Notch1 siRNA and AMD3100 Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Isolation and Differentiation of Primary Mouse Bone Marrow Cells and Neutrophils
2.4. Alternation of Macrophage Phenotypes and Assessment of NET Release
2.5. Western Blot and PCR
2.6. Migration Assay
2.7. Therapeutic Studies
2.8. Statistical Analysis
3. Results
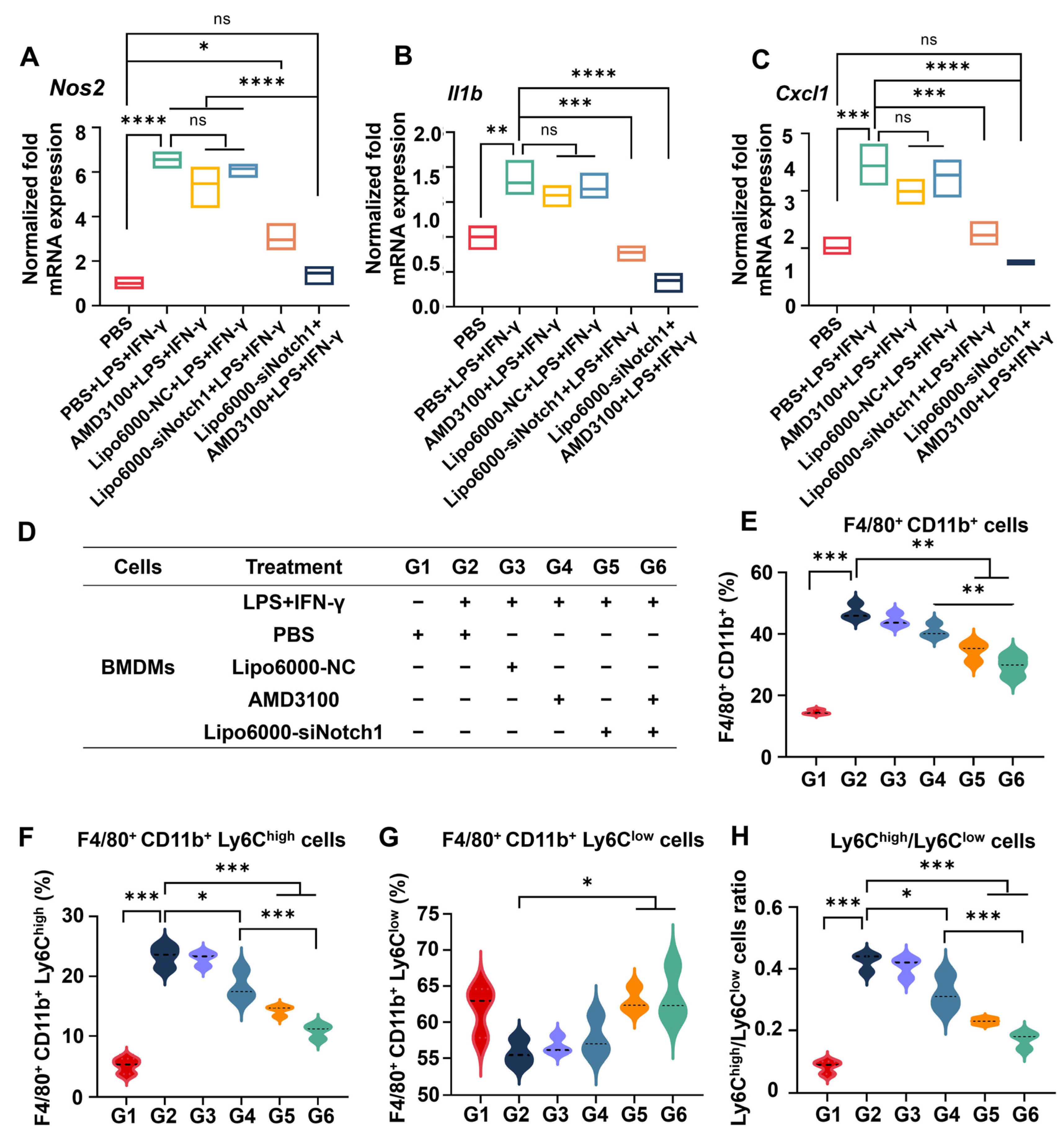
3.1. siNotch1 and AMD3100 Decreased the Expression of Notch Pathway-Related Proteins and Genes in Macrophages
3.2. siNotch1 and AMD3100 Together Regulated the Crosstalk Between Macrophages and Neutrophils
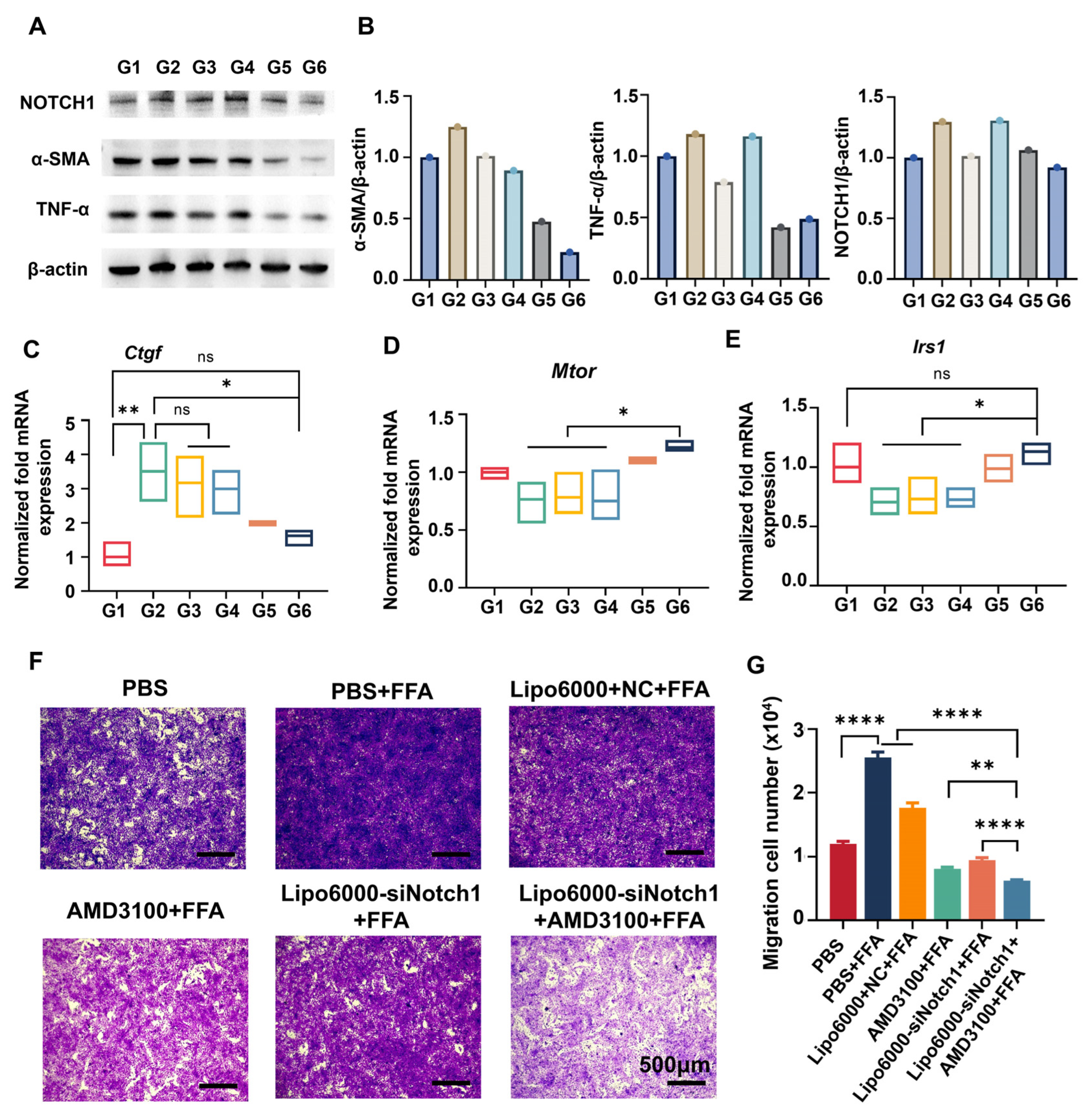
3.3. siNotch1 and AMD3100 Suppressed the Migration of BMDMs and Restored the Hepatocyte Function
3.4. siNotch1 and AMD3100 Modulated Macrophage–HSC Crosstalk
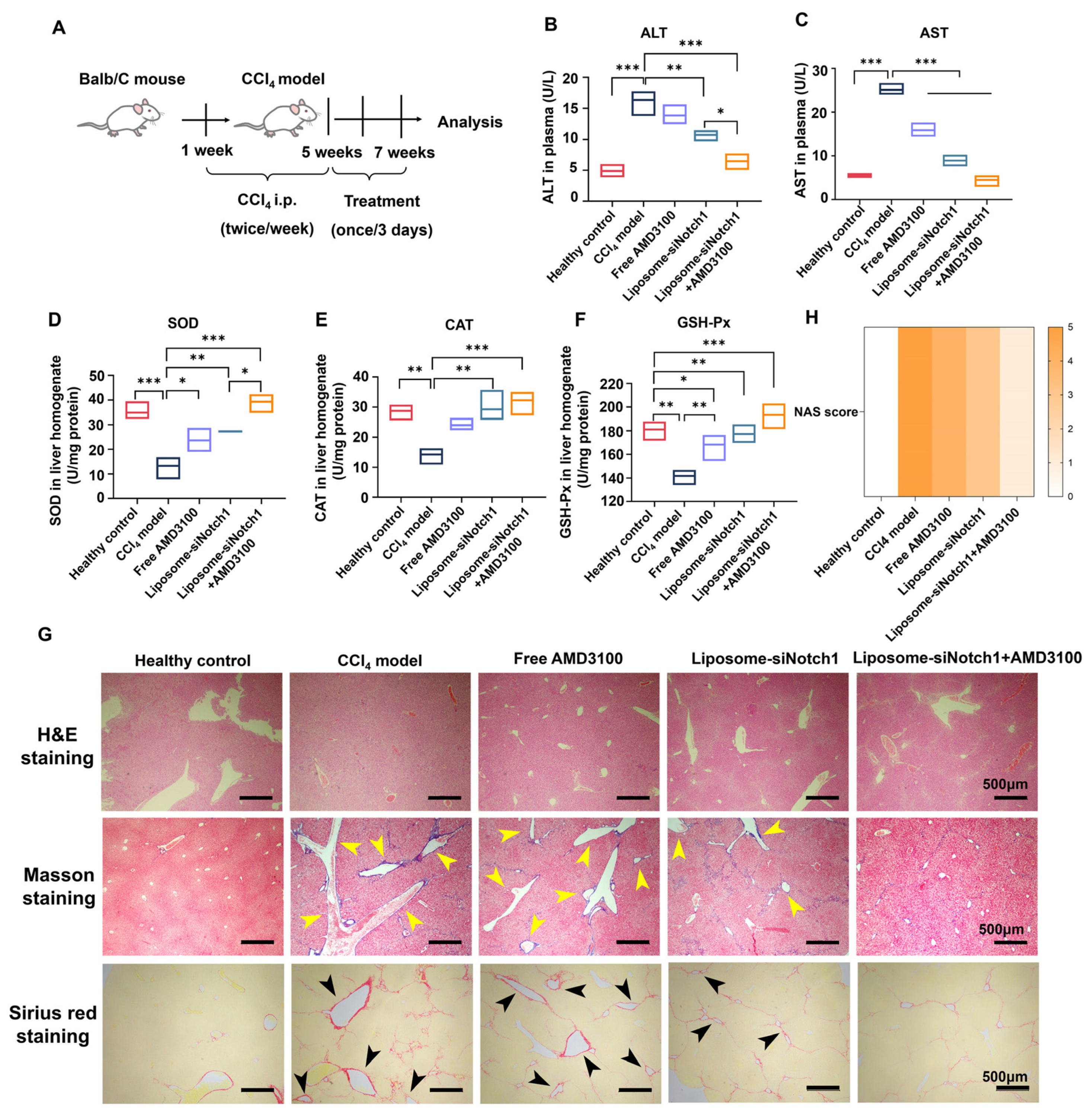
3.5. Combination of AMD3100 and siNotch1 Ameliorates Inflammation, Fibrosis, and Oxidative Stress in a CCl4-Induced MASLD Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Nassir, F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules 2022, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A Systemic Metabolic Disorder with Cardiovascular and Malignant Complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current Status and Future Trends of the Global Burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Ma, L.; Gao, W. Comprehensive Molecular Mechanisms and Clinical Therapy in Nonalcoholic Steatohepatitis: An Overview and Current Perspectives. Metabolism 2022, 134, 155264. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Yu, M.; Yang, F.; Tu, M.; Xu, H. Notch Signaling Regulates Microglial Activation and Inflammatory Reactions in a Rat Model of Temporal Lobe Epilepsy. Neurochem. Res. 2018, 43, 1269–1282. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L. The Role of Notch Signaling Pathway in Non-Alcoholic Fatty Liver Disease. Front. Mol. Biosci. 2021, 8, 792667. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Chen, J.; Ma, Y.; Song, Y.; Cen, Y.; You, M.; Yang, G. The Notch Signaling Pathway Regulates Macrophage Polarization in Liver Diseases. Int. Immunopharmacol. 2021, 99, 107938. [Google Scholar] [CrossRef]
- Palaga, T.; Wongchana, W.; Kueanjinda, P. Notch Signaling in Macrophages in the Context of Cancer Immunity. Front. Immunol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Gamrekelashvili, J.; Kapanadze, T.; Sablotny, S.; Ratiu, C.; Dastagir, K.; Lochner, M.; Karbach, S.; Wenzel, P.; Sitnow, A.; Fleig, S.; et al. Notch and TLR Signaling Coordinate Monocyte Cell Fate and Inflammation. eLife 2020, 9, e57007. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tannous, B.A.; Poznansky, M.C.; Chen, H. CXCR4 Antagonist AMD3100 (plerixafor): From an Impurity to a Therapeutic Agent. Pharmacol. Res. 2020, 159, 105010. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, S.; Li, Y.; Qian, X.; Luan, J.; Lv, X. Emerging Importance of Chemokine Receptor CXCR4 and Its Ligand in Liver Disease. Front. Cell Dev. Biol. 2021, 9, 716842. [Google Scholar] [CrossRef]
- Cui, L.N.; Zheng, X.H.; Yu, J.H.; Han, Y. Role of CXCL12-CXCR4/CXCR7 Signal Axis in Liver Regeneration and Liver Fibrosis. Zhonghua Gan Zang Bing Za Zhi 2021, 29, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Kumar, V.; Lin, F.; Kumar, V.; Bhattarai, R.; Bhatt, V.R.; Tan, C.; Mahato, R.I. Redox-Responsive Nanoplatform for Codelivery of miR-519c and Gemcitabine for Pancreatic Cancer Therapy. Sci. Adv. 2020, 6, eabd6764. [Google Scholar] [CrossRef] [PubMed]
- Sieve, I.; Ricke-Hoch, M.; Kasten, M.; Battmer, K.; Stapel, B.; Falk, C.S.; Leisegang, M.S.; Haverich, A.; Scherr, M.; Hilfiker-Kleiner, D. A Positive Feedback Loop between IL-1β, LPS and NEU1 May Promote Atherosclerosis by Enhancing a pro-Inflammatory State in Monocytes and Macrophages. Vasc. Pharmacol. 2018, 103–105, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Ogura, T.; Hokari, A.; Weisz, A.; Yamashita, J.; Esumi, H. Inducible Nitric Oxide Synthase in a Human Glioblastoma Cell Line. J. Neurochem. 1995, 64, 85–91. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Zimmermann, H.W. Macrophage Heterogeneity in Liver Injury and Fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhang, Y.; Pan, G.; Xiang, L.X.; Luo, D.C.; Shao, J.Z. Occurrences and Functions of Ly6C(hi) and Ly6C(lo) Macrophages in Health and Disease. Front. Immunol. 2022, 13, 901672. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in Chronic Inflammatory Diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil Extracellular Traps (NETs) in Autoimmune Diseases: A Comprehensive Review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, F.S.; Xu, R. Neutrophils in Liver Diseases: Pathogenesis and Therapeutic Targets. Cell Mol. Immunol. 2021, 18, 38–44. [Google Scholar] [CrossRef]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular Mechanisms of NETosis. Annu. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef]
- Fa, P.; Ke, B.G.; Dupre, A.; Tsung, A.; Zhang, H. The Implication of Neutrophil Extracellular Traps in Nonalcoholic Fatty Liver Disease. Front. Immunol. 2023, 14, 1292679. [Google Scholar] [CrossRef]
- Daemen, S.; Gainullina, A.; Kalugotla, G.; He, L.; Chan, M.M.; Beals, J.W.; Liss, K.H.; Klein, S.; Feldstein, A.E.; Finck, B.N.; et al. Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH. Cell Rep. 2022, 41, 111660. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D., Jr.; Kipp, Z.A.; Xu, M.; Yiannikouris, F.B.; Morris, A.J.; Stec, D.F.; Wahli, W.; Stec, D.E. Adipose-Specific PPARα Knockout Mice Have Increased Lipogenesis by PASK-SREBP1 Signaling and a Polarity Shift to Inflammatory Macrophages in White Adipose Tissue. Cells 2021, 11, 4. [Google Scholar] [CrossRef]
- Hung, C.T.; Su, T.H.; Chen, Y.T.; Wu, Y.F.; Chen, Y.T.; Lin, S.J.; Lin, S.L.; Yang, K.C. Targeting ER Protein TXNDC5 in Hepatic Stellate Cell Mitigates Liver Fibrosis by Repressing Non-Canonical TGFβ Signalling. Gut 2022, 71, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Tacke, F.; Schwabe, R.F.; Henderson, N.C. Understanding the Cellular Interactome of Non-Alcoholic Fatty Liver Disease. JHEP Rep. 2022, 4, 100524. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; He, H.; Wang, T.; Su, N.; Zhang, F.; Jiang, K.; Zhu, J.; Zhang, C.; Niu, K.; Wang, L.; et al. Single-Cell Transcriptomic Analysis Reveals a Hepatic Stellate Cell-Activation Roadmap and Myofibroblast Origin During Liver Fibrosis in Mice. Hepatology 2021, 74, 2774–2790. [Google Scholar] [CrossRef]
- Rahimi, S.; Angaji, S.A.; Majd, A.; Hatami, B.; Baghaei, K. A Fast and Accurate Mouse Model for Inducing Non-Alcoholic Steatohepatitis. Gastroenterol. Hepatol. Bed Bench 2022, 15, 406–414. [Google Scholar] [CrossRef]
- Oh, R.C.; Hustead, T.R. Causes and Evaluation of Mildly Elevated Liver Transaminase Levels. Am. Fam. Physician 2011, 84, 1003–1008. [Google Scholar] [PubMed]
- Grattagliano, I.; de Bari, O.; Bernardo, T.C.; Oliveira, P.J.; Wang, D.Q.; Portincasa, P. Role of Mitochondria in Nonalcoholic Fatty Liver Disease—From Origin to Propagation. Clin. Biochem. 2012, 45, 610–618. [Google Scholar] [CrossRef]
- Newsome, P.N.; Cramb, R.; Davison, S.M.; Dillon, J.F.; Foulerton, M.; Godfrey, E.M.; Hall, R.; Harrower, U.; Hudson, M.; Langford, A.; et al. Guidelines on the Management of Abnormal Liver Blood Tests. Gut 2018, 67, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Schwärzler, J.; Grabherr, F.; Grander, C.; Adolph, T.E.; Tilg, H. The Pathophysiology of MASLD: An Immunometabolic Perspective. Expert. Rev. Clin. Immunol. 2024, 20, 375–386. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Zhang, J.; Muise, E.S.; Han, S.; Kutchukian, P.S.; Costet, P.; Zhu, Y.; Kan, Y.; Zhou, H.; Shah, V.; Huang, Y.; et al. Molecular Profiling Reveals a Common Metabolic Signature of Tissue Fibrosis. Cell Rep. Med. 2020, 1, 100056. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Wong, V.W.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for Patients with Bridging Fibrosis or Compensated Cirrhosis Due to NASH: Results from Randomized Phase III STELLAR Trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef]
- Goyal, N.P.; Mencin, A.; Newton, K.P.; Durelle, J.; Carrier, C.; Ugalde-Nicalo, P.; Noel, B.; Mouton, J.; Vargas, D.; Magrez, D.; et al. An Open Label, Randomized, Multicenter Study of Elafibranor in Children with Nonalcoholic Steatohepatitis. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Boujedidi, H.; Robert, O.; Bignon, A.; Cassard-Doulcier, A.M.; Renoud, M.L.; Gary-Gouy, H.; Hemon, P.; Tharinger, H.; Prévot, S.; Bachelerie, F.; et al. CXCR4 Dysfunction in Non-Alcoholic Steatohepatitis in Mice and Patients. Clin. Sci. 2015, 128, 257–267. [Google Scholar] [CrossRef]
- Liu, C.H.; Chan, K.M.; Chiang, T.; Liu, J.Y.; Chern, G.G.; Hsu, F.F.; Wu, Y.H.; Liu, Y.C.; Chen, Y. Dual-Functional Nanoparticles Targeting CXCR4 and Delivering Antiangiogenic siRNA Ameliorate Liver Fibrosis. Mol. Pharm. 2016, 13, 2253–2262. [Google Scholar] [CrossRef]
- Bansal, R.; van Baarlen, J.; Storm, G.; Prakash, J. The Interplay of the Notch Signaling in Hepatic Stellate Cells and Macrophages Determines the Fate of Liver Fibrogenesis. Sci. Rep. 2015, 5, 18272. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, B.; Chen, Y.; Yu, F. Capsaicin Attenuates Liver Fibrosis by Targeting Notch Signaling to Inhibit TNF-α Secretion from M1 Macrophages. Immunopharmacol. Immunotoxicol. 2020, 42, 556–563. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Colombo, M.; Bulfamante, G.; Falleni, M.; Tosi, D.; Garavelli, S.; De Simone, D.; Vigolo, E.; Todoerti, K.; Neri, A.; et al. Notch Pathway Promotes Ovarian Cancer Growth and Migration via CXCR4/SDF1α Chemokine System. Int. J. Biochem. Cell Biol. 2015, 66, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Tsaouli, G.; Ferretti, E.; Bellavia, D.; Vacca, A.; Felli, M.P. Notch/CXCR4 Partnership in Acute Lymphoblastic Leukemia Progression. J. Immunol. Res. 2019, 2019, 5601396. [Google Scholar] [CrossRef]
- Soehnlein, O.; Kai-Larsen, Y.; Frithiof, R.; Sorensen, O.E.; Kenne, E.; Scharffetter-Kochanek, K.; Eriksson, E.E.; Herwald, H.; Agerberth, B.; Lindbom, L. Neutrophil Primary Granule Proteins HBP and HNP1-3 Boost Bacterial Phagocytosis by Human and Murine Macrophages. J. Clin. Investig. 2008, 118, 3491–3502. [Google Scholar] [CrossRef]
- Song, C.; Li, H.; Li, Y.; Dai, M.; Zhang, L.; Liu, S.; Tan, H.; Deng, P.; Liu, J.; Mao, Z.; et al. NETs Promote ALI/ARDS Inflammation by Regulating Alveolar Macrophage Polarization. Exp. Cell Res. 2019, 382, 111486. [Google Scholar] [CrossRef]
- Rhys, H.I.; Dell’Accio, F.; Pitzalis, C.; Moore, A.; Norling, L.V.; Perretti, M. Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages. eBioMedicine 2018, 29, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Lazzaretto, B.; Fadeel, B. Intra- and Extracellular Degradation of Neutrophil Extracellular Traps by Macrophages and Dendritic Cells. J. Immunol. 2019, 203, 2276–2290. [Google Scholar] [CrossRef]
- Haider, P.; Kral-Pointner, J.B.; Mayer, J.; Richter, M.; Kaun, C.; Brostjan, C.; Eilenberg, W.; Fischer, M.B.; Speidl, W.S.; Hengstenberg, C.; et al. Neutrophil Extracellular Trap Degradation by Differently Polarized Macrophage Subsets. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2265–2278. [Google Scholar] [CrossRef]
- Nakazawa, D.; Shida, H.; Kusunoki, Y.; Miyoshi, A.; Nishio, S.; Tomaru, U.; Atsumi, T.; Ishizu, A. The Responses of Macrophages in Interaction with Neutrophils That Undergo Netosis. J. Autoimmun. 2016, 67, 19–28. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose Tissue Remodeling and Obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Circadian Rhythms of Liver Physiology and Disease: Experimental and Clinical Evidence. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 217–226. [Google Scholar] [CrossRef]
- Bolshette, N.; Ibrahim, H.; Reinke, H.; Asher, G. Circadian Regulation of Liver Function: From Molecular Mechanisms to Disease Pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A Circadian Clock in Macrophages Controls Inflammatory Immune Responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef]
- Crouchet, E.; Dachraoui, M.; Jühling, F.; Roehlen, N.; Oudot, M.A.; Durand, S.C.; Ponsolles, C.; Gadenne, C.; Meiss-Heydmann, L.; Moehlin, J.; et al. Targeting the Liver Clock Improves Fibrosis by Restoring TGF-β Signaling. J. Hepatol. 2025, 82, 120–133. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Cheng, Y.; Yang, L.; Lyu, Y.; Li, J.; Zhao, P.; Zhu, Y.; Xin, X.; Yin, L. Notch1 siRNA and AMD3100 Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines 2025, 13, 486. https://doi.org/10.3390/biomedicines13020486
Zhu C, Cheng Y, Yang L, Lyu Y, Li J, Zhao P, Zhu Y, Xin X, Yin L. Notch1 siRNA and AMD3100 Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines. 2025; 13(2):486. https://doi.org/10.3390/biomedicines13020486
Chicago/Turabian StyleZhu, Chunli, Yiheng Cheng, Lei Yang, Yifu Lyu, Jingjing Li, Pengbo Zhao, Ying Zhu, Xiaofei Xin, and Lifang Yin. 2025. "Notch1 siRNA and AMD3100 Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease" Biomedicines 13, no. 2: 486. https://doi.org/10.3390/biomedicines13020486
APA StyleZhu, C., Cheng, Y., Yang, L., Lyu, Y., Li, J., Zhao, P., Zhu, Y., Xin, X., & Yin, L. (2025). Notch1 siRNA and AMD3100 Ameliorate Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines, 13(2), 486. https://doi.org/10.3390/biomedicines13020486







