Retrospective Clinical Investigation into the Association Between Abnormal Blood Clotting, Oral Anticoagulant Therapy, and Medium-Term Mortality in a Cohort of COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. Comorbidities Influencing COVID-19-Related Mortality
4.2. Impact of Oral Anticoagulation Therapy on COVID-19 Clinical Course
4.3. Impact of Other Pharmacological Treatments on COVID-19 Mortality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S.; He, Y.; Zuo, Q.; Liu, D.; Xiao, M.; Fan, J.; Li, X. COVID-19 Is Distinct from SARS-CoV-2-Negative Community-Acquired Pneumonia. Front. Cell. Infect. Microbiol. 2020, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Beladiya, J.; Kumar, A.; Vasava, Y.; Parmar, K.; Patel, D.; Patel, S.; Dholakia, S.; Sheth, D.; Boddu, S.H.S.; Patel, C. Safety and efficacy of COVID-19 vaccines: A systematic review and meta-analysis of controlled and randomized clinical trials. Rev. Med. Virol. 2024, 34, e2507. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin. Infect. Dis. 2023, 76, e342–e349. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Uddin, M.S.; Hossain, M.F.; Abdulhakim, J.A.; Alam, M.A.; Ashraf, G.M.; Bungau, S.G.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Aleya, L. nCOVID-19 Pandemic: From Molecular Pathogenesis to Potential Investigational Therapeutics. Front. Cell Dev. Biol. 2020, 8, 616. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Gupta, A. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Hempel, T.; Elez, K.; Krüger, N.; Raich, L.; Shrimp, J.H.; Danov, O.; Jonigk, D.; Braun, A.; Shen, M.; Hall, M.D.; et al. Synergistic Inhibition of SARS-CoV-2 Cell Entry by Otamixaban and Covalent Protease Inhibitors: Pre-Clinical Assessment of Pharmacological and Molecular Properties. Chem. Sci. 2021, 12, 12600–12609. [Google Scholar] [CrossRef]
- De Maio, F.; Rullo, M.; de Candia, M.; Purgatorio, R.; Lopopolo, G.; Santarelli, G.; Palmieri, V.; Papi, M.; Elia, G.; De Candia, E.; et al. Evaluation of Novel Guanidino-Containing Isonipecotamide Inhibitors of Blood Coagulation Factors against SARS-CoV-2 Virus Infection. Viruses 2022, 14, 1730. [Google Scholar] [CrossRef]
- Samarelli, F.; Graziano, G.; Gambacorta, N.; Graps, E.A.; Leonetti, F.; Nicolotti, O.; Altomare, C.D. Small Molecules for the Treatment of Long-COVID-Related Vascular Damage and Abnormal Blood Clotting: A Patent-Based Appraisal. Viruses 2024, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Russo, V. Chronic Oral Anticoagulation and Clinical Outcome in Hospitalized COVID-19 Patients. Cardiovasc. Drugs Ther. 2022, 36, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Topol, E. Solving the Puzzle of Long COVID. Science 2024, 383, 830–832. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; Van De Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-Associated Coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Heestermans, M.; Poenou, G.; Hamzeh-Cognasse, H.; Cognasse, F.; Bertoletti, L. Anticoagulants: A Short History, Their Mechanism of Action, Pharmacology, and Indications. Cells 2022, 11, 3214. [Google Scholar] [CrossRef]
- Mele, M.; Mele, A.; Imbrici, P.; Samarelli, F.; Purgatorio, R.; Dinoi, G.; Correale, M.; Nicolotti, O.; Luca, A.D.; Brunetti, N.D.; et al. Pleiotropic Effects of Direct Oral Anticoagulants in Chronic Heart Failure and Atrial Fibrillation: Machine Learning Analysis. Molecules 2024, 29, 2651. [Google Scholar] [CrossRef]
- Spyropoulos, A.C. Good Practice Statements for Antithrombotic Therapy in the Management of COVID-19: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2022, 20, 2226–2236. [Google Scholar] [CrossRef]
- Baumann Kreuziger, L.; Sholzberg, M.; Cushman, M. Anticoagulation in Hospitalized Patients with COVID-19. Blood 2022, 140, 809–814. [Google Scholar] [CrossRef]
- Spiegelenberg, J.P. Prior Use of Therapeutic Anticoagulation Does Not Protect against COVID-19 Related Clinical Outcomes in Hospitalized Patients: A Propensity Score-matched Cohort Study. Br. J. Clin. Pharmacol. 2021, 87, 4842–4850. [Google Scholar] [CrossRef]
- Covino, M.; De Matteis, G.; Della Polla, D.; Burzo, M.L.; Pascale, M.M.; Santoro, M.; De Cristofaro, R.; Gasbarrini, A.; De Candia, E.; Franceschi, F. Does Chronic Oral Anticoagulation Reduce In-Hospital Mortality among COVID-19 Older Patients? Aging Clin. Exp. Res. 2021, 33, 2335–2343. [Google Scholar] [CrossRef]
- Harrison, R.F.; Forte, K.; Buscher, M.G.; Chess, A.; Patel, A.; Moylan, T.; Mize, C.H.; Werdmann, M.; Ferrigno, R. The Association of Preinfection Daily Oral Anticoagulation Use and All-Cause in Hospital Mortality from Novel Coronavirus 2019 at 21 Days: A Retrospective Cohort Study. Crit. Care Explor. 2021, 3, e0324. [Google Scholar] [CrossRef] [PubMed]
- Denas, G.; Gennaro, N.; Ferroni, E.; Fedeli, U.; Lorenzoni, G.; Gregori, D.; Iliceto, S.; Pengo, V. Reduction in All-Cause Mortality in COVID-19 Patients on Chronic Oral Anticoagulation: A Population-Based Propensity Score Matched Study. Int. J. Cardiol. 2021, 329, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Trevisan, C.; Signore, S.D.; Pelagalli, G.; Volpato, S.; Gareri, P.; Mossello, E.; Malara, A.; Monzani, F.; Coin, A.; et al. COVID-19 and Atrial Fibrillation in Older Patients: Does Oral Anticoagulant Therapy Provide a Survival Benefit?—An Insight from the GeroCOVID Registry. Thromb. Haemost. 2022, 122, 105–112. [Google Scholar] [CrossRef]
- Ageno, W.; De Candia, E.; Iacoviello, L.; Di Castelnuovo, A. Protective Effect of Oral Anticoagulant Drugs in Atrial Fibrillation Patients Admitted for COVID-19: Results from the CORIST Study. Thromb. Res. 2021, 203, 138–141. [Google Scholar] [CrossRef]
- Wong, A.Y.; Tomlinson, L.; Brown, J.P.; Elson, W.; Walker, A.J.; Schultze, A. Association between Oral Anticoagulants and COVID 19-Related Outcomes: A population-based cohort study. Br. J. Gen. Pract. 2022, 72, e456–e463. [Google Scholar] [CrossRef]
- Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 7 February 2025).
- Schwartz, I.S.; Boulware, D.R.; Lee, T.C. Hydroxychloroquine for COVID19: The curtains close on a comedy of errors. Lancet Reg. Health Am. 2022, 11, 100268. [Google Scholar] [CrossRef]
- Deplanque, D. Hydroxychloroquine and COVID-19: The endgame! Therapie 2023, 78, 343–344. [Google Scholar] [CrossRef]
- Müller-Wieland, D.; Marx, N.; Dreher, M.; Fritzen, K.; Schnell, O. COVID-19 and Cardiovascular Comorbidities. Exp. Clin. Endocrinol. Diabetes 2022, 130, 178–189. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and Comorbidities: Deleterious Impact on Infected Patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, Immunity and Intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Bader, F.; Manla, Y.; Atallah, B.; Starling, R.C. Heart Failure and COVID-19. Heart Fail. Rev. 2021, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Kunichoff, D.; Garshick, M.; Shah, B.; Pillinger, M.; Hochman, J.S.; Berger, J.S. C-Reactive Protein and Clinical Outcomes in Patients with COVID-19. Eur. Heart J. 2021, 42, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, P.; Russo, V.; Carannante, N.; Imparato, M.; Rodolfi, S.; Cardillo, G.; Lodigiani, C. Clotting Factors in COVID-19: Epidemiological Association and Prognostic Values in Different Clinical Presentations in an Italian Cohort. J. Clin. Med. 2020, 9, 1371. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 Cytokine Storm: The Interplay between Inflammation and Coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH Interim Guidance on Recognition and Management of Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Russo, V.; Cardillo, G.; Viggiano, G.V.; Mangiacapra, S.; Cavalli, A.; Fontanella, A.; Agrusta, F.; Bellizzi, A.; Amitrano, M.; Iannuzzo, M.; et al. Fondaparinux Use in Patients with COVID-19: A Preliminary Multicenter Real-World Experience. J. Cardiovasc. Pharmacol. 2020, 76, 369–371. [Google Scholar] [CrossRef]
- Schiavone, M.; Gasperetti, A.; Mancone, M.; Curnis, A.; Mascioli, G.; Mitacchione, G.; Busana, M.; Sabato, F.; Gobbi, C.; Antinori, S.; et al. Oral Anticoagulation and Clinical Outcomes in COVID-19: An Italian Multicenter Experience. Int. J. Cardiol. 2021, 323, 276–280. [Google Scholar] [CrossRef]
- Tremblay, D.; van Gerwen, M.; Alsen, M.; Thibaud, S.; Kessler, A.; Venugopal, S.; Makki, I.; Qin, Q.; Dharmapuri, S.; Jun, T.; et al. Impact of Anticoagulation Prior to COVID-19 Infection: A Propensity Score–Matched Cohort Study. Blood 2020, 136, 144–147. [Google Scholar] [CrossRef]
- Russo, V.; Di Maio, M.; Attena, E.; Silverio, A.; Scudiero, F.; Celentani, D.; Lodigiani, C.; Di Micco, P. Clinical Impact of Pre-Admission Antithrombotic Therapy in Hospitalized Patients with COVID-19: A Multicenter Observational Study. Pharmacol. Res. 2020, 159, 104965. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Hadid, T.; Kafri, Z.; Al-Katib, A. Coagulation and Anticoagulation in COVID-19. Blood Rev. 2021, 47, 100761. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, F.; Silverio, A.; Di Maio, M.; Russo, V.; Citro, R.; Personeni, D.; Cafro, A.; D’Andrea, A.; Attena, E.; Pezzullo, S.; et al. Pulmonary Embolism in COVID-19 Patients: Prevalence, Predictors and Clinical Outcome. Thromb. Res. 2021, 198, 34–39. [Google Scholar] [CrossRef]
- Magrini, E.; Garlanda, C. COVID-19 Thromboinflammation: Adding Inflammatory Fibrin to the Puzzle. Trends Immunol. 2024, 45, 721–723. [Google Scholar] [CrossRef]
- Ryu, J.K.; Yan, Z.; Montano, M.; Sozmen, E.G.; Dixit, K.; Suryawanshi, R.K.; Matsui, Y.; Helmy, E.; Kaushal, P.; Makanani, S.K.; et al. Fibrin Drives Thromboinflammation and Neuropathology in COVID-19. Nature 2024, 633, 905–941. [Google Scholar] [CrossRef]
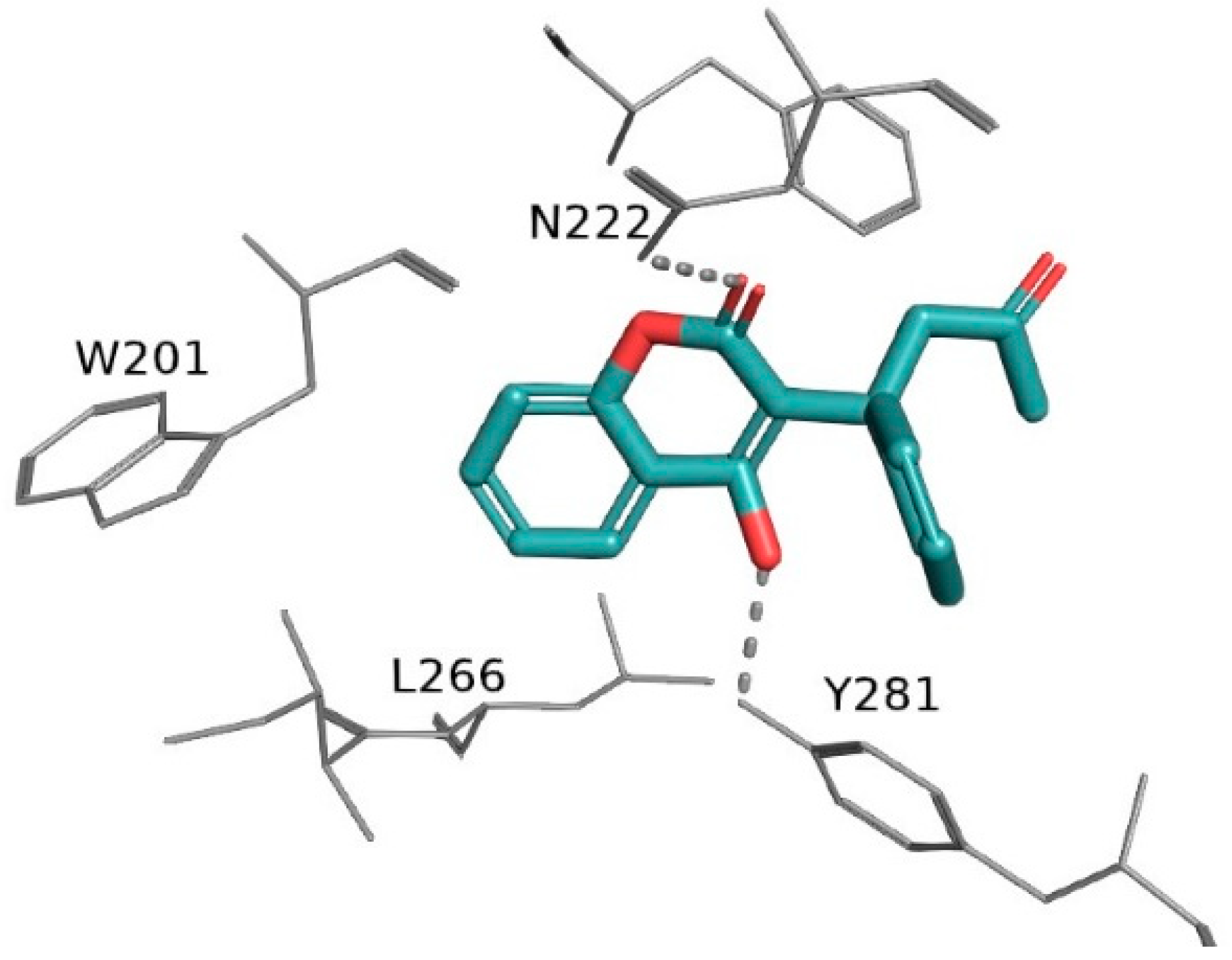
- Vicenzi, M.; Ruscica, M.; Iodice, S.; Rota, I.; Ratti, A.; Cosola, R.D.; Corsini, A.; Bollati, V.; Aliberti, S.; Blasi, F. The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients. J. Clin. Med. 2020, 9, 2943. [Google Scholar] [CrossRef]
- Kumar, N.; Zuo, Y.; Yalavarthi, S.; Hunker, K.L.; Knight, J.S.; Kanthi, Y.; Obi, A.T.; Ganesh, S.K. SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism. Viruses 2021, 13, 2209. [Google Scholar] [CrossRef]
- Fels, B.; Acharya, S.; Vahldieck, C.; Graf, T.; Käding, N.; Rupp, J.; Kusche-Vihrog, K. Mineralocorticoid Receptor-Antagonism Prevents COVID-19-Dependent Glycocalyx Damage. Pflügers Arch. Eur. J. Physiol. 2022, 474, 1069–1076. [Google Scholar] [CrossRef]
- Kim, J.; Miyazaki, K.; Shah, P.; Kozai, L.; Kewcharoen, J. Association between Mineralocorticoid Receptor Antagonist and Mortality in SARS-CoV-2 Patients: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 645. [Google Scholar] [CrossRef]
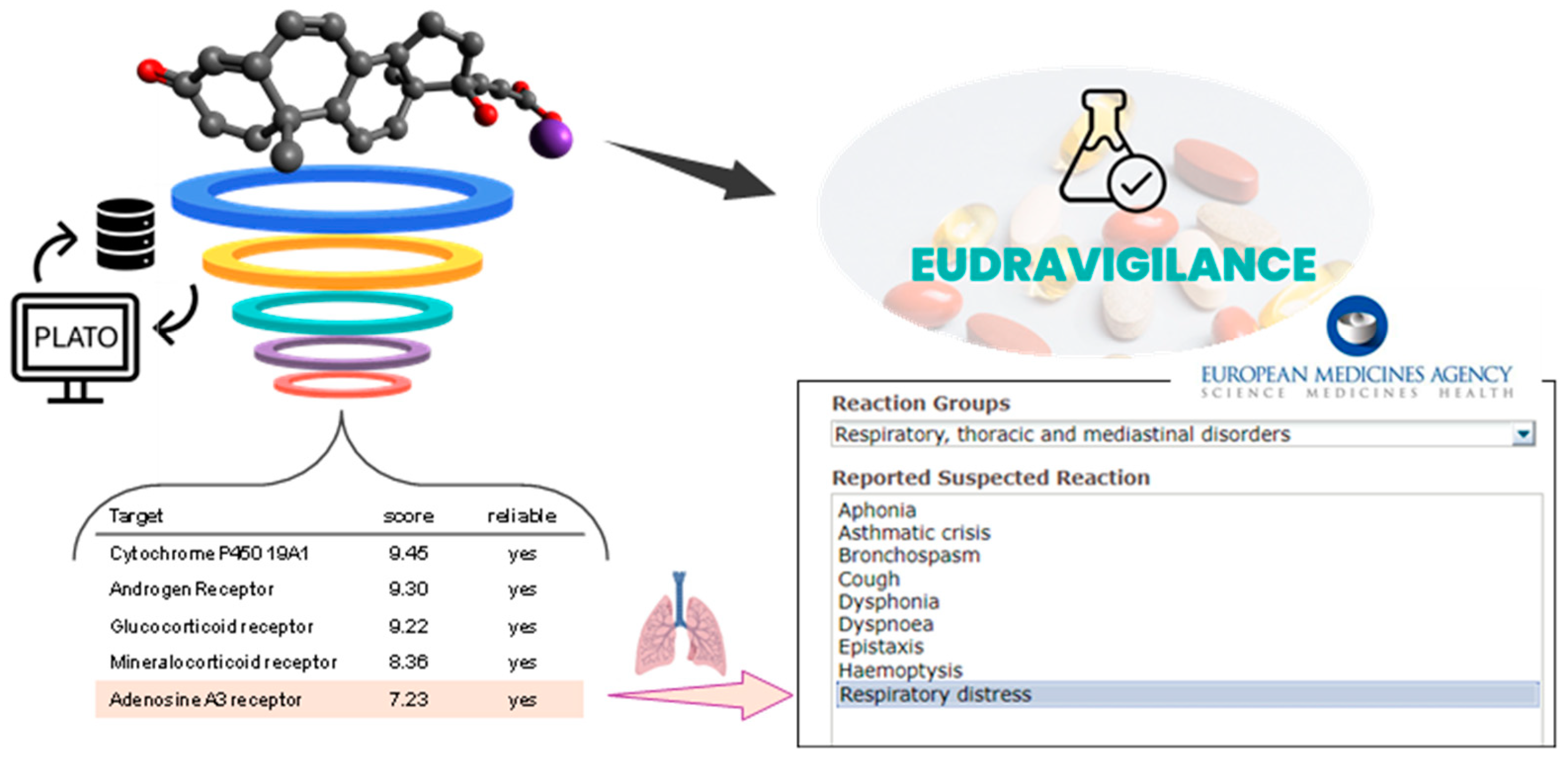
- Ciriaco, F.; Gambacorta, N.; Trisciuzzi, D.; Nicolotti, O. PLATO: A Predictive Drug Discovery Web Platform for Efficient Target Fishing and Bioactivity Profiling of Small Molecules. Int. J. Mol. Sci. 2022, 23, 5245. [Google Scholar] [CrossRef]
- Ciriaco, F.; Gambacorta, N.; Alberga, D.; Nicolotti, O. Quantitative Polypharmacology Profiling Based on a Multifingerprint Similarity Predictive Approach. J. Chem. Inf. Model. 2021, 61, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Tabrizi, M.A.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Duangrat, R.; Parichatikanond, W.; Chanmahasathien, W.; Mangmool, S. Adenosine A3 Receptor: From Molecular Signaling to Therapeutic Strategies for Heart Diseases. Int. J. Mol. Sci. 2024, 25, 5763. [Google Scholar] [CrossRef]
- Dos Anjos, F.; Simões, J.L.B.; Assmann, C.E.; Carvalho, F.B.; Bagatini, M.D. Potential Therapeutic Role of Purinergic Receptors in Cardiovascular Disease Mediated by SARS-CoV-2. J. Immunol. Res. 2020, 2020, 8632048. [Google Scholar] [CrossRef]
- Valdés, A.; Moreno, L.O.; Rello, S.R.; Orduña, A.; Bernardo, D.; Cifuentes, A. Metabolomics Study of COVID-19 Patients in Four Different Clinical Stages. Sci. Rep. 2022, 12, 1650. [Google Scholar] [CrossRef]
- Zarei, M.; Vaighan, N.S.; Ziai, S.A. Purinergic Receptor Ligands: The Cytokine Storm Attenuators, Potential Therapeutic Agents for the Treatment of COVID-19. Immunopharmacol. Immunotoxicol. 2021, 43, 633–643. [Google Scholar] [CrossRef]
- Piclidenoson for Treatment of COVID-19—A Randomized, Double-Blind, Placebo-Controlled Trial. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04333472 (accessed on 1 December 2024).
- The European Database of Sales of Veterinary Antimicrobial Agents. Available online: https://dap.ema.europa.eu/analytics/saw.dll?PortalPages (accessed on 1 December 2024).
- Gotelli, E.; Soldano, S.; Hysa, E.; Paolino, S.; Campitiello, R.; Pizzorni, C.; Sulli, A.; Smith, V.; Cutolo, M. Vitamin D and COVID-19: Narrative Review after 3 Years of Pandemic. Nutrients 2022, 14, 4907. [Google Scholar] [CrossRef]
- Getachew, B.; Tizabi, Y. Vitamin D and COVID-19: Role of ACE2, age, gender, and ethnicity. J. Med. Virol. 2021, 93, 5285–5294. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Holt, H.; Greenig, M.; Talaei, M.; Perdek, N.; Pfeffer, P.; Vivaldi, G.; Maltby, S.; Symons, J.; Barlow, N.L.; et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and COVID-19: Phase 3 randomised controlled trial (CORONAVIT). BMJ 2022, 378, e071230. [Google Scholar] [CrossRef]

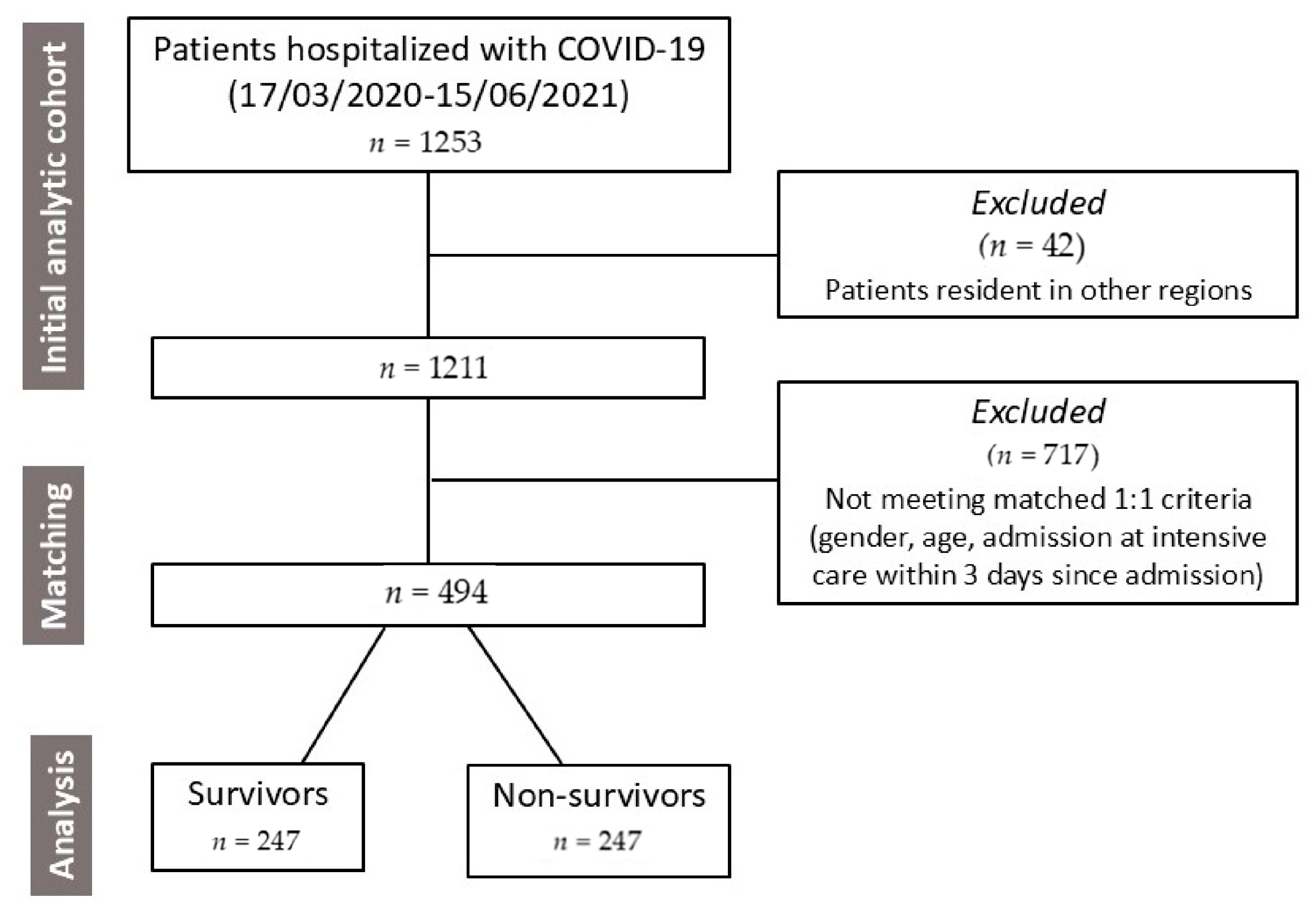

| Patients’ Characteristics | Survivors | Non-Survivors | p Value |
|---|---|---|---|
| n = 247 | n = 247 | ||
| Demographics | |||
| Age (years) | 77 ± 10 | 77 ± 10 | - |
| Males | 141 (57.1%) | 141 (57.1%) | - |
| Comorbidities | |||
| Obesity | 45 (18.2%) | 35 (14.2%) | 0.225 |
| Arterial hypertension | 195 (78.9%) | 191 (77.3%) | 0.663 |
| Dyslipidemia | 29 (11.7%) | 31 (12.6%) | 0.782 |
| Diabetes mellitus | 67 (27.1%) | 82 (33.2%) | 0.143 |
| Previous myocardial infarction | 21 (8.5%) | 28 (11.3%) | 0.307 |
| Peripheral vasculopathy | 14 (5.7%) | 20 (8.1%) | 0.289 |
| Arteriopathy | 19 (7.7%) | 39 (15.8%) | 0.007 |
| Stroke or transient ischemic attack | 20 (8.1%) | 19 (7.7%) | 0.869 |
| History of heart failure (HF) | 26 (10.5%) | 57 (23.1%) | <0.001 |
| History of cancer | 38 (15.4%) | 61 (24.7%) | 0.011 |
| Asthma | 20 (8.1%) | 39 (15.8%) | 0.007 |
| Chronic obstructive pulmonary disease (COPD) | 61 (24.7%) | 83 (33.6%) | 0.038 |
| Atrial fibrillation (AF) | 33 (13.4%) | 55 (22.3%) | 0.009 |
| Chronic liver disease | 22 (8.9%) | 24 (9.7%) | 0.758 |
| Kidney failure (KF) | 52 (21.1%) | 74 (30.0%) | 0.016 |
| Pharmacotherapy at hospital admission | |||
| ACE inhibitors a | 76 (30.8%) | 58 (23.5%) | 0.061 |
| AT2 receptor blockers b | 62 (25.1%) | 69 (27.9%) | 0.490 |
| Antidiabetics c | 51 (20.6%) | 43 (17.4%) | 0.317 |
| Aspirin | 55 (22.3%) | 74 (30.0%) | 0.051 |
| Heparins d | 11 (4.5%) | 14 (5.7%) | 0.664 |
| DOACs e | 21 (8.5%) | 25 (10.1%) | 0.547 |
| VKA (warfarin) | 12 (4.9%) | 14 (5.7%) | 0.839 |
| Vitamin D | 7 (2.8%) | 17 (6.9%) | 0.052 |
| Diuretics f | 89 (36.0%) | 103 (41.7%) | 0.174 |
| Corticosteroids g | 21 (8.5%) | 27 (10.9%) | 0.355 |
| Aldosterone antagonists h | 34 (13.8%) | 74 (30.0%) | <0.001 |
| Hypocholesterolemic agents i | 24 (9.7%) | 18 (7.3%) | 0.289 |
| Clinical Data and Interventions | Survivors | Non-Survivors | p Value |
|---|---|---|---|
| n = 247 | n = 247 | ||
| Intensive care within 3 days since admission | 25 (10.1%) | 25 (10.1%) | - |
| Systolic blood pressure (mm Hg) | 137 ± 22 | 131 ± 25 | 0.072 |
| Diastolic blood pressure (mm Hg) | 73 ± 13 | 72 ± 14 | 0.311 |
| Heart rate (b/min) | 80 ± 15 | 85 ± 17 | <0.001 |
| Severe COVID-19 | 162 (65.6%) | 190 (76.9%) | 0.003 |
| Respiratory insufficiency | 195 (78.9%) | 229 (92.7%) | <0.001 |
| Cough | 133 (53.8%) | 48 (19.4%) | <0.001 |
| Dyspnea | 199 (80.6%) | 206 (83.4%) | 0.406 |
| Fever | 134 (54.3%) | 101 (40.9%) | 0.003 |
| Asthenia | 145 (58.7%) | 174 (70.4%) | 0.006 |
| PEEP (cmH2O) a | 54 (21.9%) | 140 (56.7%) | <0.001 |
| PaO2 (mmHg) a | 70 ± 23 | 67 ± 24 | 0.087 |
| FiO2 (%) a | 28 ± 16 | 30 ± 17 | 0.241 |
| PaO2_FiO2 (mmHg) a | 285 ± 105 | 264 ± 111 | 0.007 |
| PaCO2 (mmHg) a | 38 ± 27 | 35 ± 10 | 0.542 |
| Respiratory support | |||
| Noninvasive ventilation | 39 (15.8%) | 86 (34.8%) | <0.001 |
| Mask | 146 (59.1%) | 103 (41.7%) | <0.001 |
| Mechanical ventilation | 26 (10.5%) | 84 (34.0%) | <0.001 |
| Low-flow oxygen devices | 67 (27.1%) | 31 (12.6%) | <0.001 |
| High-flow oxygen devices | 131 (53.0%) | 200 (81.0%) | <0.001 |
| Pharmacological treatment | |||
| Remdesivir | 12 (4.9%) | 3 (1.2%) | 0.035 |
| Lopinavir | 10 (4.0%) | 8 (3.2%) | 0.727 |
| Hydroxychloroquine | 24 (9.7%) | 13 (5.3%) | 0.007 |
| Azithromycin | 106 (42.9%) | 87 (35.2%) | 0.084 |
| Low-molecular-weight heparins (LMWHs) | 176 (71.3%) | 160 (64.8%) | 0.106 |
| High-molecular-weight heparins (HMWHs) | 42 (17.0%) | 88 (35.6%) | <0.001 |
| Oral anticoagulant therapy (OAT: DOACs or VKA) b | 52 (21.1%) | 53 (21.5%) | 0.904 |
| Corticosteroids c | 179 (72.5%) | 202 (81.8%) | 0.006 |
| Insulin | 71 (28.7%) | 95 (38.5%) | 0.022 |
| Aspirin | 60 (24.3%) | 82 (33.2%) | 0.026 |
| Statins d | 64 (25.9%) | 56 (22.7%) | 0.404 |
| Vitamin D | 106 (42.9%) | 69 (27.9%) | <0.001 |
| Vitamin C | 30 (12.1%) | 38 (15.4%) | 0.194 |
| Laboratory Data | Survivors | Non-Survivors | p Value |
|---|---|---|---|
| n = 247 | n = 247 | ||
| Azotemia | 62 ± 40 | 82 ± 51 | <0.001 |
| Estimated glomerular filtration rate (eGFR) | 67 ± 29 | 58 ± 30 | 0.001 |
| Potassium (mmol/L) | 4.18 ± 0.68 | 4.10 ± 0.68 | 0.179 |
| Sodium (mmol/L) | 139 ± 5 | 140 ± 7 | 0.212 |
| Lactate dehydrogenase (U/L) | 323 ± 152 | 393 ± 250 | <0.001 |
| Lipase (U/L) | 215 ± 214 | 218 ± 370 | 0.225 |
| C-reactive protein (CRP, mg/L) | 7 ± 6 | 10 ± 7 | <0.001 |
| Pro-calcitonin (ng/mL) | 0.6 ± 4.2 | 0.8 ± 2.0 | <0.001 |
| Erythrocyte sedimentation rate (mm/h) | 54 ± 30 | 58 ± 27 | 0.287 |
| Interleukin 6 (pg/mL) | 47 ± 50 | 141 ± 539 | <0.001 |
| Prothrombin time INR | 1.29 ± 0.73 | 1.23 ± 0.46 | 0.261 |
| Prothrombin time Ratio | 1.28 ± 0.70 | 1.23 ± 0.44 | 0.258 |
| Vitamin 25-OH-D3 (ng/mL) | 16 ± 10 | 19 ± 16 | 0.128 |
| Activated partial thromboplastin time ratio | 1.02 ± 0.23 | 1.15 ± 0.64 | 0.081 |
| Calcium (mg/dL) | 8.47 ± 0.61 | 8.35 ± 0.82 | 0.016 |
| Amylase (U/L) | 67 ± 46 | 68 ± 63 | 0.228 |
| Direct bilirubin (mg/dL) | 0.23 ± 0.18 | 0.38 ± 0.96 | 0.025 |
| Total bilirubin (mg/dL) | 0.63 ± 0.46 | 0.80 ± 1.22 | 0.208 |
| D-dimers (ng/mL) | 3427 ± 9634 | 6227 ± 18,858 | <0.001 |
| Ferritin (ng/mL) | 958 ± 2853 | 1197 ± 2313 | 0.007 |
| Gamma-GT (U/L) | 58 ± 82 | 88 ± 158 | 0.132 |
| Glycemia (mg/dL) | 130 ± 70 | 136 ± 63 | 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinoi, G.; Togo, M.V.; Guida, P.; Deruvo, C.; Samarelli, F.; Imbrici, P.; Nicolotti, O.; De Luca, A.; Mastroianni, F.; Liantonio, A.; et al. Retrospective Clinical Investigation into the Association Between Abnormal Blood Clotting, Oral Anticoagulant Therapy, and Medium-Term Mortality in a Cohort of COVID-19 Patients. Biomedicines 2025, 13, 535. https://doi.org/10.3390/biomedicines13030535
Dinoi G, Togo MV, Guida P, Deruvo C, Samarelli F, Imbrici P, Nicolotti O, De Luca A, Mastroianni F, Liantonio A, et al. Retrospective Clinical Investigation into the Association Between Abnormal Blood Clotting, Oral Anticoagulant Therapy, and Medium-Term Mortality in a Cohort of COVID-19 Patients. Biomedicines. 2025; 13(3):535. https://doi.org/10.3390/biomedicines13030535
Chicago/Turabian StyleDinoi, Giorgia, Maria Vittoria Togo, Pietro Guida, Caterina Deruvo, Francesco Samarelli, Paola Imbrici, Orazio Nicolotti, Annamaria De Luca, Franco Mastroianni, Antonella Liantonio, and et al. 2025. "Retrospective Clinical Investigation into the Association Between Abnormal Blood Clotting, Oral Anticoagulant Therapy, and Medium-Term Mortality in a Cohort of COVID-19 Patients" Biomedicines 13, no. 3: 535. https://doi.org/10.3390/biomedicines13030535
APA StyleDinoi, G., Togo, M. V., Guida, P., Deruvo, C., Samarelli, F., Imbrici, P., Nicolotti, O., De Luca, A., Mastroianni, F., Liantonio, A., & Altomare, C. D. (2025). Retrospective Clinical Investigation into the Association Between Abnormal Blood Clotting, Oral Anticoagulant Therapy, and Medium-Term Mortality in a Cohort of COVID-19 Patients. Biomedicines, 13(3), 535. https://doi.org/10.3390/biomedicines13030535










