 93.39 ± 2.60 | 90.15 ± 2.36 | 0.001 |
| Range | 86–98 | 95–99 | 89–98 | 86–95 | ||
| FEV1% | ||||||
| Mean ± SD | 39.41 ± 13.12 | 83.25 ± 2.84 | 0.001 | 47.58 ± 13.01 | 31.23 ± 6.47 | 0.001 |
| Range | 20–75 | 80–88 | 20–75 | 20–50 | ||
| FVC% | ||||||
| Mean ± SD | 54.31 ± 10.63 | 79.90 ± 1.64 | 0.001 | 60.06 ± 9.64 | 48.56 ± 8.25 | 0.001 |
| Range | 30–86.6 | 77–83 | 30–86.6 | 31–72 | ||
| FEV1/FVC | ||||||
| Mean ± SD | 57.56 ± 11.44 | 86.17 ± 3.22 | 0.001 | 51.14 ± 12.56 | 63.98 ± 4.75 | 0.001 |
| Range | 34–76 | 83–91 | 34–76 | 54–69.4 | ||
| VC% | ||||||
| Mean ± SD | 39.91 ± 14.56 | 86.19 ± 2.22 | 0.001 | 28.33 ± 6.94 | 51.48 ± 7.49 | 0.001 |
| Range | 14–75 | 83–89 | 14–53 | 35–75 | ||
| FEF% | ||||||
| Mean ± SD | 36.19 ± 11.25 | 29.40 ± 6.69 | 0.001 | 29.22 ± 7.36 | 40.83 ± 10.00 | 0.001 |
| Range | 13–55 | 20–40 | 19–52 | 13–55 | ||
| WBCs (103/mm3) | ||||||
| Mean ± SD | 8.32 ± 4.11 | 6.40 ± 1.23 | 0.002 | 6.38 ± 1.13 | 10.25 ± 2.78 | 0.001 |
| Range | 4.32–19 | 4.65–9.5 | 4.32–8.2 | 4.8–19 | ||
| RBCs (103/mm3) | ||||||
| Mean ± SD | 5.16 ± 0.41 | 5.17 ± 0.31 | NS | 5.20 ± 0.35 | 5.13 ± 0.47 | NS |
| Range | 4.1–5.9 | 4.5–5.6 | 4.5–5.9 | 4.1–5.8 | ||
| Hb (gm/dL) | ||||||
| Mean ± SD | 14.15 ± 10.14 | 13.54 ± 0.79 | NS | 14.01 ± 1.05 | 14.96 ± 1.64 | NS |
| Range | 11.4–15.5 | 11.9–14.8 | 11.9–15.5 | 11.4–18 | ||
| HCT% | ||||||
| Mean ± SD | 45.98 ± 4.14 | 44.90 ± 1.27 | NS | 45.61 ± 3.05 | 46.35 ± 5.01 | NS |
| Range | 35.7–55 | 42–47.5 | 35.7–51 | 37–55 | ||
| MCV (µm3) | ||||||
| Mean ± SD | 87.84 ± 4.09 | 86.78 ± 3.65 | NS | 87.60 ± 4.97 | 88.07 ± 3.00 | NS |
| Range | 64.1–94.4 | 81.4–93 | 64.1–94.4 | 80.2–93.8 | ||
| MCHC (gm/dL) | ||||||
| Mean ± SD | 32.78 ± 1.54 | 33.62 ± 1.28 | 0.001 | 33.00 ± 1.40 | 32.56 ± 1.65 | NS |
| Range | 29–36 | 31.4–35.3 | 29.9–35.3 | 29–36 | ||
| PLT (103/mm3) | ||||||
| Mean ± SD | 293.00 ± 57.81 | 233.77 ± 51.67 | 0.001 | 276.77 ± 61.47 | 309.23 ± 49.38 | 0.005 |
| Range | 180–421 | 170–370 | 180–385 | 206–421 | ||
| CRP (gm/L) | ||||||
| Mean ± SD | 8.55 ± 2.53 | 3.46 ± 0.78 | 0.001 | 5.38 ± 1.36 | 11.71 ± 2.55 | 0.001 |
| Range | 2.6–26.2 | 2.1–4.5 | 2.6–9.1 | 6.4–26.2 | ||
| AK155(IL–26) (pg/mL) | ||||||
| Mean ± SD | 31.47 ± 8.06 | 13.87 ± 2.71 | 0.001 | 21.79 ± 4.72 | 41.15 ± 7.58 | 0.001 |
| Range | 12.1–53 | 9.9–22 | 12.1–34 | 23–53 | ||
| TLR–9 | Group (n) | COPD Group (n) | ||||
|---|---|---|---|---|---|---|
| rs5743836SNP | COPD (96) | Control (48) | OR (95% CI) | Exacerbation (48) | Stable (48) | OR (95% CI) |
| Genotypes | ||||||
| TT | 31 (32.1%) | 32 (66.7%) | Reference | 11 (22.9%) | 20 (41.7%) | Reference |
| TC | 50 (52.1%) | 16 (33.3%) | 3.22 (1.5–6.82) * | 27 (56.3%) | 23 (47.9%) | 2.13 (0.84–5.37) |
| CC | 15 (15.6%) | 0 (0.0%) | 31.98 (1.8–57.7) * | 10 (20.8%) | 5 (10.4%) | 3.64 (0.9–13.36) * |
| Alleles frequency | ||||||
| T | 112 (58.3%) | 80 (83.3%) | Reference | 49 (51.0%) | 63 (65.6%) | Reference |
| C | 80 (41.7%) | 16 (16.7%) | 3.57 (1.94–6.56) * | 47 (49.0%) | 33 (34.4%) | 1.83 (1.02–3.27) * |
| Dominant model | ||||||
| TT | 31 (32.1%) | 32 (66.7%) | Reference | 11 (22.9%) | 20 (41.7%) | Reference |
| TC + CC | 65 (67.7%) | 16 (33.3%) | 4.19 (2.01–8.76) * | 37 (77.1%) | 28 (58.3%) | 2.40 (0.99–5.82) * |
| Recessive model | ||||||
| TT + TC | 81 (84.4%) | 48 (100.0%) | Reference | 38 (79.2%) | 43 (89.6%) | Reference |
| CC | 15 (15.6%) | 0 (0.0%) | 18.45 (1.1–35.3) * | 10 (20.8%) | 5 (10.4%) | 2.26 (0.71–7.2) |
| AK155(IL−26) (pg/mL) in the COPD Group | ||||
|---|---|---|---|---|
| Exacerbated (n = 48) | Stable (n = 48) | |||
| Variables | r-Value | p-Value | r-Value | p-Value |
| Age (years) | −0.155 | NS | −0.07 | NS |
| Smoking index | −0.124 | NS | 0.172 | NS |
| BMI [kg/m2] | 0.790 | 0.001 | 0.974 | 0.001 |
| pH | −0.164 | NS | −0.111 | NS |
| PCO2 (mmHg) | 0.268 | NS | 0.126 | NS |
| PO2 (mmHg) | −0.188 | NS | 0.058 | NS |
| HCO3 (mmol/L) | 0.222 | NS | 0.027 | NS |
| S O2% | −0.181 | NS | 0.005 | NS |
| FEV1% | −0.915 | 0.001 | −0.873 | 0.001 |
| FVC% | 0.008 | NS | −0.190 | NS |
| FEV1/FVC | −0.731 | 0.001 | −0.87 | 0.001 |
| VC% | 0.061 | NS | 0.326 | 0.024 |
| FEF% | −0.066 | NS | −0.062 | NS |
| WBCs (103/mm3) | 0.151 | NS | 0.23 | NS |
| RBCs (103/mm3) | 0.029 | NS | 0.116 | NS |
| Hb (gm/dL) | 0.034 | NS | 0.269 | NS |
| HCT% | 0.052 | NS | 0.180 | NS |
| MCV (µm3) | −0.002 | NS | −0.151 | NS |
| MCHC (gm/dL) | 0.094 | NS | −0.112 | NS |
| PLT (103/mm3) | 0.238 | NS | −0.273 | NS |
| CRP (gm/L) | 0.755 | 0.001 | 0.963 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, E.R.; Elshennawy, S.I.; Elhakeem, H.; Saleh, R.A.M.; Elsawy, S.B.; Salama, K.S.M.; Mohamed, M.F.; Bahi, R.H.; Mansour, H.H.; Kasim Mahmoud, S.A.; et al. The Association of Toll-like Receptor-9 Gene Single-Nucleotide Polymorphism and AK155(IL-26) Serum Levels with Chronic Obstructive Pulmonary Disease Exacerbation Risk: A Case-Controlled Study with Bioinformatics Analysis. Biomedicines 2025, 13, 613. https://doi.org/10.3390/biomedicines13030613
Mokhtar ER, Elshennawy SI, Elhakeem H, Saleh RAM, Elsawy SB, Salama KSM, Mohamed MF, Bahi RH, Mansour HH, Kasim Mahmoud SA, et al. The Association of Toll-like Receptor-9 Gene Single-Nucleotide Polymorphism and AK155(IL-26) Serum Levels with Chronic Obstructive Pulmonary Disease Exacerbation Risk: A Case-Controlled Study with Bioinformatics Analysis. Biomedicines. 2025; 13(3):613. https://doi.org/10.3390/biomedicines13030613
Chicago/Turabian StyleMokhtar, Entsar R., Salwa I. Elshennawy, Heba Elhakeem, Rayyh A. M. Saleh, Sawsan Bakr Elsawy, Khadiga S. M. Salama, Maha Fathy Mohamed, Rania Hamid Bahi, Hayam H. Mansour, Sammar Ahmed Kasim Mahmoud, and et al. 2025. "The Association of Toll-like Receptor-9 Gene Single-Nucleotide Polymorphism and AK155(IL-26) Serum Levels with Chronic Obstructive Pulmonary Disease Exacerbation Risk: A Case-Controlled Study with Bioinformatics Analysis" Biomedicines 13, no. 3: 613. https://doi.org/10.3390/biomedicines13030613
APA StyleMokhtar, E. R., Elshennawy, S. I., Elhakeem, H., Saleh, R. A. M., Elsawy, S. B., Salama, K. S. M., Mohamed, M. F., Bahi, R. H., Mansour, H. H., Kasim Mahmoud, S. A., Hassan, M. M., Elhadad, S. M., Eid El Sayed, H. M., Mohamed, A. N., & Hamdy, N. M. (2025). The Association of Toll-like Receptor-9 Gene Single-Nucleotide Polymorphism and AK155(IL-26) Serum Levels with Chronic Obstructive Pulmonary Disease Exacerbation Risk: A Case-Controlled Study with Bioinformatics Analysis. Biomedicines, 13(3), 613. https://doi.org/10.3390/biomedicines13030613








