Mechanisms for Orofacial Pain: Roles of Immunomodulation, Metabolic Reprogramming, Oxidative Stress and Epigenetic Regulation
Abstract
1. Introduction
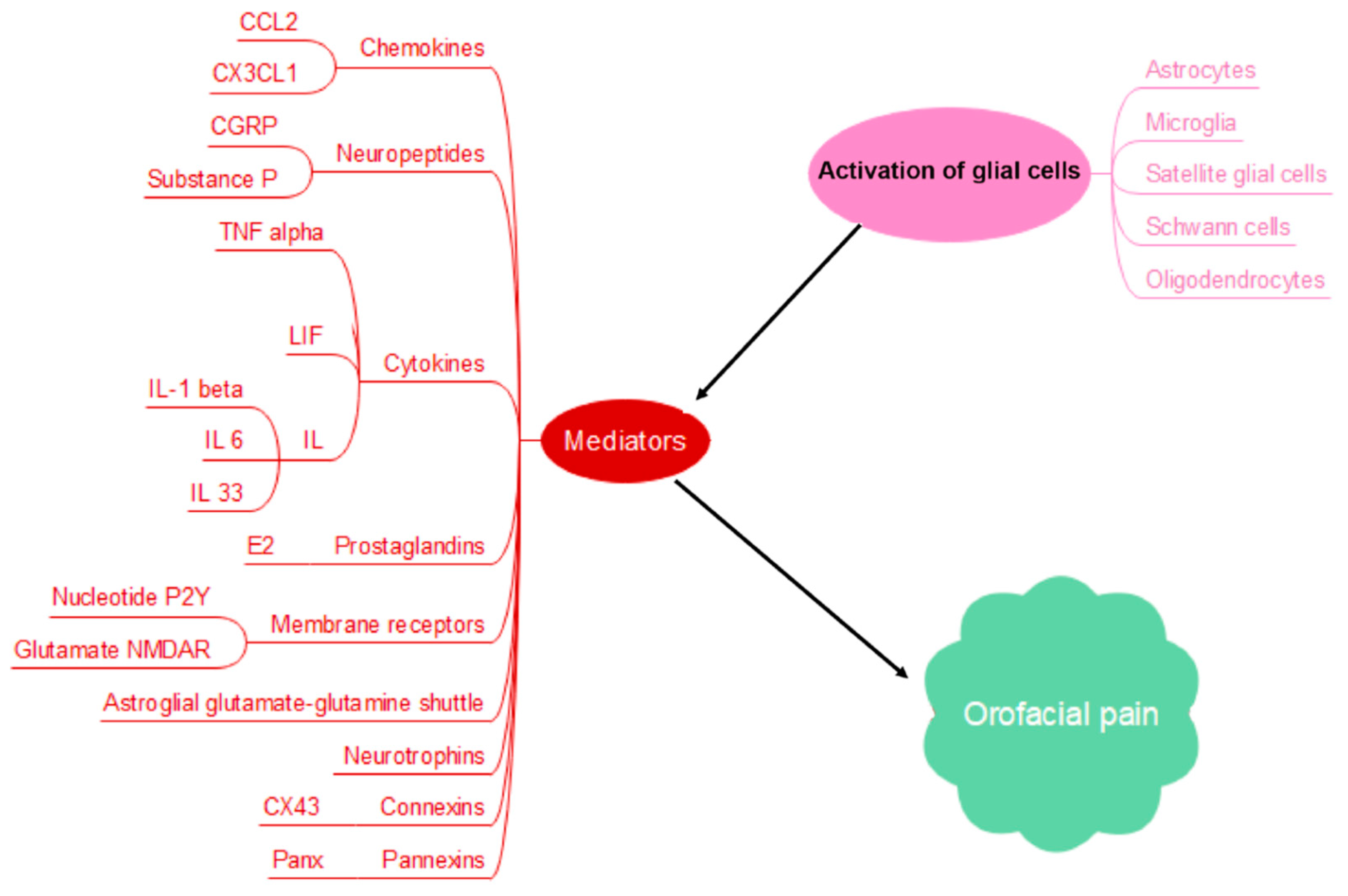
2. Glial Cell Activation and Orofacial Pain Chronicity
3. Metabolic Reprogramming and Orofacial Pain
4. Oxidative Stress and Mitochondrial Dysfunction in the Pathogenesis of Orofacial Pain
5. Epigenetic Regulation of Orofacial Pain
6. Primary Experimental or Clinical Data in Orofacial Pain Research
7. Roles of Psychosocial Factors and Systemic Health Conditions in Orofacial Pain
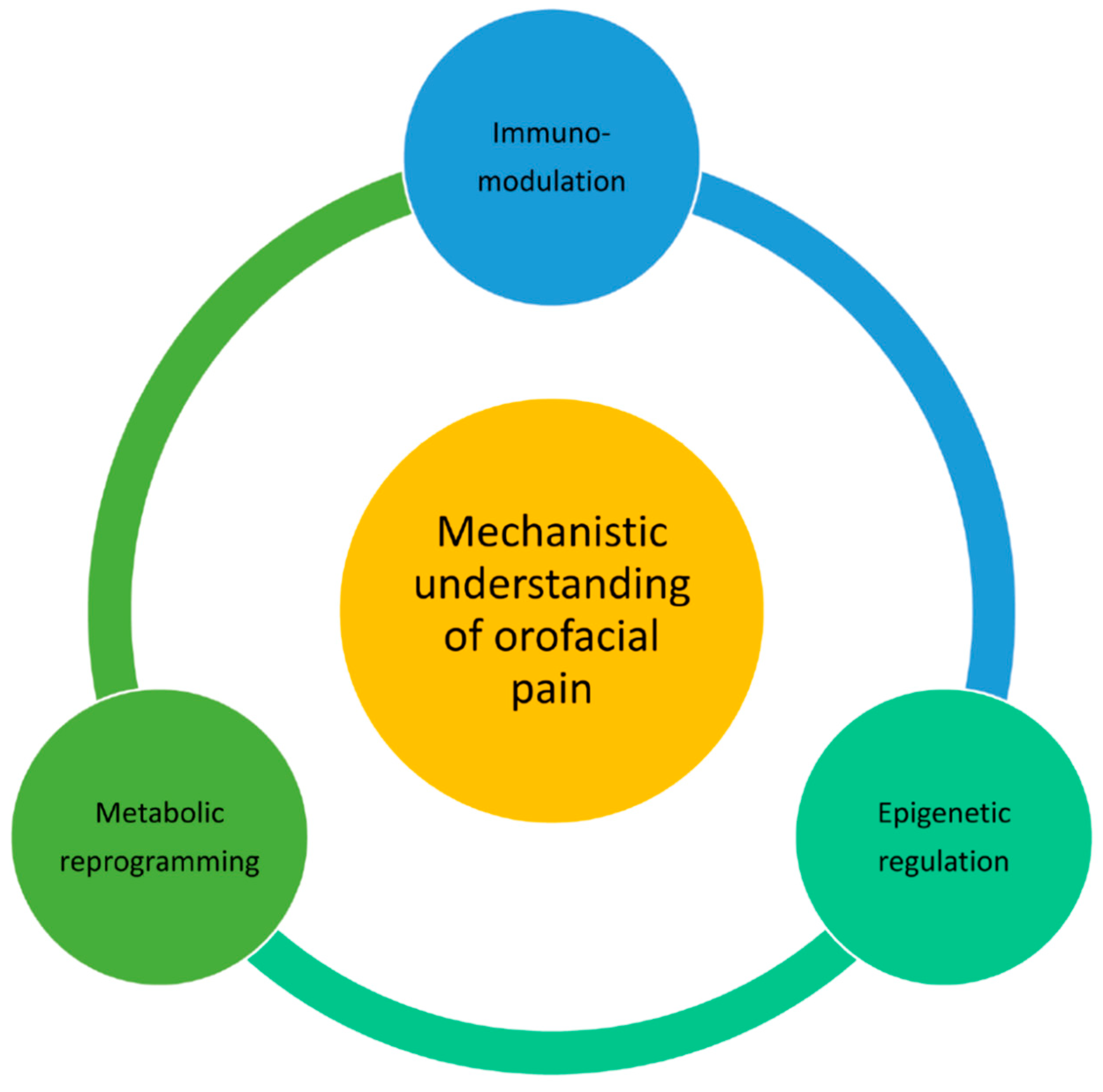
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia 2017, 37, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Labanca, M.; Gianò, M.; Franco, C.; Rezzani, R. Orofacial Pain and Dentistry Management: Guidelines for a More Comprehensive Evidence-Based Approach. Diagnostics 2023, 13, 2854. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef]
- Pigg, M.; Nixdorf, D.R.; Law, A.S.; Renton, T.; Sharav, Y.; Baad-Hansen, L.; List, T. New International Classification of Orofacial Pain: What Is in It For Endodontists? J. Endod. 2021, 47, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Imamura, Y.; Hayashi, Y.; Noma, N.; Okada-Ogawa, A.; Hitomi, S.; Iwata, K. Orofacial Neuropathic Pain-Basic Research and Their Clinical Relevancies. Front. Mol. Neurosci. 2021, 14, 691396. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.; Ye, L. A Review on Autophagy in Orofacial Neuropathic Pain. Cells 2022, 11, 3842. [Google Scholar] [CrossRef]
- Willemen, H.L.D.M.; Santos Ribeiro, P.S.; Broeks, M.; Meijer, N.; Versteeg, S.; Tiggeler, A.; De Boer, T.P.; Małecki, J.M.; Falnes, P.Ø.; Jans, J.; et al. Inflammation-induced mitochondrial and metabolic disturbances in sensory neurons control the switch from acute to chronic pain. Cell Rep. Med. 2023, 4, 101265. [Google Scholar] [CrossRef]
- Kong, E.; Li, Y.; Deng, M.; Hua, T.; Yang, M.; Li, J.; Feng, X.; Yuan, H. Glycometabolism Reprogramming of Glial Cells in Central Nervous System: Novel Target for Neuropathic Pain. Front. Immunol. 2022, 13, 861290. [Google Scholar] [CrossRef]
- Bai, G.; Ren, K.; Dubner, R. Epigenetic regulation of persistent pain. Transl. Res. 2015, 165, 177–199. [Google Scholar] [CrossRef]
- Norris, G.T.; Kipnis, J. Immune cells and CNS physiology: Microglia and beyond. J. Exp. Med. 2019, 216, 60–70. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Marrocco, F.; Limatola, C. Microglial cells: Sensors for neuronal activity and microbiota-derived molecules. Front. Immunol. 2022, 13, 1011129. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Foster, S.L.; Woolf, C.J. Neuroimmunity: Physiology and Pathology. Annu. Rev. Immunol. 2016, 34, 421–447. [Google Scholar] [CrossRef]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Kubo, A.; Hayashi, Y.; Iwata, K. Peripheral and Central Mechanisms of Persistent Orofacial Pain. Front. Neurosci. 2019, 13, 1227. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, J. Heterogeneity of macrophages in injured trigeminal nerves: Cytokine/chemokine expressing vs. phagocytic macrophages. Brain Behav. Immun. 2012, 26, 891–903. [Google Scholar] [CrossRef]
- Batbold, D.; Shinoda, M.; Honda, K.; Furukawa, A.; Koizumi, M.; Akasaka, R.; Yamaguchi, S.; Iwata, K. Macrophages in trigeminal ganglion contribute to ectopic mechanical hypersensitivity following inferior alveolar nerve injury in rats. J. Neuroinflamm. 2017, 14, 249. [Google Scholar] [CrossRef]
- Ye, Y.; Salvo, E.; Romero-Reyes, M.; Akerman, S.; Shimizu, E.; Kobayashi, Y.; Michot, B.; Gibbs, J. Glia and Orofacial Pain: Progress and Future Directions. Int. J. Mol. Sci. 2021, 22, 5345. [Google Scholar] [CrossRef]
- Zhang, S.; Azubuine, J.; Schmeer, C. A systematic literature review on the role of glial cells in the pathomechanisms of migraine. Front. Mol. Neurosci. 2023, 16, 1219574. [Google Scholar] [CrossRef]
- Epstein, J.B.; Hong, C.; Logan, R.M.; Barasch, A.; Gordon, S.M.; Oberlee-Edwards, L.; McGuire, D.; Napenas, J.J.; Elting, L.S.; Spijkervet, F.K.L.; et al. A systematic review of orofacial pain in patients receiving cancer therapy. Support. Care Cancer 2010, 18, 1023–1031. [Google Scholar] [CrossRef]
- Hidaka, K.; Ono, K.; Harano, N.; Sago, T.; Nunomaki, M.; Shiiba, S.; Nakanishi, O.; Fukushima, H.; Inenaga, K. Central glial activation mediates cancer-induced pain in a rat facial cancer model. Neuroscience 2011, 180, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Sago, T.; Ono, K.; Harano, N.; Furuta-Hidaka, K.; Hitomi, S.; Nunomaki, M.; Yoshida, M.; Shiiba, S.; Nakanishi, O.; Matsuo, K.; et al. Distinct time courses of microglial and astrocytic hyperactivation and the glial contribution to pain hypersensitivity in a facial cancer model. Brain Res. 2012, 1457, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, T.; Shinoda, M.; Honda, K.; Furukawa, A.; Kaji, K.; Nagashima, H.; Akasaka, R.; Chen, J.; Sessle, B.J.; Yonehara, Y.; et al. Involvement of Microglial P2Y12 Signaling in Tongue Cancer Pain. J. Dent. Res. 2016, 95, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Saraithong, P.; Curtin, J.G.; Janal, M.N.; Ye, Y. Reciprocal interactions between cancer and Schwann cells contribute to oral cancer progression and pain. Heliyon 2019, 5, e01223. [Google Scholar] [CrossRef]
- Salvo, E.; Tu, N.H.; Scheff, N.N.; Dubeykovskaya, Z.A.; Chavan, S.A.; Aouizerat, B.E.; Ye, Y. TNFα promotes oral cancer growth, pain, and Schwann cell activation. Sci. Rep. 2021, 11, 1840. [Google Scholar] [CrossRef]
- Eising, E.; De Leeuw, C.; Min, J.L.; Anttila, V.; Verheijen, M.H.; Terwindt, G.M.; Dichgans, M.; Freilinger, T.; Kubisch, C.; Ferrari, M.D.; et al. Involvement of astrocyte and oligodendrocyte gene sets in migraine. Cephalalgia 2016, 36, 640–647. [Google Scholar] [CrossRef]
- Villa, G.; Ceruti, S.; Zanardelli, M.; Magni, G.; Jasmin, L.; Ohara, P.T.; Abbracchio, M.P. Temporomandibular Joint Inflammation Activates Glial and Immune Cells in Both the Trigeminal Ganglia and in the Spinal Trigeminal Nucleus. Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef]
- Magni, G.; Merli, D.; Verderio, C.; Abbracchio, M.P.; Ceruti, S. P2Y2 receptor antagonists as anti-allodynic agents in acute and sub-chronic trigeminal sensitization: Role of satellite glial cells. Glia 2015, 63, 1256–1269. [Google Scholar] [CrossRef]
- Spray, D.C.; Hanani, M. Gap junctions, pannexins and pain. Neurosci. Lett. 2019, 695, 46–52. [Google Scholar] [CrossRef]
- Ahmed, F.; Rahman, M.; Thompson, R.; Bereiter, D.A. Role of Connexin 43 in an Inflammatory Model for TMJ Hyperalgesia. Front. Pain Res. 2021, 2, 715871. [Google Scholar] [CrossRef]
- Zhang, P.; Gan, Y.-H. Prostaglandin E2 Upregulated Trigeminal Ganglionic Sodium Channel 1.7 Involving Temporomandibular Joint Inflammatory Pain in Rats. Inflammation 2017, 40, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Liu, S.; Shu, H.; Tang, Y.; George, S.; Dong, T.; Schmidt, B.L.; Tao, F. TNFα in the Trigeminal Nociceptive System Is Critical for Temporomandibular Joint Pain. Mol. Neurobiol. 2019, 56, 278–291. [Google Scholar] [CrossRef]
- Kim, W.; Angulo, M.C. Unraveling the role of oligodendrocytes and myelin in pain. J. Neurochem. 2024, 169, e16206. [Google Scholar] [CrossRef]
- Jha, M.K.; Song, G.J.; Lee, M.G.; Jeoung, N.H.; Go, Y.; Harris, R.A.; Park, D.H.; Kook, H.; Lee, I.-K.; Suk, K. Metabolic Connection of Inflammatory Pain: Pivotal Role of a Pyruvate Dehydrogenase Kinase-Pyruvate Dehydrogenase-Lactic Acid Axis. J. Neurosci. 2015, 35, 14353–14369. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflügers Arch.—Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, S.; Sanal, N.; Marzano, M. Energy metabolic pathways in neuronal development and function. Oxf. Open Neurosci. 2023, 2, kvad004. [Google Scholar] [CrossRef] [PubMed]
- Nimgampalle, M.; Chakravarthy, H.; Devanathan, V. Glucose metabolism in the brain: An update. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 77–88. [Google Scholar]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Zhang, W.; Jiao, B.; Yu, S.; Zhang, C.; Zhang, K.; Liu, B.; Zhang, X. Histone deacetylase as emerging pharmacological therapeutic target for neuropathic pain: From epigenetic to selective drugs. CNS Neurosci. Ther. 2024, 30, e14745. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; He, Y.; Chen, J.; Yan, J.; Zhang, Q.; Li, L.; Cai, X. Cellular succinate metabolism and signaling in inflammation: Implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef]
- Certo, M.; Llibre, A.; Lee, W.; Mauro, C. Understanding lactate sensing and signalling. Trends Endocrinol. Metab. 2022, 33, 722–735. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Wan, J.; Dong, B.; Sun, J. Lactate: A Novel Signaling Molecule in Synaptic Plasticity and Drug Addiction. BioEssays 2019, 41, 1900008. [Google Scholar] [CrossRef] [PubMed]
- Errea, A.; Cayet, D.; Marchetti, P.; Tang, C.; Kluza, J.; Offermanns, S.; Sirard, J.-C.; Rumbo, M. Lactate Inhibits the Pro-Inflammatory Response and Metabolic Reprogramming in Murine Macrophages in a GPR81-Independent Manner. PLoS ONE 2016, 11, e0163694. [Google Scholar] [CrossRef] [PubMed]
- Nasi, A.; Fekete, T.; Krishnamurthy, A.; Snowden, S.; Rajnavölgyi, E.; Catrina, A.I.; Wheelock, C.E.; Vivar, N.; Rethi, B. Dendritic Cell Reprogramming by Endogenously Produced Lactic Acid. J. Immunol. 2013, 191, 3090–3099. [Google Scholar] [CrossRef]
- Cai, M.; Wan, J.; Cai, K.; Song, H.; Wang, Y.; Sun, W.; Hu, J. Understanding the Contribution of Lactate Metabolism in Cancer Progress: A Perspective from Isomers. Cancers 2022, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E. What Is the Role of Lactate in Brain Metabolism, Plasticity, and Neurodegeneration? Neurology 2024, 102, e209378. [Google Scholar] [CrossRef]
- Ma, X.; Qi, Q.; Wang, W.; Huang, M.; Wang, H.; Luo, L.; Xu, X.; Yuan, T.; Shi, H.; Jiang, W.; et al. Astrocytic pyruvate dehydrogenase kinase-lactic acid axis involvement in glia-neuron crosstalk contributes to morphine-induced hyperalgesia in mice. Fundam. Res. 2024, 4, 820–828. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Y.; Zhang, C.; Zhou, X. Astrocyte-neuron lactate transport in the ACC contributes to the occurrence of long-lasting inflammatory pain in male mice. Neurosci. Lett. 2021, 764, 136205. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ishikura, K.I.; Kume, K.; Ohsawa, M. Astrocyte-neuron lactate shuttle sensitizes nociceptive transmission in the spinal cord. Glia 2019, 67, 27–36. [Google Scholar] [CrossRef]
- Valério, D.; Ferreira, F.; Cunha, T.; Alves-Filho, J.; Lima, F.; De Oliveira, R., Jr.; Ferreira, S.; Cunha, F.; Queiroz, R.; Verri, W., Jr. Fructose-1,6-bisphosphate reduces inflammatory pain-like behaviour in mice: Role of adenosine acting on A1 receptors. Br. J. Pharmacol. 2009, 158, 558–568. [Google Scholar] [CrossRef]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10, 1147. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Ruparel, S. Role of Oxidized Lipids and TRP Channels in Orofacial Pain and Inflammation. J. Dent. Res. 2016, 95, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Singer, P.; Ariel, A. Beyond the classic eicosanoids: Peripherally-acting oxygenated metabolites of polyunsaturated fatty acids mediate pain associated with tissue injury and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflamm. 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Gianò, M.; Franco, C.; Castrezzati, S.; Rezzani, R. Involvement of Oxidative Stress and Nutrition in the Anatomy of Orofacial Pain. Int. J. Mol. Sci. 2023, 24, 13128. [Google Scholar] [CrossRef]
- Viggiano, E.; Monda, M.; Viggiano, A.; Viggiano, A.; Aurilio, C.; De Luca, B. Persistent facial pain increases superoxide anion production in the spinal trigeminal nucleus. Mol. Cell. Biochem. 2010, 339, 149–154. [Google Scholar] [CrossRef]
- Rodríguez De Sotillo, D.; Velly, A.M.; Hadley, M.; Fricton, J.R. Evidence of oxidative stress in temporomandibular disorders: A pilot study. J. Oral. Rehabil. 2011, 38, 722–728. [Google Scholar] [CrossRef]
- Eslami, H.; Katebi, K.; Ghaffaripour Saleh, S.; Mirizadeh, L.; Hashemi, M. The relationship between oxidative stress markers and temporomandibular disorders: A systematic review and meta-analysis. J. Res. Med. Sci. 2024, 29, 33. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ngo, L.Q.; Promsudthi, A.; Surarit, R. Salivary oxidative stress biomarkers in chronic periodontitis and acute coronary syndrome. Clin. Oral. Investig. 2017, 21, 2345–2353. [Google Scholar] [CrossRef]
- Goriuc, A.; Cojocaru, K.-A.; Luchian, I.; Ursu, R.-G.; Butnaru, O.; Foia, L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. Int. J. Mol. Sci. 2024, 25, 1425. [Google Scholar] [CrossRef]
- Xu, M.; Fang, L.; Xue, Q.; Zhang, X.; He, Y. The Nrf2 Pathway Alleviates Overloading Force-Induced TMJ Degeneration by Downregulating Oxidative Stress Reactions. J. Inflamm. Res. 2023, 16, 5601–5612. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [PubMed]
- Van Den Ameele, J.; Fuge, J.; Pitceathly, R.D.S.; Berry, S.; McIntyre, Z.; Hanna, M.G.; Lee, M.; Chinnery, P.F. Chronic pain is common in mitochondrial disease. Neuromuscul. Disord. 2020, 30, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.-L.; Sui, B.-D.; Wang, X.-Y.; Wei, Y.-Y.; Huang, J.; Chen, J.; Wu, S.-X.; Li, Y.-Q.; Wang, Y.-Y.; Yang, Y.-L. Significant changes in mitochondrial distribution in different pain models of mice. Mitochondrion 2013, 13, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.A.; Tiwari, L.; Farah, C.S. Epigenetics and oral disease. In Translational Systems Medicine and Oral Disease; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–206. [Google Scholar]
- Nirvanie-Persaud, L.; Millis, R.M. Epigenetics and Pain: New Insights to an Old Problem. Cureus 2022, 14, e29353. [Google Scholar] [CrossRef]
- Massart, R.; Dymov, S.; Millecamps, M.; Suderman, M.; Gregoire, S.; Koenigs, K.; Alvarado, S.; Tajerian, M.; Stone, L.S.; Szyf, M. Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Sci. Rep. 2016, 6, 19615. [Google Scholar] [CrossRef]
- Luo, D.; Li, X.; Tang, S.; Song, F.; Li, W.; Xie, G.; Liang, J.; Zhou, J. Epigenetic modifications in neuropathic pain. Mol. Pain 2021, 17, 174480692110567. [Google Scholar] [CrossRef]
- Smith, S.B.; Mir, E.; Bair, E.; Slade, G.D.; Dubner, R.; Fillingim, R.B.; Greenspan, J.D.; Ohrbach, R.; Knott, C.; Weir, B.; et al. Genetic Variants Associated With Development of TMD and Its Intermediate Phenotypes: The Genetic Architecture of TMD in the OPPERA Prospective Cohort Study. J. Pain 2013, 14, T91–T101.e3. [Google Scholar] [CrossRef]
- Xiao, J.-L.; Meng, J.-H.; Gan, Y.-H.; Li, Y.-L.; Zhou, C.-Y.; Ma, X.-C. DNA methylation profiling in different phases of temporomandibular joint osteoarthritis in rats. Arch. Oral Biol. 2016, 68, 105–115. [Google Scholar] [CrossRef]
- Bonomini, F.; Favero, G.; Castrezzati, S.; Borsani, E. Role of Neurotrophins in Orofacial Pain Modulation: A Review of the Latest Discoveries. Int. J. Mol. Sci. 2023, 24, 12438. [Google Scholar] [CrossRef]
- Nakae, A.; Nakai, K.; Tanaka, T.; Hosokawa, K.; Mashimo, T.; Oro-facial Study Group. Epigenetic regulation of BDNF genes in rat orofacial neuropathic pain model: 14AP3-1. Eur. J. Anaesthesiol. 2013, 30, 210–211. [Google Scholar] [CrossRef]
- Westlund, K.N.; Montera, M.; Goins, A.E.; Shilling, M.W.; Afaghpour-Becklund, M.; Alles, S.R.A.; Elise Hui, S. Epigenetic HDAC5 Inhibitor Reverses Craniofacial Neuropathic Pain in Mice. J. Pain 2024, 25, 428–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, Y.-Q.; Zou, F.; Bie, B.; Pan, Z.Z. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat. Med. 2011, 17, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Roganović, J.; Petrović, N. Clinical Perspectives of Non-Coding RNA in Oral Inflammatory Diseases and Neuropathic Pain: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 8278. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Makunin, I.V. Non-Coding RNA. Hum. Mol. Genet. 2006, 15, R17–R29. [Google Scholar] [CrossRef]
- Fu, X.D. Non-Coding RNA: A New Frontier in Regulatory Biology. Natl. Sci. Rev. 2014, 1, 190–204. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-Coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-Coding RNAs in Disease: From Mechanisms to Therapeutics. Nat. Rev. Genet. 2023, 25, 211–232. [Google Scholar] [CrossRef]
- Soutschek, M.; Schratt, G. Non-Coding RNA in the Wiring and Remodeling of Neural Circuits. Neuron 2023, 111, 2140–2154. [Google Scholar] [CrossRef]
- El-Gewely, M.R. Dysregulation of Regulatory NcRNAs and Diseases. Int. J. Mol. Sci. 2023, 25, 24. [Google Scholar] [CrossRef]
- Loganathan, T.; Doss C, G.P. Non-Coding RNAs in Human Health and Disease: Potential Function as Biomarkers and Therapeutic Targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef]
- Malgundkar, S.H.; Tamimi, Y. The Pivotal Role of Long Non-Coding RNAs as Potential Biomarkers and Modulators of Chemoresistance in Ovarian Cancer (OC). Hum. Genet. 2024, 143, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qiu, W.; Wu, B.; Fang, F. Long Non-Coding RNAs Are Novel Players in Oral Inflammatory Disorders, Potentially Premalignant Oral Epithelial Lesions and Oral Squamous Cell Carcinoma (Review). Int. J. Mol. Med. 2020, 46, 535. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Ferreira, D.; Lawless, N.; Bird, E.V.; Atkins, S.; Collier, D.; Sher, E.; Malki, K.; Lambert, D.W.; Boissonade, F.M. Correlation of MiRNA Expression with Intensity of Neuropathic Pain in Man. Mol. Pain 2019, 15, 1744806919860323. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Byun, J.-S.; Jung, J.-K.; Choi, J.-K. Profiling of Salivary Exosomal Micro RNAs in Burning Mouth Syndrome Patients. J. Oral Med. Pain 2019, 44, 25–30. [Google Scholar]
- Li, X.; Wang, D.; Zhou, J.; Yan, Y.; Chen, L. Evaluation of Circulating MicroRNA Expression in Patients with Trigeminal Neuralgia: An Observational Study. Medicine 2020, 99, e22972. [Google Scholar] [CrossRef]
- Li, G.; Jiang, H.; Zheng, C.; Zhu, G.; Xu, Y.; Sheng, X.; Wu, B.; Guo, J.; Zhu, S.; Zhan, Y.; et al. Long Noncoding RNA MRAK009713 Is a Novel Regulator of Neuropathic Pain in Rats. Pain 2017, 158, 2042–2052. [Google Scholar] [CrossRef]
- Fang, Z.H.; Liao, H.L.; Tang, Q.F.; Liu, Y.J.; Zhang, Y.Y.; Lin, J.; Yu, H.P.; Zhou, C.; Li, C.J.; Liu, F.; et al. Interactions Among Non-Coding RNAs and MRNAs in the Trigeminal Ganglion Associated with Neuropathic Pain. J. Pain Res. 2022, 15, 2967–2988. [Google Scholar] [CrossRef]
- Walczyńska-Dragon, K.; Kurek-Górecka, A.; Niemczyk, W.; Nowak, Z.; Baron, S.; Olczyk, P.; Nitecka-Buchta, A.; Kempa, W.M. Cannabidiol Intervention for Muscular Tension, Pain, and Sleep Bruxism Intensity—A Randomized, Double-Blind Clinical Trial. J. Clin. Med. 2024, 13, 1417. [Google Scholar] [CrossRef]
- Crescente, G.; Minervini, G.; Spagnuolo, C.; Moccia, S. Cannabis Bioactive Compound-Based Formulations: New Perspectives for the Management of Orofacial Pain. Molecules 2022, 28, 106. [Google Scholar] [CrossRef]
- Sitnikova, V.; Kämppi, A.; Kämppi, L.; Alvesalo, E.; Burakova, M.; Kemppainen, P.; Teronen, O. Clinical Benefit of Botulinum Toxin for Treatment of Persistent TMD-Related Myofascial Pain: A Randomized, Placebo-Controlled, Cross-over Trial. Pain Pract. 2024, 24, 1014–1023. [Google Scholar] [CrossRef]
- Ma, S.J.; Iovoli, A.J.; Wang, K.; Neimanis, D.; Smith, K.A.; Attwood, K.; Farrugia, M.; Hermann, G.; Singh, A.K. Efficacy of Prophylactic High-Dose Gabapentin and Venlafaxine on Reducing Oral Mucositis Pain Among Patients Treated With Chemoradiation for Head and Neck Cancer: A Single-Institution, Phase 2, Randomized Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Sulukdjian, A.; L’Homme, R.; Chanlon, A.; Moreau, N. Gabapentinoid Prescription in Oral Medicine and Oral Surgery Practice. Part I—Experience from a French Orofacial Pain Clinic. J. Oral Med. Oral Surg. 2020, 26, 13. [Google Scholar] [CrossRef][Green Version]
- De la Torre Canales, G.; Poluha, R.L.; Bonjardim, L.R.; Ernberg, M.; Conti, P.C.R. Botulinum Toxin-A Effects on Pain, Somatosensory and Psychosocial Features of Patients with Refractory Masticatory Myofascial Pain: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2024, 14, 4201. [Google Scholar] [CrossRef]
- Cook, A.; Modh, A.; Ali, H.; Sheqwara, J.; Chang, S.; Ghanem, T.; Momin, S.; Wu, V.; Tam, S.; Money, S.; et al. Randomized Phase 3, Double-Blind, Placebo-Controlled Study of Prophylactic Gabapentin for the Reduction of Oral Mucositis Pain During the Treatment of Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 926–937. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising Advances in Clinical Trials of Dental Tissue-Derived Cell-Based Regenerative Medicine. Stem Cell Res. Ther. 2020, 11, 175. [Google Scholar] [CrossRef]
- Hanif, M.; Sheikh, M.A. Efficacy of Platelet Rich Plasma (PRP) on Mouth Opening and Pain after Surgical Extraction of Mandibular Third Molars. J. Oral Med. Oral Surg. 2021, 27, 9. [Google Scholar] [CrossRef]
- Li, F.; Wu, C.; Sun, H.; Zhou, Q. Effect of Platelet-Rich Plasma Injections on Pain Reduction in Patients with Temporomandibular Joint Osteoarthrosis: A Meta-Analysis of Randomized Controlled Trials. J. Oral Facial Pain Headache 2020, 34, 149–156. [Google Scholar] [CrossRef]
- Clinical Trial of the Safety and Efficacy of Peripheral Nerve Stimulation in the Treatment of Postherpetic Neuralgia. Clinical Research Trial Listing. Available online: https://www.centerwatch.com/clinical-trials/listings/NCT06290661/clinical-trial-of-the-safety-and-efficacy-of-peripheral-nerve-stimulation-in-the-treatment-of-postherpetic-neuralgia (accessed on 22 January 2025).
- UCSF Pain Trial → Transcranial Magnetic Stimulation for Chronic Neuropathic Pain. Available online: https://clinicaltrials.ucsf.edu/trial/NCT05593237 (accessed on 22 January 2025).
- Liao, M.; Diao, Y.; Pan, J.; Wong, L.S.; Subramaniam, G.; Vasanthi, R.K.; Liao, L. Efficacy and Safety of Repetitive Transcranial Magnetic Stimulation with Different Frequencies on Neuropathic Orofacial Pain: A Systematic Literature Review and Meta-Analysis. J. Oral Facial Pain Headache 2024, 38, 48–67. [Google Scholar] [CrossRef]
- Di Francesco, F.; Minervini, G.; Siurkel, Y.; Cicciù, M.; Lanza, A. Efficacy of Acupuncture and Laser Acupuncture in Temporomandibular Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Oral Health 2024, 24, 174. [Google Scholar] [CrossRef]
- Ferrando, M.; Galdón, M.J.; Durá, E.; Andreu, Y.; Jiménez, Y.; Poveda, R. Enhancing the Efficacy of Treatment for Temporomandibular Patients with Muscular Diagnosis through Cognitive-Behavioral Intervention, Including Hypnosis: A Randomized Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 81–89. [Google Scholar] [CrossRef]
- Patil, D.J.; Dheer, D.S. Psychological Assessment and Cognitive Behavioral Therapy in Temporomandibular Joint Disorders: A Randomized Controlled Study. J. Indian Acad. Oral Med. Radiol. 2023, 35, 326–330. [Google Scholar] [CrossRef]
- Reviewing the Evidence: Can Cognitive Behavioral Therapy Improve Outcomes for Patients with Chronic Orofacial Pain? Available online: https://www.jofph.com/articles/10.11607/jofph.24.2.04 (accessed on 22 January 2025).
- Shi, Y.; Wu, W. Multimodal Non-Invasive Non-Pharmacological Therapies for Chronic Pain: Mechanisms and Progress. BMC Med. 2023, 21, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Aboushaar, N.; Serrano, N. The Mutually Reinforcing Dynamics between Pain and Stress: Mechanisms, Impacts and Management Strategies. Front. Pain Res. 2024, 5, 1445280. [Google Scholar] [CrossRef]
- De Ridder, D.; Adhia, D.; Vanneste, S. The Anatomy of Pain and Suffering in the Brain and Its Clinical Implications. Neurosci. Biobehav. Rev. 2021, 130, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Assiri, K. Relationships between Personality Factors and DC/TMD Axis II Scores of Psychosocial Impairment among Patients with Pain Related Temporomandibular Disorders. Sci. Rep. 2024, 14, 26869. [Google Scholar] [CrossRef]
- Hekmati, A.; Mortazavi, N.; Ozouni-Davaji, R.B.; Vakili, M. Personality Traits and Anxiety in Patients with Temporomandibular Disorders. BMC Psychol. 2022, 10, 86. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, J.; Liang, Y.; Sun, J.; Zheng, Z. Causal Relationship between Psychiatric Traits and Temporomandibular Disorders: A Bidirectional Two-Sample Mendelian Randomization Study. Clin. Oral Investig. 2023, 27, 7513–7521. [Google Scholar] [CrossRef]
- Ye, C.; Pu, D.; Zhang, J.; Jia, M.; Zhang, Y.; Du, S.; Wang, J.; Xiong, X. Unlocking the Link between Temporomandibular Disorders and Suicide Ideation in Pre-Orthodontic Patients: A Moderated Mediation Model of Depression and Anxiety. J. Affect. Disord. 2024, 349, 486–493. [Google Scholar] [CrossRef]
- Lee, Y.H.; Auh, Q.S. Clinical Factors Affecting Depression in Patients with Painful Temporomandibular Disorders during the COVID-19 Pandemic. Sci. Rep. 2022, 12, 14667. [Google Scholar] [CrossRef]
- Calixtre, L.B.; Da Grüninger, B.L.S.; Chaves, T.C.; De Oliveira, A.B. Is There an Association between Anxiety/Depression and Temporomandibular Disorders in College Students? J. Appl. Oral Sci. 2014, 22, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, P.; Long, L.; He, H.; Zhang, J.; Sun, S. Diabetes Mellitus in Classical Trigeminal Neuralgia: A Predisposing Factor for Its Development. Clin. Neurol. Neurosurg. 2016, 151, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.M.; Karlsson, P.; Bonjardim, L.R.; Conti, P.C.R.; Tankisi, H.; Jensen, T.S.; Nyengaard, J.R.; Svensson, P.; Baad-Hansen, L. Trigeminal Nociceptive Function and Oral Somatosensory Functional and Structural Assessment in Patients with Diabetic Peripheral Neuropathy. Sci. Rep. 2019, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, K.; Jain, A.; Sharma, R.; Prashar, S.; Jain, R. Diabetes and Periodontitis. J. Indian Soc. Periodontol. 2010, 14, 207–212. [Google Scholar] [CrossRef]
- Salvi, G.E.; Carollo-Bittel, B.; Lang, N.P. Effects of Diabetes Mellitus on Periodontal and Peri-Implant Conditions: Update on Associations and Risks. J. Clin. Periodontol. 2008, 35, 398–409. [Google Scholar] [CrossRef]
- Gurav, A.; Jadhav, V. Periodontitis and Risk of Diabetes Mellitus. J. Diabetes 2011, 3, 21–28. [Google Scholar] [CrossRef]
- Pontes Andersen, C.C.; Flyvbjerg, A.; Buschard, K.; Holmstrup, P. Relationship between Periodontitis and Diabetes: Lessons from Rodent Studies. J. Periodontol. 2007, 78, 1264–1275. [Google Scholar] [CrossRef]
- López-López, J.; Garcia-Vicente, L.; Jané-Salas, E.; Estrugo-Devesa, A.; Chimenos-Küstner, E.; Roca-Elias, J. Orofacial Pain of Cardiac Origin: Review Literature and Clinical Cases. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e538. [Google Scholar] [CrossRef]
- Kreiner, M.; Okeson, J.; Tanco, V.; Waldenström, A.; Isberg, A. Orofacial Pain and Toothache as the Sole Symptom of an Acute Myocardial Infarction Entails a Major Risk of Misdiagnosis and Death. J. Oral Facial Pain Headache 2020, 34, 53–60. [Google Scholar] [CrossRef]
- Choi, E.; Lee, Y.H.; Park, H.K. Orofacial Pain with Cardiac Origin of Coronary Artery Disease: A Case Report and Literature Review. Case Rep. Dent. 2023, 2023, 6304637. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Tao, F. Mechanisms for Orofacial Pain: Roles of Immunomodulation, Metabolic Reprogramming, Oxidative Stress and Epigenetic Regulation. Biomedicines 2025, 13, 434. https://doi.org/10.3390/biomedicines13020434
Khan S, Tao F. Mechanisms for Orofacial Pain: Roles of Immunomodulation, Metabolic Reprogramming, Oxidative Stress and Epigenetic Regulation. Biomedicines. 2025; 13(2):434. https://doi.org/10.3390/biomedicines13020434
Chicago/Turabian StyleKhan, Saniyya, and Feng Tao. 2025. "Mechanisms for Orofacial Pain: Roles of Immunomodulation, Metabolic Reprogramming, Oxidative Stress and Epigenetic Regulation" Biomedicines 13, no. 2: 434. https://doi.org/10.3390/biomedicines13020434
APA StyleKhan, S., & Tao, F. (2025). Mechanisms for Orofacial Pain: Roles of Immunomodulation, Metabolic Reprogramming, Oxidative Stress and Epigenetic Regulation. Biomedicines, 13(2), 434. https://doi.org/10.3390/biomedicines13020434




