MRI T2 Mapping of Dorsal Root Ganglia Reveals Increased T2 Relaxation Time in Classical Fabry Disease
Abstract
1. Introduction
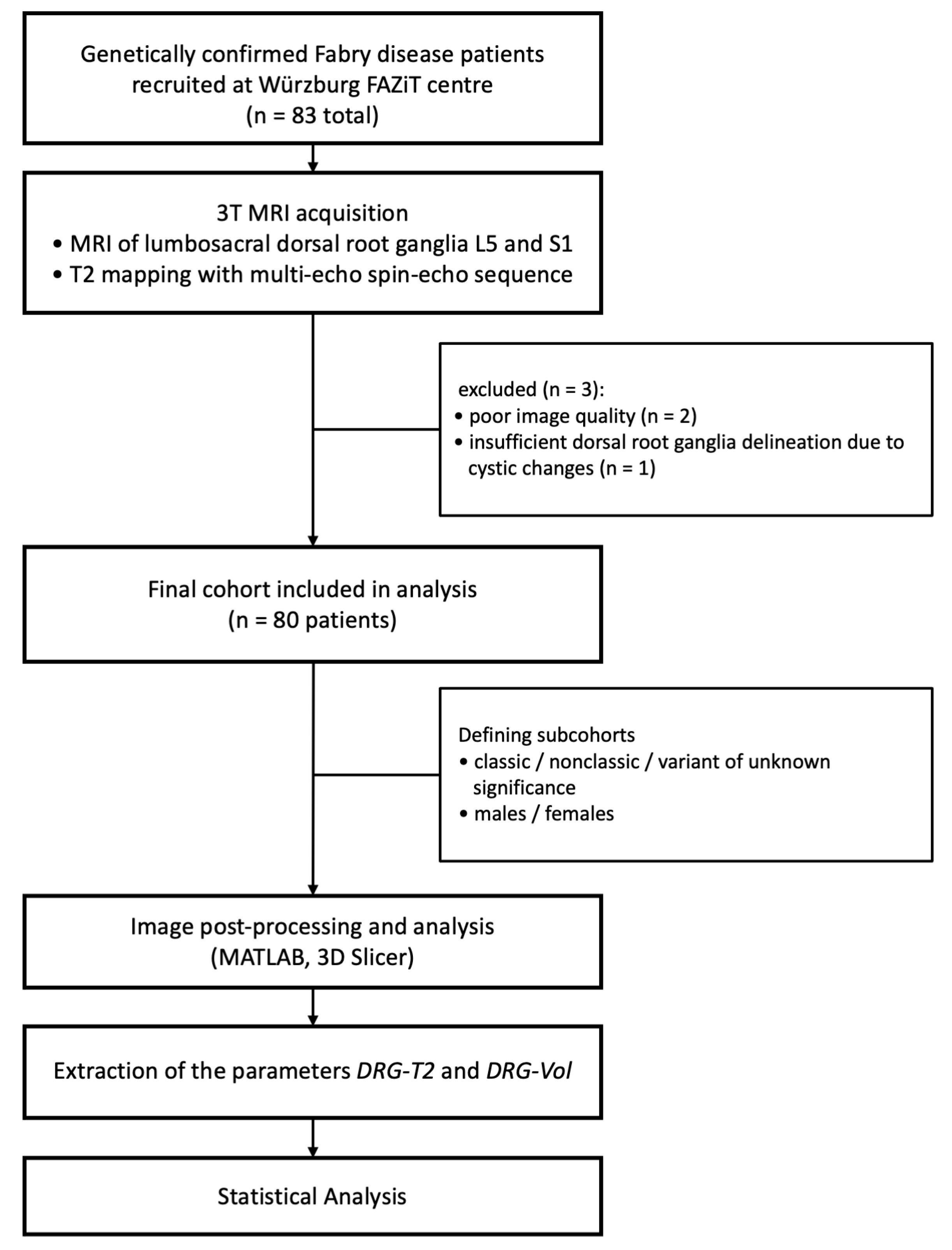
2. Materials and Methods
2.1. Ethical Approval and Patient Consent
2.2. Study Population
2.3. MRI Acquisition
2.4. Image Postprocessing and Evaluation
2.5. Laboratory Data
2.6. Statistical Analysis
3. Results
3.1. Study Cohort
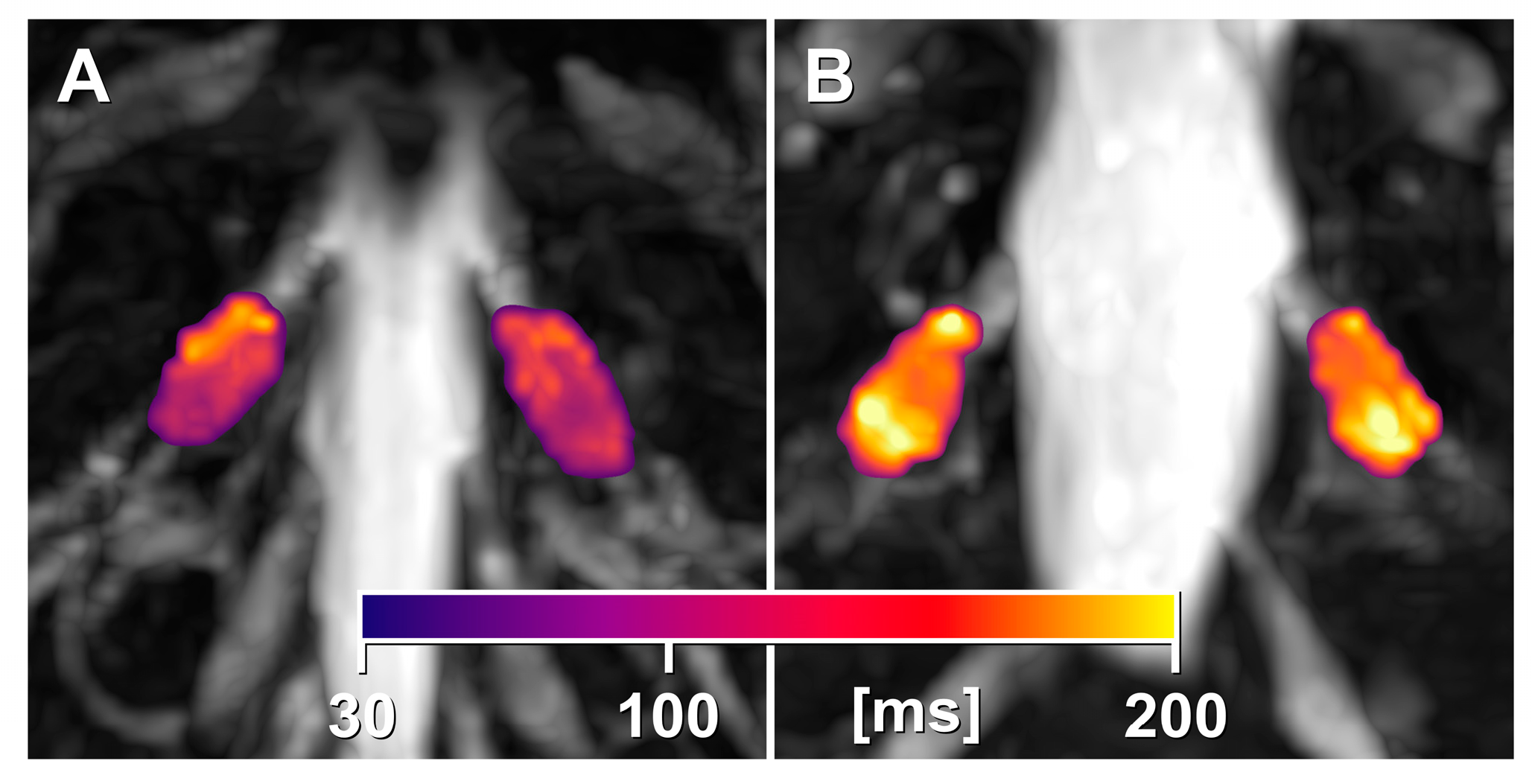
3.2. DRG Imaging Features
3.3. Sex-Specific Differences
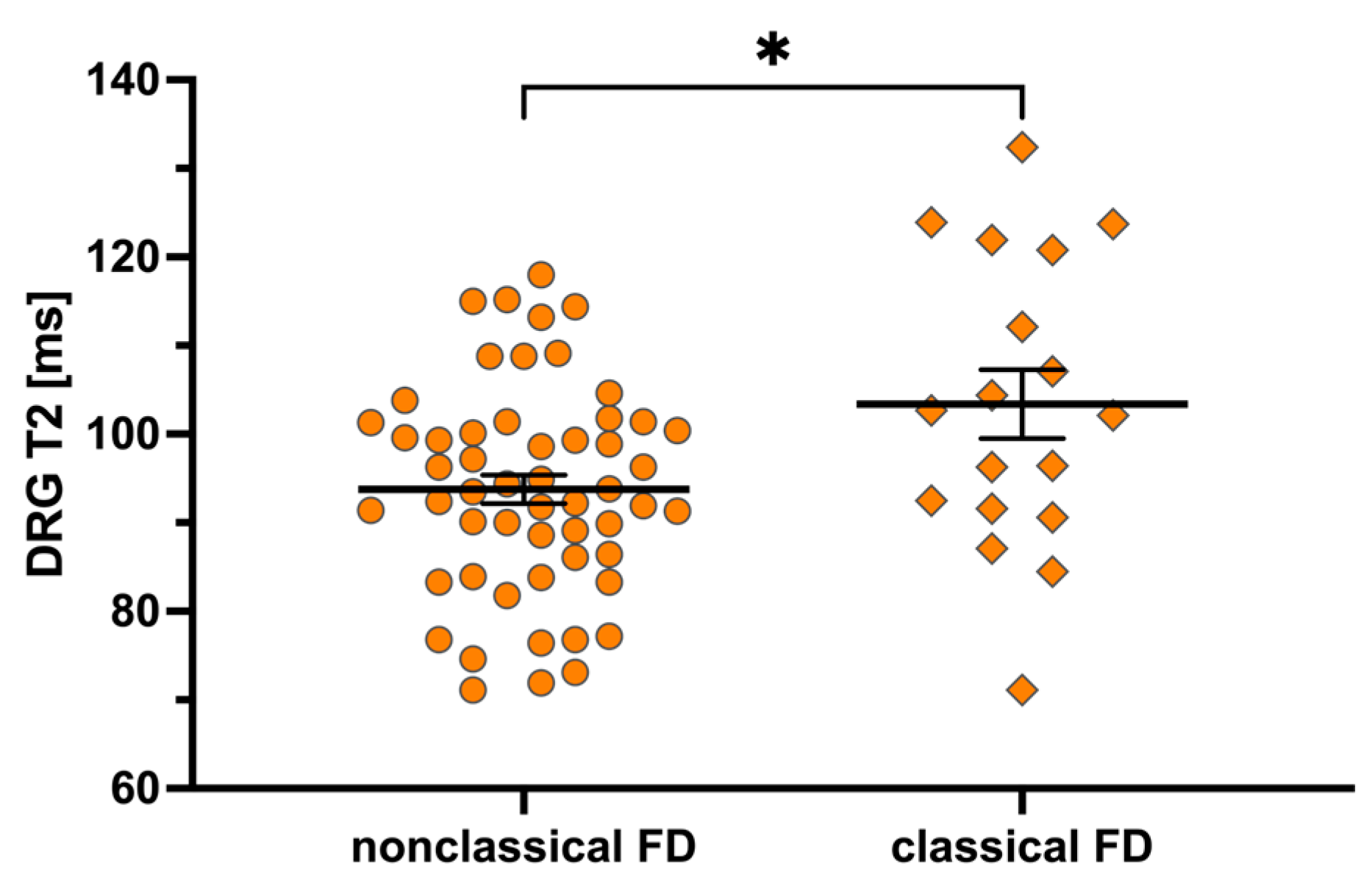
3.4. Mutation-Specific Differences
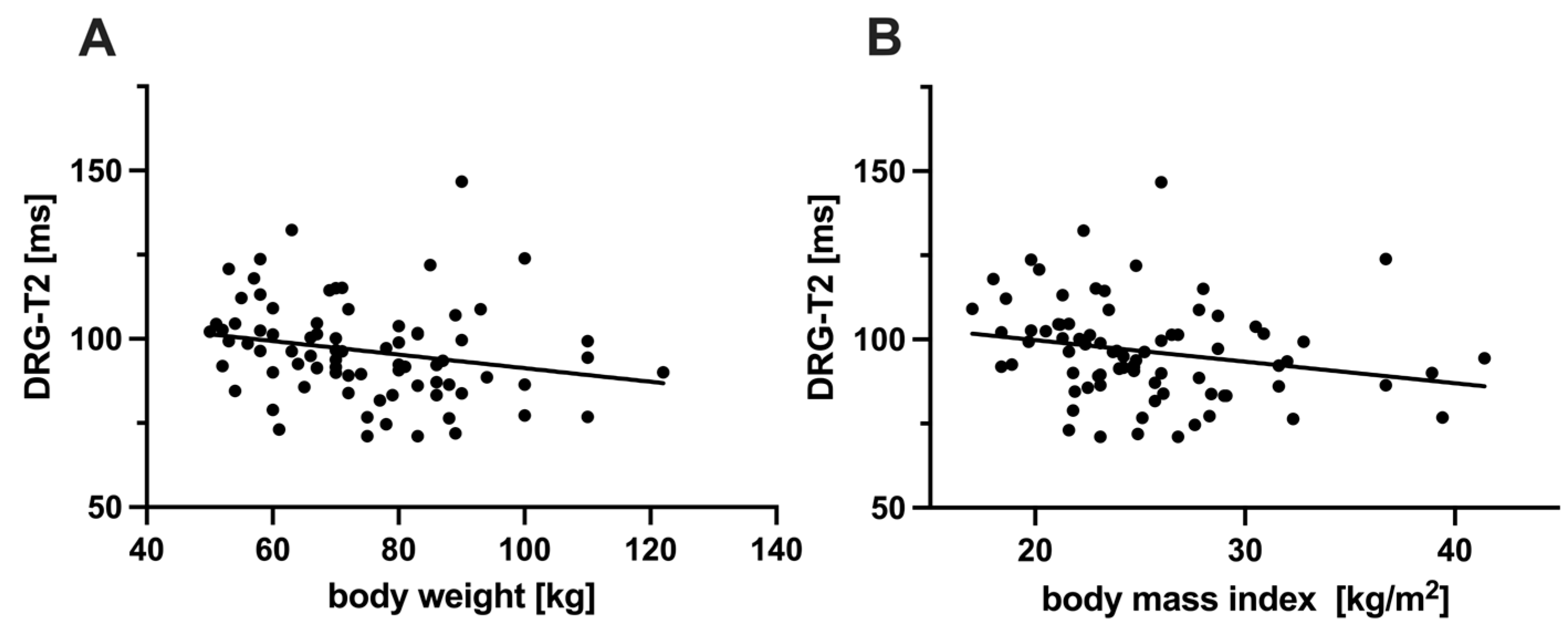
3.5. Correlation Between DRG-T2 and Other Characteristics
3.6. Inter-Rater and Test–Retest Reliability of DRG-T2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DRG | Dorsal root ganglion/ganglia |
| DRG-T2 | T2 relaxation time of the dorsal root ganglia |
| ERT | Enzyme replacement therapy |
| FD | Fabry disease |
| Gb3 | Globotriaosylceramide |
| GLA | Alpha-galactosidase A enzyme |
| Lyso-Gb3 | Globotriaosylsphingosine |
| T2 | T2 relaxation time |
References
- Meikle, P.J. Prevalence of Lysosomal Storage Disorders. JAMA 1999, 281, 249. [Google Scholar] [CrossRef]
- Poorthuis, B.J.H.M.; Wevers, R.A.; Kleijer, W.J.; Groener, J.E.M.; de Jong, J.G.N.; van Weely, S.; Niezen-Koning, K.E.; van Diggelen, O.P. The Frequency of Lysosomal Storage Diseases in The Netherlands. Hum. Genet. 1999, 105, 151–156. [Google Scholar] [CrossRef]
- Brady, R.O.; Gal, A.E.; Bradley, R.M.; Martensson, E.; Warshaw, A.L.; Laster, L. Enzymatic Defect in Fabry’s Disease. Ceramidetrihexosidase Deficiency. N. Engl. J. Med. 1967, 276, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry Disease, an under-Recognized Multisystemic Disorder: Expert Recommendations for Diagnosis, Management, and Enzyme Replacement Therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Pecoraro, R.; Simonetta, I.; Miceli, S.; Pinto, A.; Licata, G. Anderson-Fabry Disease: A Multiorgan Disease. Curr. Pharm. Des. 2013, 19, 5974–5996. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.; Jensen, T. Neurological Manifestations in Fabry’s Disease. Nat. Clin. Pract. Neurol. 2007, 3, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.; Vernon, J.; Kopach, O. Fabry Disease-Associated Li- Pid Lyso-Gb3 Enhances Voltage-Gated Calcium Currents Sensory Neurons Causes Pain. Neurosci. Lett. 2015, 594, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell. Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Godel, T.; Bäumer, P.; Pham, M.; Köhn, A.; Muschol, N.; Kronlage, M.; Kollmer, J.; Heiland, S.; Bendszus, M.; Mautner, V.-F. Human Dorsal Root Ganglion in Vivo Morphometry and Perfusion in Fabry Painful Neuropathy. Neurology 2017, 89, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Godel, T.; Köhn, A.; Muschol, N.; Kronlage, M.; Schwarz, D.; Kollmer, J.; Heiland, S.; Bendszus, M.; Mautner, V.-F.; Bäumer, P. Dorsal Root Ganglia in Vivo Morphometry and Perfusion in Female Patients with Fabry Disease. J. Neurol. 2018, 265, 2723–2729. [Google Scholar] [CrossRef]
- Godel, T.; Bäumer, P.; Stumpfe, K.; Muschol, N.; Kronlage, M.; Brunnée, M.; Kollmer, J.; Heiland, S.; Bendszus, M.; Mautner, V.-F. Dorsal Root Ganglia Volume Is Increased in Patients with the Fabry-Related GLA Variant p.D313Y. J. Neurol. 2019, 266, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Schindehütte, M.; Weiner, S.; Klug, K.; Hölzli, L.; Nauroth-Kreß, C.; Hessenauer, F.; Kampf, T.; Homola, G.A.; Nordbeck, P.; Wanner, C.; et al. Dorsal Root Ganglion Magnetic Resonance Imaging Biomarker Correlations with Pain in Fabry Disease. Brain Commun. 2024, 6, fcae155. [Google Scholar] [CrossRef]
- Sollmann, N.; Weidlich, D.; Cervantes, B.; Klupp, E.; Ganter, C.; Kooijman, H.; Rummeny, E.J.; Zimmer, C.; Kirschke, J.S.; Karampinos, D.C. High Isotropic Resolution T2 Mapping of the Lumbosacral Plexus with T2-Prepared 3D Turbo Spin Echo. Clin. Neuroradiol. 2019, 29, 223–230. [Google Scholar] [CrossRef]
- Filler, A.G.; Kliot, M.; Howe, F.A.; Hayes, C.E.; Saunders, D.E.; Goodkin, R.; Bell, B.A.; Winn, H.R.; Griffiths, J.R.; Tsuruda, J.S. Application of Magnetic Resonance Neurography in the Evaluation of Patients with Peripheral Nerve Pathology. J. Neurosurg. 1996, 85, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; du Boulay, E.P. Towards Quantitative Measurements of Relaxation Times and Other Parameters in the Brain. Neuroradiology 1990, 32, 407–415. [Google Scholar] [CrossRef]
- Caredda, G.; Bassareo, P.P.; Cherchi, M.V.; Pontone, G.; Suri, J.S.; Saba, L. Anderson-Fabry Disease: Role of Traditional and New Cardiac MRI Techniques. Br. J. Radiol. 2021, 94, 20210020. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Augusto, J.B.; Nordin, S.; Kozor, R.; Camaioni, C.; Xue, H.; Hughes, R.K.; Manisty, C.; Brown, L.A.E.; Kellman, P.; et al. Quantitative Myocardial Perfusion in Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e008872. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.; Wanner, C.; Hughes, D.; Mehta, A.; Oder, D.; Watkinson, O.T.; Elliott, P.M.; Linthorst, G.E.; Wijburg, F.A.; Biegstraaten, M.; et al. Characterization of Classical and Nonclassical Fabry Disease: A Multicenter Study. J. Am. Soc. Nephrol. 2017, 28, 1631–1641. [Google Scholar] [CrossRef]
- Rickert, V.; Wagenhäuser, L.; Nordbeck, P.; Wanner, C.; Sommer, C.; Rost, S.; Üçeyler, N. Stratification of Fabry Mutations in Clinical Practice: A Closer Look at α-Galactosidase A-3D Structure. J. Intern. Med. 2020, 288, 593–604. [Google Scholar] [CrossRef]
- Neumann, D.; Blaimer, M.; Jakob, P.M.; Breuer, F.A. Simple Recipe for Accurate T(2) Quantification with Multi Spin-Echo Acquisitions. MAGMA 2014, 27, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Tofts, P. Quantitative MRI of the Brain: Measuring Changes Caused by Disease; Tofts, P., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2004; ISBN 9780470014295. [Google Scholar]
- Hopkin, R.J.; Bissler, J.; Banikazemi, M.; Clarke, L.; Eng, C.M.; Germain, D.P.; Lemay, R.; Tylki-Szymanska, A.; Wilcox, W.R. Characterization of Fabry Disease in 352 Pediatric Patients in the Fabry Registry. Pediatr. Res. 2008, 64, 550–555. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry Disease: Clinical Manifestations and Impact of Disease in a Cohort of 98 Hemizygous Males. J. Med. Genet. 2001, 38, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Wolf, M.; West, M.L.; Tonelli, M.; Ruthazer, R.; Pastores, G.M.; Obrador, G.T. Patients with Fabry Disease on Dialysis in the United States. Kidney Int. 2002, 61, 249–255. [Google Scholar] [CrossRef]
- Weiner, S.; Strinitz, M.; Herfurth, J.; Hessenauer, F.; Nauroth-Kreß, C.; Kampf, T.; Homola, G.A.; Üçeyler, N.; Sommer, C.; Pham, M.; et al. Dorsal Root Ganglion Volumetry by MR Gangliography. AJNR Am. J. Neuroradiol. 2022, 43, 769–775. [Google Scholar] [CrossRef]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated Globotriaosylsphingosine Is a Hallmark of Fabry Disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G.; Aerts, J.M.; Auray-Blais, C.; Maruyama, H.; Mehta, A.B.; Skuban, N.; Krusinska, E.; Schiffmann, R. Assessment of Plasma Lyso-Gb3 for Clinical Monitoring of Treatment Response in Migalastat-Treated Patients with Fabry Disease. Genet. Med. 2021, 23, 192–201. [Google Scholar] [CrossRef]
- Effraimidis, G.; Feldt-Rasmussen, U.; Rasmussen, Å.K.; Lavoie, P.; Abaoui, M.; Boutin, M.; Auray-Blais, C. Globotriaosylsphingosine (Lyso-Gb3) and Analogues in Plasma and Urine of Patients with Fabry Disease and Correlations with Long-Term Treatment and Genotypes in a Nationwide Female Danish Cohort. J. Med. Genet. 2021, 58, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martinez, D.; Aguiar, P.; Auray-Blais, C.; Beck, M.; Bichet, D.G.; Burlina, A.; Cole, D.; Elliott, P.; Feldt-Rasmussen, U.; Feriozzi, S.; et al. Standardising Clinical Outcomes Measures for Adult Clinical Trials in Fabry Disease: A Global Delphi Consensus. Mol. Genet. Metab. 2021, 132, 234–243. [Google Scholar] [CrossRef]
- Nowak, A.; Beuschlein, F.; Sivasubramaniam, V.; Kasper, D.; Warnock, D.G. Lyso-Gb3 Associates with Adverse Long-Term Outcome in Patients with Fabry Disease. J. Med. Genet. 2022, 59, 287–293. [Google Scholar] [CrossRef]
- Arends, M.; Wijburg, F.A.; Wanner, C.; Vaz, F.M.; van Kuilenburg, A.B.P.; Hughes, D.A.; Biegstraaten, M.; Mehta, A.; Hollak, C.E.M.; Langeveld, M. Favourable Effect of Early versus Late Start of Enzyme Replacement Therapy on Plasma Globotriaosylsphingosine Levels in Men with Classical Fabry Disease. Mol. Genet. Metab. 2017, 121, 157–161. [Google Scholar] [CrossRef]
- van der Tol, L.; Sminia, M.L.; Hollak, C.E.M.; Biegstraaten, M. Cornea Verticillata Supports a Diagnosis of Fabry Disease in Non-Classical Phenotypes: Results from the Dutch Cohort and a Systematic Review. Br. J. Ophthalmol. 2016, 100, 3–8. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D. Anderson-Fabry Disease: Clinical Manifestations and Impact of Disease in a Cohort of 60 Obligate Carrier Females. J. Med. Genet. 2001, 38, 769–775. [Google Scholar] [CrossRef]
- Ries, M.; Ramaswami, U.; Parini, R.; Lindblad, B.; Whybra, C.; Willers, I.; Gal, A.; Beck, M. The Early Clinical Phenotype of Fabry Disease: A Study on 35 European Children and Adolescents. Eur. J. Pediatr. 2003, 162, 767–772. [Google Scholar] [CrossRef]
- Ramaswami, U.; Whybra, C.; Parini, R.; Pintos-Morell, G.; Mehta, A.; Sunder-Plassmann, G.; Widmer, U.; Beck, M.; FOS European Investigators. Clinical Manifestations of Fabry Disease in Children: Data from the Fabry Outcome Survey. Acta Paediatr. 2006, 95, 86–92. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Median (IQR) or Number (Percentage) |
|---|---|
| age [years] | 42.0 (33.0–57.0) |
| male | 38 (47.5%) |
| height [cm] | 168.0 (165.0–178.0) |
| weight [kg] | 71.5 (62.5–85.2) |
| body mass index [kg/m2] | 24.5 (21.9–27.8) |
| classical mutation | 18 (22.5%) |
| ↳ male/female | 10 (55.6%)/8 (44.4%) |
| nonclassical mutation | 54 (67.5%) |
| ↳ male/female | 24 (44.4%)/30 (55.6%) |
| variant of unknown significance | 8 (10%) |
| ↳ male/female | 4 (50.0%)/4 (50.0%) |
| alpha-galactosidase A activity [nmol/min/mg protein] | 0.17 (0.04–0.29) |
| Lyso-Gb3 level [ng/mL] | 11.1 (2.0–22.6) |
| previous cerebrovascular event | 13 (16.2%) |
| neuropathy or any involvement of the PNS | 53 (66.2%) |
| small fibre neuropathy | 25 (31.2%) |
| pain | 48 (60.%) |
| previous FD-specific therapy | 47 (58.8%) |
| previous neuropathy-specific therapy | 18 (22.5%) |
| Parameter | FD Men (n = 38) Median (IQR) or Number (%) | FD Women (n = 42) Median (IQR) or Number (%) | p-Value (Men vs. Women) |
|---|---|---|---|
| DRG-T2 [ms] | 94.4 (88.7–108.4) | 95.6 (86.2–102.0) | 0.75 |
| DRG-T2L5 [ms] | 91.1 (85.8–101.4) | 93.4 (88.1–101.6) | 0.77 |
| DRG-T2S1 [ms] | 97.5 (89.8–114.4) | 95.3 (85.4–102.2) | 0.49 |
| DRG-Vol [mm3] | 1342.1 (1012.6–1610.7) | 987.8 (769.1–1187.5) | <0.001 |
| DRG-VolL5 [mm3] | 1049.8 (780.0–1303.5) | 761.1 (582.5–972.5) | <0.001 |
| DRG-VolS1 [mm3] | 1595.1 (1239.3–1947.9) | 1144.5 (827.4–1434.7) | <0.001 |
| age [years] | 36.0 (29.2–44.0) | 52.0 (35.2–62.8) | 0.002 |
| height [cm] | 178.0 (173.5–183.8) | 165.0 (163.0–167.8) | <0.001 |
| weight [kg] | 80.0 (70.2–89.0) | 67.0 (58.0–78.8) | <0.001 |
| body mass index [kg/m2] | 24.7 (23.0–26.4) | 24.0 (21.6–28.9) | 0.84 |
| classical mutation | 10 (26.3%) | 8 (19%) | 0.61 |
| nonclassical mutation | 24 (63.2%) | 30 (71.4%) | 0.58 |
| variant of unknown significance | 4 (10.5%) | 4 (9.5%) | 1.0 |
| α-Gal [nmol/min/mg protein] | 0.04 (0.04–0.06) | 0.28 (0.23–0.39) | <0.001 |
| Lyso-Gb3 [ng/mL] | 20.1 (6.9–60.8) | 4.2 (1.1–13.8) | <0.001 |
| previous FD-specific therapy | 28 (73.7%) | 19 (45.2%) | 0.75 |
| Parameter | Total Cohort (n = 80) Median (IQR) or Number (%) | Classical Mutation (n = 18) Median (IQR) or Number (%) | Nonclassical Mutation (n = 54) Median (IQR) or Number (%) | p-Value (Classical vs. Nonclassical Mutation) |
|---|---|---|---|---|
| DRG-T2 [ms] | 95.6 (87.0–103.0) | 102.4 (91.8–118.6) | 93.6 (86.2–101.0) | 0.026 |
| DRG-T2L5 [ms] | 92.9 (86.5–101.5) | 96.5 (89.6–105.6) | 91.8 (85.8–101.0) | 0.14 |
| DRG-T2S1 [ms] | 96.8 (87.8–105.2) | 106.2 (93.2–121.7) | 96.1 (83.1–102.0) | 0.038 |
| DRG-Vol [mm3] | 1097.1 (838.7–1392.4) | 1097.1 (850.7–1470.4) | 1044.7 (788.4–1356.7) | 0.79 |
| DRG-VolL5 [mm3] | 905.4 (691.1–1120.5) | 895.2 (672.9–1277.9) | 815.0 (632.0–1043.2) | 0.41 |
| DRG-VolS1 [mm3] | 1323.9 (977.6–1676.7) | 1221.8 (998.0–1570.3) | 1302.0 (866.1–1694.2) | 0.87 |
| age [years] | 42.0 (33.0–57.0) | 38.5 (33.5–47.8) | 43.5 (33.2–59.8) | 0.38 |
| male | 38 (47.5%) | 10 (55.6%) | 24 (44.4%) | 0.59 |
| height [cm] | 168.0 (165.0–178.0) | 169.0 (164.2–176.0) | 168.0 (165.0–177.8) | 0.73 |
| weight [kg] | 71.5 (62.5–85.2) | 63.5 (54.2–82.2) | 75.0 (67.0–86.0) | 0.035 |
| body mass index [kg/m2] | 24.5 (21.9–27.8) | 22.1 (19.9–25.1) | 25.1 (23.0–28.6) | 0.016 |
| classical mutation | 18 (22.5%) | 18 (100%) | 0 (0%) | <0.001 |
| nonclassical mutation | 54 (67.5%) | 0 (0%) | 54 (100%) | <0.001 |
| variant of unknown significance | 8 (10%) | 0 (0%) | 0 (0%) | 1.0 |
| α-Gal [nmol/min/mg protein] | 0.17 (0.04–0.29) | 0.05 (0.04–0.19) | 0.21 (0.05–0.32) | 0.025 |
| Lyso-Gb3 [ng/mL] | 11.1 (2.0–22.6) | 40.3 (16.8–86.6) | 4.9 (1.3–13.1) | <0.001 |
| previous FD-specific therapy | 47 (58.8%) | 13 (72.2%) | 30 (55.6%) | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiner, S.; Perleth, S.; Kampf, T.; Lau, K.; Hessenauer, F.; Homola, G.; Nordbeck, P.; Üçeyler, N.; Sommer, C.; Pham, M.; et al. MRI T2 Mapping of Dorsal Root Ganglia Reveals Increased T2 Relaxation Time in Classical Fabry Disease. Biomedicines 2025, 13, 592. https://doi.org/10.3390/biomedicines13030592
Weiner S, Perleth S, Kampf T, Lau K, Hessenauer F, Homola G, Nordbeck P, Üçeyler N, Sommer C, Pham M, et al. MRI T2 Mapping of Dorsal Root Ganglia Reveals Increased T2 Relaxation Time in Classical Fabry Disease. Biomedicines. 2025; 13(3):592. https://doi.org/10.3390/biomedicines13030592
Chicago/Turabian StyleWeiner, Simon, Sarah Perleth, Thomas Kampf, Kolja Lau, Florian Hessenauer, György Homola, Peter Nordbeck, Nurcan Üçeyler, Claudia Sommer, Mirko Pham, and et al. 2025. "MRI T2 Mapping of Dorsal Root Ganglia Reveals Increased T2 Relaxation Time in Classical Fabry Disease" Biomedicines 13, no. 3: 592. https://doi.org/10.3390/biomedicines13030592
APA StyleWeiner, S., Perleth, S., Kampf, T., Lau, K., Hessenauer, F., Homola, G., Nordbeck, P., Üçeyler, N., Sommer, C., Pham, M., & Schindehütte, M. (2025). MRI T2 Mapping of Dorsal Root Ganglia Reveals Increased T2 Relaxation Time in Classical Fabry Disease. Biomedicines, 13(3), 592. https://doi.org/10.3390/biomedicines13030592







