A Perceived Dissociation Between Systemic Chronic Inflammation, Age, and the Telomere/Telomerase System in Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Subjects
2.2. Data Collection
2.3. Ethical Issues
2.4. Blood Sample Collection
2.5. Clinical Chemistry Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA): Plasma Insulin, Cytokines, and Telomerase Measurement
2.6.1. Insulin
2.6.2. Inflammatory Markers
2.6.3. Telomerase
2.7. DNA Extraction
2.8. Estimation of the Absolute Leukocyte Telomere Length via Real-Time PCR
2.9. Selection of TE Genes SNPs and Real-Time PCR (rtPCR) Genotyping
2.10. Statistical Analysis
3. Results
3.1. Characteristics of the Study Subjects
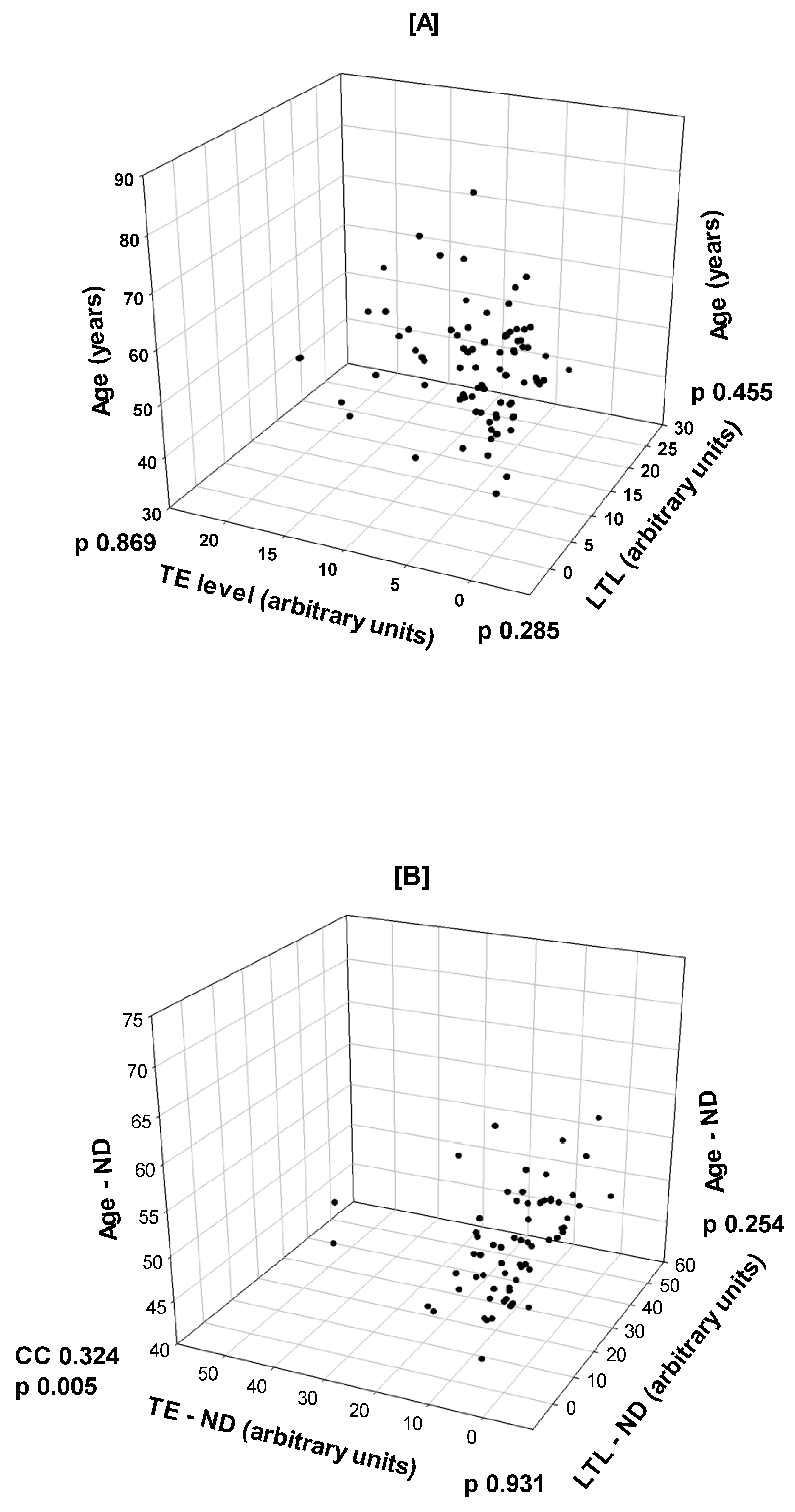
3.2. Correlations Between Age, LTL, and TE in T2D and Non-Diabetic Healthy Subjects
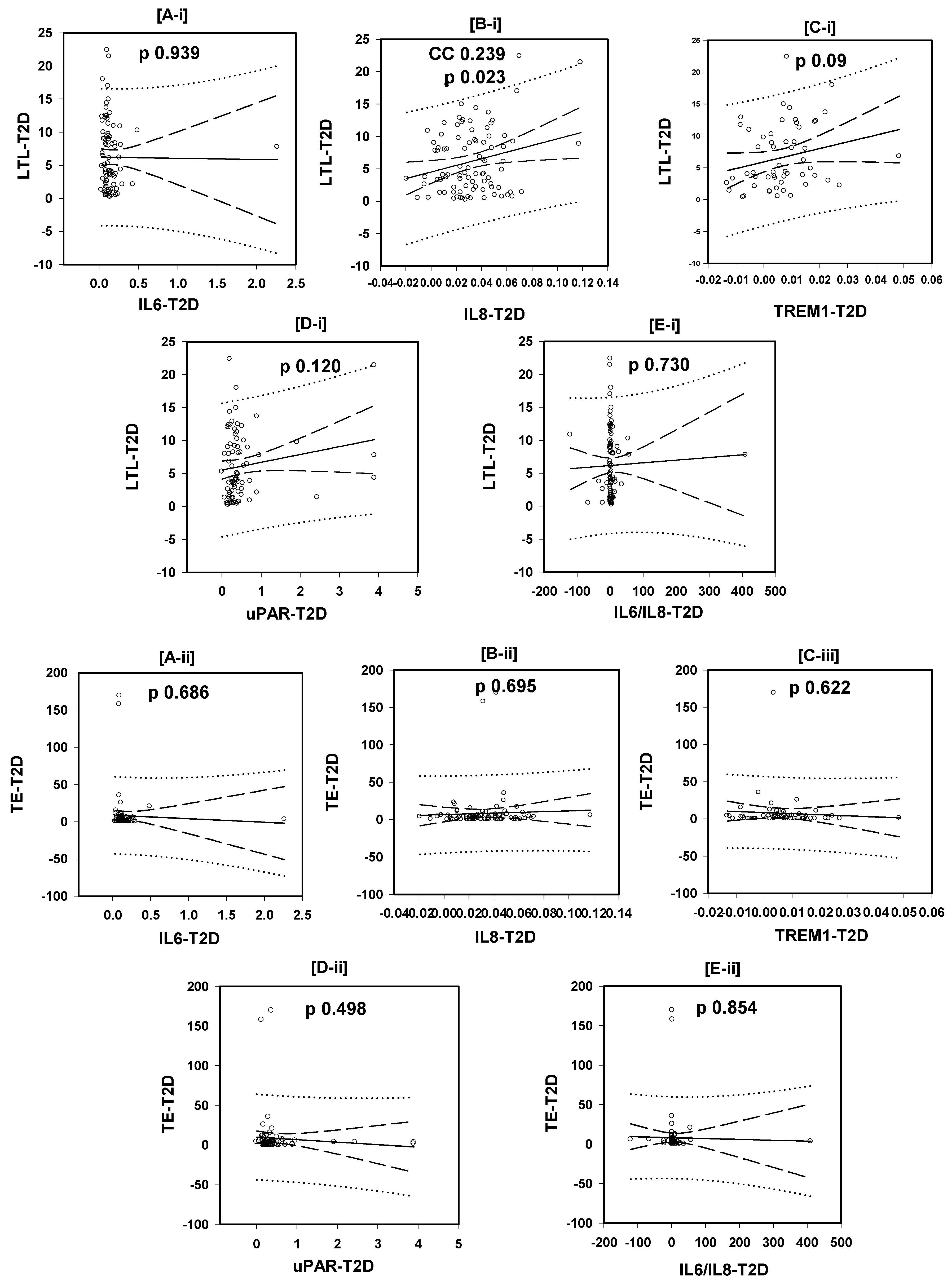
3.3. Correlations of the LTL with the Plasma Levels of the Tested Inflammatory Markers
3.4. Correlations of the Plasma TE with the Plasma Levels of the Tested Inflammatory Markers
3.5. Inter-Quartile Comparisons of the Plasma Levels of Inflammatory Markers in T2D Patients in the Different LTL and TE Quartiles
3.6. Comparisons of the Plasma Levels of the Tested Inflammatory Markers Between Diabetic Patients Carrying Different Genotypes of the Telomerase Genes’ SNPs
3.7. Correlations of the LTL and Plasma TE Concentration with the Plasma Levels of the Tested Inflammatory Markers in Healthy Subjects
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liston, A.; Masters, S.L. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat. Rev. Immunol. 2017, 17, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in aging, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-grade inflammation, diet composition, and health: Current research evidence and its translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Henao-Mejia, J.; Flavell, R.A. Innate immune receptors: Key regulators of metabolic disease progression. Cell Metab. 2013, 17, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Burcelin, R.; Garidou, L.; Pomie, C. Immuno-microbiota cross and talk: The new paradigm of metabolic diseases. Semin. Immunol. 2012, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Sater, M.S.; AlDehaini, D.M.B.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. Plasma IL-6, TREM1, uPAR, and IL6/IL8 biomarkers increment further witnessing the chronic inflammation in type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2023, 44, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.B.; Justice, J.N.; Nicklas, B.J.; Kirkland, J.L. Physiological Aging: Links Among Adipose Tissue Dysfunction, Diabetes, and Frailty. Physiology 2017, 32, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]
- von Zglinicki, T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann. N. Y. Acad. Sci. 2000, 908, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B. Kleemeier Award Lecture 2008—The canary in the coal mine: Telomeres and human healthspan. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 511–515. [Google Scholar] [CrossRef]
- Hohensinner, P.J.; Goronzy, J.J.; Weyand, C.M. Telomere dysfunction, autoimmunity, and aging. Aging Dis. 2011, 2, 524–537. [Google Scholar]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.-C.; Satterfield, S.; et al. Health Aging and Body Composition Study. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging, and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Meier, U.T. Architecture and assembly of mammalian H/ACA small nucleolar and telomerase ribonucleoproteins. EMBO J. 2004, 23, 1857–1867. [Google Scholar] [CrossRef]
- Bekaert, S.; Derradji, H.; Baatout, S. Telomere biology in mammalian germ cells and during development. Dev. Biol. 2004, 274, 15–30. [Google Scholar] [CrossRef]
- AlDehaini, D.M.B.; Al-Bustan, S.A.; Ali, M.E.; Malalla, Z.H.A.; Sater, M.; Giha, H.A. Shortening of the leucocytes’ telomeres length in T2DM independent of age and telomerase activity. Acta Diabetol. 2020, 57, 1287–1295. [Google Scholar] [CrossRef]
- AlDehaini, D.M.B.; Al-Bustan, S.A.; Malalla, Z.H.A.; Ali, M.E.; Sater, M.; Giha, H.A. The influence of TERC, TERT, and ACYP2 genes polymorphisms on plasma telomerase concentration, telomeres length, and T2DM. Gene 2021, 766, 145127. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.L.; Kronmal, R.A.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Walston, J.; Kimura, M.; Aviv, A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 2007, 165, 14–21. [Google Scholar] [CrossRef]
- Masi, S.; Nightingale, C.M.; Day, I.N.; Guthrie, P.; Rumley, A.; Lowe, G.D.; von Zglinicki, T.; D’Aiuto, F.; Taddei, S.; Klein, N.; et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2029–2034. [Google Scholar] [CrossRef]
- Opresko, P.L.; Fan, J.; Danzy, S.; Wilson, D.M., 3rd; Bohr, V.A. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005, 33, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Zeka, A.; Sullivan, J.R.; Vokonas, P.S.; Sparrow, D.; Schwartz, J. Inflammatory markers and particulate air pollution: Characterizing the pathway to disease. Int. J. Epidemiol. 2006, 35, 1347–1354. [Google Scholar] [CrossRef]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Aging and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Riera, C.E.; Merkwirth, C.; De Magalhaes Filho, C.D.; Dillin, A. Signaling Networks Determining Life Span. Annu. Rev. Biochem. 2016, 85, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Laterveer, L.; Lindley, I.J.; Hamilton, M.S.; Willemze, R.; Fibbe, W.E. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood 1995, 85, 2269–2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Ge, Y.; Liu, J.; Zhao, Y. TERC promotes cellular inflammatory response independent of telomerase. Nucleic Acids Res. 2019, 47, 8084–8095. [Google Scholar] [CrossRef]
- Jaiswal, R.K.; Yadava, P.K. TGF-β-mediated regulation of plasminogen activators is human telomerase reverse transcriptase dependent in cancer cells. Biofactors 2019, 45, 803–817. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Abdi, S.; Sabico, S.; Alnaami, A.M.; Wani, K.A.; Ansari, M.G.A.; Khattak, M.N.K.; Khan, N.; Tripathi, G.; Chrousos, G.P.; et al. Gut-Derived Endotoxin and Telomere Length Attrition in Adults with and without Type 2 Diabetes. Biomolecules 2021, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhu, W.; Hu, S.; Yu, X.; Yang, Y. Association between oxidative stress and telomere length in Type 1 and Type 2 diabetic patients. J. Endocrinol. Investig. 2013, 36, 1032–1037. [Google Scholar]
- Qi Nan, W.; Ling, Z.; Bing, C. The influence of the telomere-telomerase system on diabetes mellitus and its vascular complications. Expert. Opin. Ther. Targets 2015, 19, 849–864. [Google Scholar] [CrossRef]
- Zhou, Y.; Ning, Z.; Lee, Y.; Hambly, B.D.; McLachlan, C.S. Shortened leukocyte telomere length in type 2 diabetes mellitus: Genetic polymorphisms in mitochondrial uncoupling proteins and telomeric pathways. Clin. Transl. Med. 2016, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Pudas, S.; Josefsson, M.; Nordin Adolfsson, A.; Landfors, M.; Kauppi, K.; Veng-Taasti, L.M.; Hultdin, M.; Adolfsson, R.; Degerman, S. Short Leukocyte Telomeres, But Not Telomere Attrition Rates, Predict Memory Decline in the 20-Year Longitudinal Betula Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef] [PubMed]
- Atzmon, G.; Cho, M.; Cawthon, R.M.; Budagov, T.; Katz, M.; Yang, X.; Siegel, G.; Bergman, A.; Huffman, D.M.; Schechter, C.B.; et al. Evolution in health and medicine Sackler colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 1), 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.M.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008, 11, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Kuhlow, D.; Florian, S.; von Figura, G.; Weimer, S.; Schulz, N.; Petzke, K.J.; Zarse, K.; Pfeiffer, A.F.; Rudolph, K.L.; Ristow, M. Telomerase deficiency impairs glucose metabolism and insulin secretion. Aging 2010, 2, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 1997, 33, 787–791. [Google Scholar] [CrossRef] [PubMed]

| Variables | T2D Patients | Healthy Controls | p-Value | Stat Test |
|---|---|---|---|---|
| Number | 118 | 74 | ||
| Sex (M/F) | 53/53 * | 27/47 | ||
| Age | 56.5, 52.0–64.0 | 51.0, 49.0–55.000 | <0.001 | MW |
| BMI | 30.81, 26.83–35.59 | 28.89, 26.56–33.30 | 0.149 | MW |
| HOMA-IR | 12.32, 7.85–17.60 | 2.76, 1.30–3.99 | <0.001 | MW |
| Insulin | 34.09, 25.78–50.38 | 12.23, 8.72–16.37 | <0.001 | MW |
| HbA1c | 7.65, 7.00–8.60 | 5.90, 5.65–6.25 | <0.001 | MW |
| Inflammatory Marker | Levels (Arbitrary Units) | p-Value | |||
|---|---|---|---|---|---|
| LTL | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | KW |
| IL6 | 0.137, 0.092–0.189 | 0.139, 0.101–0.163 | 0.123, 0.081–0.160 | 0.110, 0.092–0.129 | 0.486 |
| IL8 | 0.033 ± 0.022 | 0.023 ± 0.019 | 0.036 ± 0.025 | 0.038 ± 0.027 | 0.169 * |
| TREM1 | 0.0001 ± 0.0072 | 0.0058 ± 0.0110 | 0.0112 ± 0.0132 | 0.0089 ± 0.010 | 0.100 * |
| uPAR | 0.258, 0.198–0.409 | 0.353, 0.280–0.428 | 0.399, 0.226–0.568 | 0.356, 0.209–0.399 | 0.329 |
| IL6/IL8 | 3.635, 2.493–6.918 | 3.797, 2.143–9.022 | 3.828, 1.679–9.008 | 2.733, 1.832–4.842 | 0.672 |
| TE Levels | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |
| IL6 | 0.100, 0.075–0.166 | 0.143, 0.102–0.203 | 0.123, 0.104–0.173 | 0.100, 0.086–0.125 | 0.067 |
| IL8 | 0.0336 ± 0.0176 | 0.0313 ± 0.0215 | 0.0285 ± 0.0298 | 0.0355 ± 0.0171 | 0.765 * |
| TREM1 | 0.0064 ± 0.0111 | 0.0093 ± 0.0157 | 0.0052 ±0.0098 | 0.0055 ± 0.0073 | 0.801 * |
| uPAR | 0.355, 0.270–0.549 | 0.324, 0.201–0.506 | 0.368, 0.229–0.437 | 0.268, 0.173–0.388 | 0.312 |
| IL6/IL8 | 3.90, 1.89–5.82 | 3.85, 2.13–8.84 | 3.19, 1.96–5.05 | 2.41, 1.99–3.99 | 0.538 |
| Genotype | IL6 Level | IL8 Level | TREM1 Level | uPAR Level | IL6/IL8 Ratio |
|---|---|---|---|---|---|
| Tested SNPs of TE genes TERC rs12696304 G/C | |||||
| GG | 0.116, 0.090–0.215 | 0.024 ± 0.021 | 0.000 ± 0.007 | 0.296, 0.233–0.372 | 3.04, 2.00–4.67 |
| GC | 0.132, 0.103–0.174 | 0.033 ± 0.024 | 0.008 ± 0.014 | 0.339, 0.205–0.410 | 3.77, 2.29–7.40 |
| CC | 0.112, 0.094–0.159 | 0.039 ± 0.027 | 0.008 ± 0.009 | 0.315, 0.235–0.527 | 2.88, 1.62–5.32 |
| p-value, KW | p = 0.463 | p = 0.179 * | p = 0.060 * | p = 0.741 | p = 0.473 |
| TERT rs2736100 C/A | |||||
| CC | 0.125, 0.100–0.180 | 0.051, 0.043–0.056 | 0.007 ± 0.000 | 0.369, 0.255–0.577 | 2.52, 1.67–4.73 |
| CA | 0.124, 0.093–0.183 | 0.033, 0.019–0.045 | 0.007 ± 0.013 | 0.321, 0.248–0.409 | 3.52, 2.20–4.88 |
| AA | 0.123, 0.092–0.160 | 0.027, 0.016–0.043 | 0.006 ± 0.010 | 0.296, 0.198–0.452 | 3.13, 1.65–7.08 |
| p-value, KW | p = 0.974 | p = 0.057 | p = 0.552 * | p = 0.782 | p = 0.880 |
| ACYP2 rs6713088 G/C | |||||
| GG | 0.124, 0.095–0.216 | 0.033 ± 0.019 | 0.007 ± 0.012 | 0.365, 0.294–0.579 | 3.64, 2.69–7.76 |
| GC | 0.123, 0.094–0.163 | 0.030 ± 0.023 | 0.005 ± 0.009 | 0.296, 0.202–0.394 | 3.15, 1.91–6.51 |
| CC | 0.126, 0.087–0.156 | 0.041 ± 0.029 | 0.007 ± 0.015 | 0.340, 0.237–0.476 | 2.88, 1.53–5.19 |
| p-value, KW | p = 0.778 | p = 0.200 * | p = 0.841 * | p = 0.210 | p = 0.392 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sater, M.S.; AlDehaini, D.M.B.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. A Perceived Dissociation Between Systemic Chronic Inflammation, Age, and the Telomere/Telomerase System in Type 2 Diabetes. Biomedicines 2025, 13, 531. https://doi.org/10.3390/biomedicines13030531
Sater MS, AlDehaini DMB, Malalla ZHA, Ali ME, Giha HA. A Perceived Dissociation Between Systemic Chronic Inflammation, Age, and the Telomere/Telomerase System in Type 2 Diabetes. Biomedicines. 2025; 13(3):531. https://doi.org/10.3390/biomedicines13030531
Chicago/Turabian StyleSater, Mai S., Dhuha M. B. AlDehaini, Zainab H. A. Malalla, Muhalab E. Ali, and Hayder A. Giha. 2025. "A Perceived Dissociation Between Systemic Chronic Inflammation, Age, and the Telomere/Telomerase System in Type 2 Diabetes" Biomedicines 13, no. 3: 531. https://doi.org/10.3390/biomedicines13030531
APA StyleSater, M. S., AlDehaini, D. M. B., Malalla, Z. H. A., Ali, M. E., & Giha, H. A. (2025). A Perceived Dissociation Between Systemic Chronic Inflammation, Age, and the Telomere/Telomerase System in Type 2 Diabetes. Biomedicines, 13(3), 531. https://doi.org/10.3390/biomedicines13030531







